Abstract
This study evaluated the effect of chitosan, poly vinyl alcohol (PVA) and poly (2-hydroxyethyl methacrylate) (pHEMA) on delaying the human fibroblast senescence. Cells could form suspending multicellular spheroids on these biomaterials, but only chitosan was capable of decreasing the SA β-gal activity and increasing the proliferation ability of senescent fibroblasts. Therefore, in addition to the structure of multicellular spheroids, chitosan itself should play an important role in delaying fibroblast senescence. The main difference of senescence-related protein expressions for cells cultured on chitosan, PVA and pHEMA occurred on the TGF-β signaling pathway. In addition to the intracellular TGF-β expression, the extracellular TGF-β expression was also downregulated. Chitosan with cationic amino structure was assumed to bind with anionic TGF-β by forming polyelectrolyte complexes. This assumption was demonstrated by directly adding chitosan into the medium to downregulate the cell TGF-β expression and further to delay cell senescence, indicating TGF-β signaling pathway was involved in the chitosan-mediating fibroblast senescence process. Finally, the delaying cell senescence ability of chitosan increased with increasing the amount of amino groups in chitosan and its ionization degree. In summary, these results provide important information for considering the application of chitosan in the future cell therapy and regeneration medicine.
Introduction
Cell senescence indicates a state of stopped division after limited proliferation, generally followed by specific features such as enlargement morphology [Citation1–3], high senescence-associated β-galactosidase (SA β-gal) activity [Citation4,Citation5] and low proliferation rate ability [Citation6,Citation7]. Cell senescence in vitro would increase the time of cell expansion, decrease the cell viability, and impair the cell functions for following cell therapy. Many triggers have been used to induce cellular senescence such as DNA damage [Citation8], telomere shorten [Citation9], reactive oxygen species (ROS) [Citation10–12], senescence-associated secretory phenotypes (SASPs) [Citation13,Citation14] and oncogenic signaling [Citation15,Citation16]. Conversely, cell senescence might be modulated or delayed by appropriate chemicals or techniques [Citation17–20]. For example, lower oxygen level has been shown to be an important environmental factor to mediate cell proliferation and senescence [Citation11,Citation21]. Our previous study showed chitosan could suspend fibroblasts to form multicellular spheroids to effectively delay the cell senescence process [Citation22]. Therefore, the purpose of this study is to investigate whether the formation of multicellular spheroids played an important role in delaying cell senescence. For comparison, hydrophilic polymers poly vinyl alcohol (PVA) and poly (2-hydroxyethyl methacrylate) (pHEMA) with cell unfavorable detachment property were included in this study.
Chitosan, modified from chitin by deacetylation, exhibits biodegradable, biocompatible and pH-sensitive characteristics [Citation23,Citation24]. For tissue engineering applications, chitosan has been confirmed to enhance the salivary gland branching morphogenesis [Citation25], the stemness capabilities of adipose-derived stem cells [Citation26], and control of cell attachment/detachment [Citation27]. To our knowledge, cellular senescence modulated by biomaterial properties and the senescence-related signaling events involved are poorly understood [Citation28]. Our previous study demonstrated that senescent fibroblasts treated by chitosan were accompanied by a continuous decrease in the retinoblastoma protein (pRB) [Citation22], a checkpoint for controlling cell cycle [Citation29,Citation30]. Chitosan is a cationic polyelectrolyte, which can easily bind to anionic growth factors by forming polyelectrolyte complexes to regulate biological function of growth factors [Citation31,Citation32]. Thus, in addition to the formation of multicellular structure, the senescence-modulating effect of chitosan stems possibly from its interaction with senescent stimulants. For example, transforming growth factor-β (TGF-β), one of SASPs, is able to phosphorylate Smad proteins and is well known to be involved in senescence-related pathways [Citation33,Citation34]. Therefore, this study also investigates whether chitosan itself with specific structure played a vital role in delaying fibroblast senescence. These results would provide important information for considering the effects of chitosan treatment for further clinical application of cell therapy and regeneration medicine.
Materials and methods
Preparation biomaterial substrates
Biomaterials were coated on tissue culture polystyrene (TCPS) using the method described in our previous study [Citation22]. 1% (w/w) chitosan (Sigma-Aldrich, C3646, 77% degree of deacetylation (DDA)), 1% (w/w) PVA (Chemika Fluka, MW 72,000 g/mol) and 0.6% (w/w) pHEMA (Sigma-Aldrich, 25249–16-5) were dissolved in acetic acid, dimethyl sulfoxide and ethanol, respectively. The polymer solutions were coated to plates which were following dried in an oven at 60 °C. Before cells were seeded, these plates were sterilized by the ultraviolet light.
For preparation of chitosan with different DDA, 10% (w/v) chitosan was added into 50% (w/w) sodium hydroxide solution. Then, the chitosan solutions were reacted in oil bath at 80 °C for 30 min (85% DDA chitosan) and 2 h (90% DDA chitosan). After reacting, these products were washed by distilled water and filtered until the pH of waste water was neutral. Finally, the products were dried and dissolved in acetic acid for subsequent experiments as described above.
Cell culture
Human foreskin fibroblasts were isolated from specimens of donors who needed to undergo circumcision. The ages of donors were about fifteen to thirty, and five strains were used in this study. The study protocol was approved by the Institutional Review Board of the National Taiwan University Hospital. The isolated and cultured protocols have been described [Citation35]. The cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Gibco), which contained 10% fetal bovine serum (FBS, Biological Industries), 100 U/ml penicillin G, 100 μg/ml streptomycin, 250 μg/ml amphotericin B (Biological Industries) at 37 °C in a humidified chamber with 5% CO2 and 95% air atmosphere.
Cells were seeded on six-well TCPS at the density about 5 × 103/cm2 routinely and subcultured for calculating the population doublings. Cells were visualized under a microscope (Olympus IX71). For biomaterial treatments, cells were collected from TCPS and seeded on biomaterial-coated plates. Polymer-adding medium were prepared as previous studies [Citation25,Citation36]. 3% Chitosan, 5% PVA and 1.2% pHEMA solutions were prepared and mixed with the medium for specified concentrations.
Viability assay
Cell viabilities were observed by the Live/Dead kit (Invitrogen, 3224) and Alexa Fluor® 488 Annexin V/Dead Cell Apoptosis Kit (Invitrogen, V13241). Live cells stained with calcein AM (green) and dead cells labeled with ethidium homodimer (red) were observed under a confocal fluorescence microscope (LEICA, SP5). Moreover, fibroblast spheroids were dissociated by trypsinization, and cells were stained with the annexin V and propidium iodide (PI) according to the manufacture’s protocol. The cell viabilities were quantified by flow cytometry (BD Biosciences, FACSCalibur).
SA β-gal staining
Cells were fixed in 4% formaldehyde and incubated with X-gal in SA β-gal staining solution at pH 6 in the absence of CO2 at 37 °C [Citation4,Citation5,Citation37]. The stained cells were photographed, and the percentages of cells with blue sedimentation in the cytoplasm were counted. For calculating the percentages of cells stained SA β-gal, at least 400 cells were calculated from ten randomly selected fields for each case.
5-Bromo-2′-deoxyuridine (BrdU) incorporation assay
Cells were incubated with BrdU for 24 h, and then BrdU incorporation was assayed according to the Cell Proliferation Enzyme-Linked ImmunoSorbent Assay (ELISA) BrdU kit (Roche, 11 647 229 001) protocol. Results were quantified using an ELISA reader (Molecular Devices, Spectra Max M2e) at dual wavelength of 450 and 690 nm.
Western blotting
Cells were lysed with the lysis buffer (RIPA, Roche Diagnostics GmbH). Proteins from lysed cells were quantitated by Bio-Rad total protein assay, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred onto nitrocellulose membranes. The membranes were probed with human primary antibodies against p53 (Merck OP09; PAb1891; 1:200), p16 (BD Pharmingen 551153; G175–405; 1:1000), p21 (Cell Signaling #2946; DSC60; 1:2000), pRB (Merck OP66; LM95.1; 1:100), TGF-β (Cell signaling 3709; 56E4; 1:1000), phosphorylated Smad2/3 (pSmad2/3; Cell Signaling #8828; D27F4; 1:1000), Smad2/3 (Cell Signaling #8828; D7G7; 1:1000), and GAPDH (Abcam ab22555; polyclone; 1:10000), incubated in HRP-conjugated secondary antibodies (Goat anti-rabbit IgG (Abcam ab97051; 1:5000) and Goat anti-mouse IgG (Abcam ab97023; 1:5000)), and then visualized by enhanced chemiluminescence (ECL, Millipore).
Quantitative of extracellular TGF-β concentration
Cells were cultured on biomaterial-coated plates for 3 days, and then the cultured medium was collected. Cultured medium was mix with 1 N HCl solution. After incubating at room temperature for 10 min, 1.2 N NaOH/0.5 M HEPES was added to neutralize. Then, the Human TGF-β1 Quantikine ELISA Kit (R&D systems, DB100B) was used according to the manufacture’s protocol. Results were quantified using an ELISA reader at dual wavelength of 450 and 540 nm.
Statistical analysis
All data were shown as the mean ± standard deviation (SD) from at least three independent experiments. Statistical significance was determined using one-way analysis of variance (ANOVA) followed by Duncan’s test. p Values of <.05 and .01 were considered significant.
Results
Optimization of culture condition for senescent fibroblast spheroids on chitosan
In this study, senescent fibroblasts (about 45–50 population doublings) were cultured on TCPS and collected for all subsequent tests. shows senescent fibroblasts seeded on chitosan for 1 day (1 DIV), 3 days (3 DIV) and 5 days (5 DIV) could suspend on chitosan to form multicellular spheroids and the size of spheroids increased with increasing cell seeding density and culture time (). The majority of cells in the spheroids for 1 and 3DIV were viable, stained by calcein AM (green), but many cells in spheroids for 5 DIV were stained by ethidium homodimer (red), marked by dead cells. These spheroids were collected from the chitosan-coated wells and then reseeded on TCPS for additional 5 days. Spheroids could attach onto TCPS wells and cells could migrate out of the spheroids. Compared to fibroblasts always on TCPS without culturing on chitosan (w/o treatment), cells after chitosan treatment for 3 days significantly decreased the percentage of SA β-gal-positive cells from about 20% to less than 10% (, p < .01). Conversely, cells cultured on chitosan for 5 DIV increased the percentage of SA β-gal-positive cells (p < .01). Especially, most of cells at 5 × 104/cm2 seeding density were dead and could not attach onto TCPS (). For cell proliferation assay, BrdU incorporation assay showed cells cultured on chitosan for 1 and 3 DIV exhibited significantly higher proliferation rate than cells without chitosan treatment (, p < .01). Overall, cells at 2.5 × 104/cm2 seeding density treated by chitosan for 3 DIV possessed the lowest percentage of SA β-gal-positive cells and the highest BrdU incorporation absorbance. Therefore, senescent cells at 2.5 × 104/cm2 seeding density for 3-day chitosan treatment were chosen for the subsequent experimental tests.
Figure 1. Fibroblasts were cultured on chitosan-coated plates. (A) Optical and confocal microscopic live/dead staining images of fibroblast multicellular spheroids. Scale bar = 100 μm. (B) SA β-gal staining images of cells. Scale bar = 200 μm. (C) The percentages of cells stained by SA β-gal. N/A means no attached cells could be counted. (D). BrdU incorporation assay of cells (n = 4). #p < .05 and ##p < .01 mean that these groups were compared to the w/o treatment group. *p < .05.
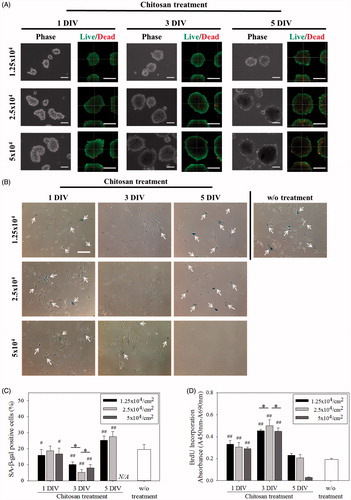
Table 1. Diameters of fibroblast multicellular spheroids on chitosan-coated plates during 5-day cultured period. Unit: μm.
Senescent fibroblast spheroids on PVA and pHEMA
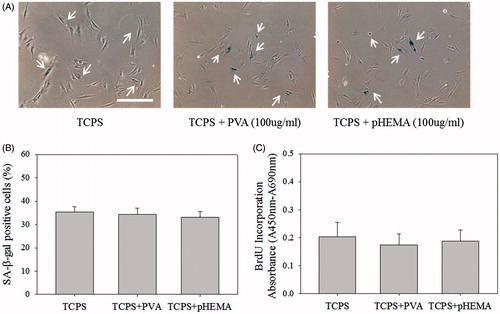
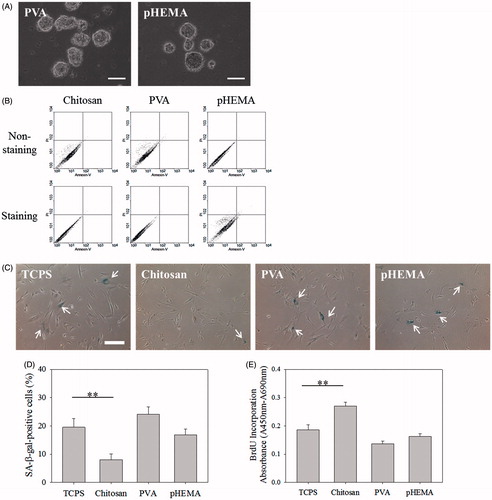
In addition to chitosan, PVA and pHEMA also could suspend fibroblasts to form multicellular spheroids, as shown in . Flow cytometry assay showed no obvious apoptosis and necrosis for senescent fibroblasts cultured on these biomaterials for 3 days, stained by annexin V and PI, (). However, compared with senescent fibroblasts always cultured on TCPS, cells cultured on PVA or pHEMA for 3 DIV did not decrease the percentage of SA β-gal-positive cells () and did not increase the BrdU incorporation absorbance (). These results indicate, dissimilar to chitosan, PVA and pHEMA could not delay the senescence of fibroblasts, even these two biomaterials could induce the aggregation of suspending cells to form multicellular spheroids.
Figure 2. Fibroblasts were cultured on PVA and pHEMA-coated plates. (A) Optical images of multicellular spheroids on PVA and pHEMA. Scale bar = 100 μm. (B) Viability of cells within the multicellular spheroids on chitosan, PVA and pHEMA determined by PI/annexin-V-FITC labeling. (C) SA β-gal staining images of biomaterial treatment cells. Scale bar = 200 μm. (D) The percentages of biomaterial treatment cells stained by SA β-gal. (E) BrdU incorporation assay of biomaterial treatment cells (n = 4). **p < .01.
Expression of senescence-related proteins
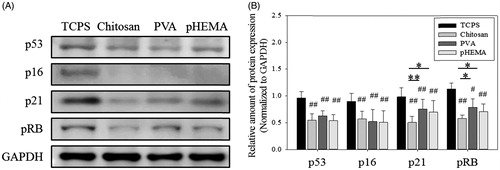
Western blot assay was used to analyze the effect of biomaterial treatment on the expression of senescence-related proteins. Compared with TCPS culture without further treatment, the expression of p53 and p16 proteins of senescent cells could be significantly downregulated by the treatment of chitosan, PVA and pHEMA for 3 days (, p < .01). P53 and p16 are two tumor suppressor proteins and have been demonstrated in different cell senescence pathways [Citation38–40]. Although these two proteins’ expression of senescent fibroblasts treated by these biomaterials could be successfully downregulated, the expression of their downstream protein p21 and common cell cycle mediator pRB exhibited significant difference (p < .05 and p < .01).
Figure 3. Senescence-related protein expression of fibroblasts on biomaterials. (A) Western blot results of p53, p16, p21, and pRB expressions in cells. (B) The relative amount of p53, p16, p21, and pRB protein expressions in cells (n > 3). #p < .05 and ##p < .01 mean these groups were compared to the TCPS groups. *p < .05, **p < .01.
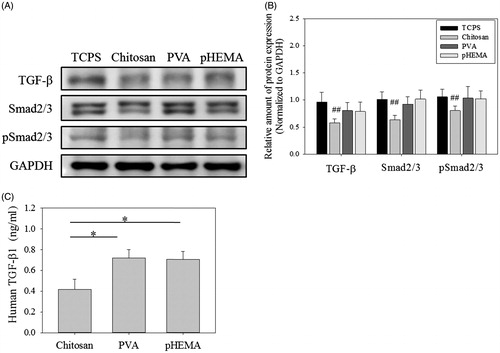
TGF-β has been reported to play an important role in inducing senescence in several cell types [Citation41–43] and Smad2/3 is well known to be involved in TGF-β-related pathways [Citation33], so the senescent effect of TGF-β on human foreskin fibroblasts was also assessed in this study. As shown in Figure S1, the tendency of cell senescence increased with increasing the TGF-β concentration. Therefore, we assumed chitosan could suppress TGF-β signaling to delay the progress of fibroblast senescence. shows the intracellular protein expression of TGF-β, Smad2/3 and pSmad2/3 of senescent fibroblasts cultured on chitosan, PVA and pHEMA for 3 days. Clearly, compared to cells cultured on TCPS, only cells treated by chitosan showed significantly lower expression of TGF-β, Smad2/3 and pSmad2/3 (p < .01). For cells treated by PVA and pHEMA, they could not decline the expression of TGF-β, Smad2/3 and pSmad2/3. In addition, shows the extracellular TGF-β concentration of senescent fibroblasts cultured on chitosan was also significantly lower than those on PVA and pHEMA (p < .05). Therefore, the main difference of senescence-related protein expression for cells cultured on chitosan, PVA and pHEMA was TGF-β and Smad2/3 signaling pathway.
Figure 4. TGF-β pathway protein expression of fibroblasts on biomaterials. (A) Western blot results of TGF-β, Smad2/3 and pSmad2/3 expressions in cells. (B) The relative amount of TGF-β, Smad2/3 and pSmad2/3 protein expressions in cells (n > 3). ##p < .01 means these groups were compared to the TCPS groups. (C) Human TGF-β concentration of the medium which cells were cultured for 3 days (n = 3). *p < .05.
Effect of soluble-form chitosan on cell senescence
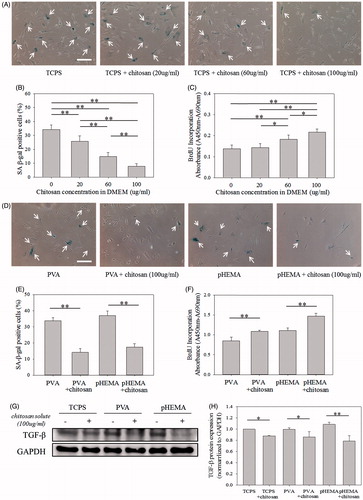
To further investigate the role of chitosan in mediating cell senescence, chitosan was directly added into the medium with concentration ranging from 20 to 100 μg/ml instead of the chitosan-coated wells. shows SA β-gal activity and BrdU incorporation absorbance of senescent fibroblasts were mediated on all culture wells, regardless of monolayered cells on TCPS or multicellular spheroids on PVA and pHEMA, when senescent fibroblasts were cultured in chitosan-containing medium for 3 days. For cells cultured on TCPS, the decreasing tendency of SA β-gal-positive cells and the increasing tendency of BrdU incorporation absorbance correlated with the chitosan concentration, suggesting that chitosan mediated cell senescence in a dose-dependent manner. Furthermore, shows the TGF-β expression of all cells were significantly downregulated when 100 μg/ml of chitosan were added into the medium (p < .05 and p < .01). On the other hand, senescent fibroblasts did not exhibit lower SA β-gal activity and higher BrdU incorporation absorbance when 100 μg/ml of PVA or pHEMA was added into the medium (). These results assured that chitosan was specific to mediate fibroblast senescence through the TGF-β protein expression, which was independent of the structure of multicellular spheroids developed by biomaterial-coated surface.
Figure 5. Soluble-form chitosan directly added into cells cultured on TCPS, PVA and pHEMA-coated plates. (A) SA β-gal staining images of cells cultured on TCPS. Scale bar =200 μm. (B) The percentages of cells cultured on TCPS stained by SA β-gal. (C) BrdU incorporation assay of cells cultured on TCPS (n = 4). (D) SA β-gal staining images of cells cultured on PVA and pHEMA. Scale bar = 200 μm. (E) The percentages of cells cultured on PVA and pHEMA stained by SA β-gal. The concentration of chitosan in the medium was 100 μg/ml. (F) BrdU incorporation assay of cells cultured on PVA and pHEMA (n = 4). The concentration of chitosan in the medium was 100 μg/ml. (G) Western blot results of TGF-β expressions in cells. (H) The relative amount of TGF-β protein expressions in cells (n > 3). *p < .05, **p < .01.
Interaction between chitosan and TGF-β
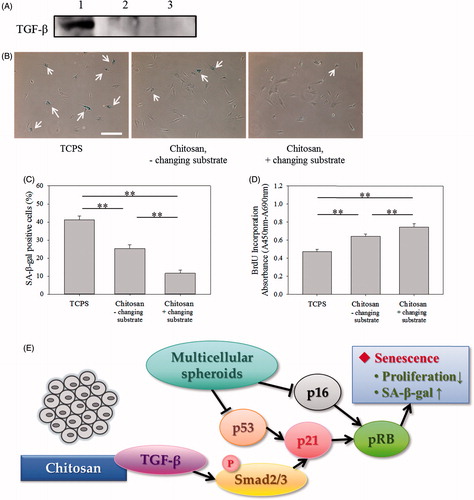
Chitosan, composed of N-acetyl-glucosamine and glucosamine groups, is a cationic polymer at neutral pH, which is able to bind with anionic growth factors by forming polyelectrolyte complexes to regulate biological function of growth factors [Citation31,Citation32]. Thus, chitosan was hypothesized to remove TGF-β by ionic interaction to suppress TGF-β signaling senescence. This assumption was investigated by four experiments. Firstly, chitosan and fibroblast lysis protein solution were mixed for 1 h, and then the supernatant was analyzed by western blot assay. shows TGF-β protein was almost removed by precipitation after chitosan was added into the solution. Subsequently, when senescent fibroblasts were seeded on chitosan for 3 days to delay the cellular senescence, multicellular spheroids were collected and reseeded on fresh chitosan-coated wells every day. It is possible that more TGF-β could be removed by forming polyelectrolyte complexes with fresh chitosan. shows that significantly lower ratio of SA β-gal-positive cells and higher absorbance of BrdU incorporation could be prepared by changing substrates every day than those without changing substrates (p < .01).
Figure 7. Interaction between chitosan and TGF-β. (A) Western blot results of TGF-β expression. First lane was 25 μg PD50 cell lysis protein only, second land was the supernatant which was collected from 25 μg PD50 cell lysis protein adding into 0.5% chitosan solution, and third land was 0.5% chitosan solution only. (B) SA β-gal staining images of cells. Scale bar = 200 μm. Changing substrate group means that the chitosan-coated substrate was changed every day within the 3-day culture. (C) The percentages of cells stained by SA β-gal. (D) BrdU incorporation assay of cells (n = 4). **p < .01. (E) The diagram of chitosan affecting senescent-related pathways.
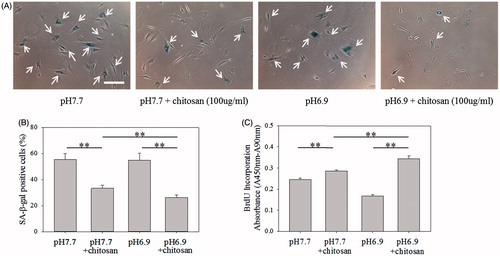
Furthermore, the amount of amino groups (–NH2) in chitosan and its ionization degree (–NH3+) were regulated to increase the interaction between chitosan and TGF-β to mediate cell senescence [Citation44,Citation45]. shows the decreasing tendency of SA β-gal-positive cells and the increasing tendency of BrdU incorporation absorbance correlated with the DDA of chitosan, analyzed by Nuclear Magnetic Resonance spectrometer (NMR, Figure S2), suggesting chitosan with more capability to delay cell senescence when chitosan possessing more cationic amino groups to bind with TGF-β. shows the SA β-gal-positive cells could be significantly decreased (p < .01) and the BrdU incorporation absorbance could be significantly increased (p < .01) when the medium pH was decreased from 7.7 to 6.9 to increase the ionization degree of amino groups in chitosan. These results indicate the role of amino groups of chitosan, different from the chemical structure of PVA and HEMA, in mediating cell senescence.
Figure 8. Effect of DDA of chitosan on senescence of fibroblasts. (A) SA β-gal staining images of cells cultured on 77% DDA, 85% DDA and 90% DDA chitosan-coated plates. Scale bar = 200 μm. (B) The percentages of cells cultured on different DDA chitosan-coated plates stained by SA β-gal. (C) BrdU incorporation assay of cells cultured on different DDA chitosan-coated plates (n = 4). **p < .01.
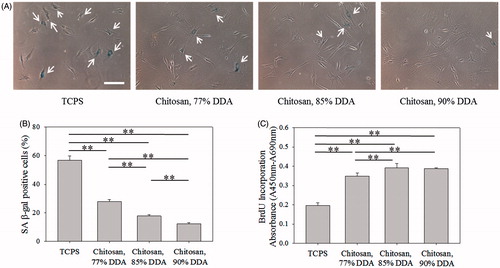
Figure 9. Effect of medium pH on senescence of fibroblasts. (A) SA β-gal staining images of cells cultured in different cultured medium pH. Scale bar = 200 μm. (B) The percentages of cells stained by SA β-gal. (C) BrdU incorporation assay of cells (n = 4). The concentration of chitosan in the medium was 100 μg/ml. **p < .01.
Discussion
It has been known that cell extracellular microenvironment can regulate cell adhesion, migration, proliferation and function. In this study, fibroblasts were cultured on chitosan to form multicellular spheroids. Consistent with our previous study [Citation22], senescent fibroblasts treated by chitosan with appropriate seeding density (2.5 × 104 cells/cm2) and culture time (3 days) could optimize cell properties with lower cell death, lower SA β-gal activity and higher proliferation rate (). Similar to chitosan, PVA and pHEMA, extensively used for various biomedical applications [Citation46,Citation47], could suspend fibroblasts to form multicellular spheroids, but they could not mediate cell senescence as chitosan to decrease SA β-gal activity or increase proliferation rate (). Therefore, in addition to the formation of the multicellular spheroids, chitosan itself should play an important role in mediating fibroblast senescence due to its specific structure.
It is reasonable to assume that senescence-related protein expression of fibroblasts could be especially downregulated by chitosan. Generally, senescence signals engage either the p53 or the p16 tumor suppressor pathways [Citation38–40]. shows the cells after making spheroids by the treatment of PVA and pHEMA also downregulated p53, p21, p16 and pRB expression as that by the chitosan treatment, but there was substantial difference for the expression of pRB and p21 among them. Many factors activate senescence-related pathways that converge on the downstream protein p21 and the activation pRB, cell cycle inhibitor [Citation29,Citation30]. Therefore, other senescence pathways to regulate p21 and pRB to delay fibroblast senescence by chitosan were analyzed in this study. This suggests that the formation of cell spheroids induced by appropriate biomaterials could mediate the senescence-related protein expression, but cells also might be mediated by other factors to exhibit different senescent phenotypes.
It is known that TGF-β signaling pathway is involved in many cellular processes to regulate cell function. In our previous study, TGF-β expression of senescent fibroblasts has been demonstrated to be downregulated after chitosan treatment [Citation22]. For TGF-β-related senescence pathway, TGF-β phosphorylates Smad2/3 protein to mediate p21 protein and then induce the senescence in several cell types [Citation33,Citation48,Citation49]. Therefore, it has been reported that attenuation or blockade of TGF-β/Smad signaling could increase cell proliferation to suppress premature senescence [Citation50,Citation51]. As expected, chitosan treatment for 3 days exhibited lower expression of TGF-β, Smad2/3, pSmad2/3, p21 and pRB, but the effect of PVA and pHEMA treatment was not so obvious (). In addition, shows the extracellular TGF-β concentration of senescent fibroblasts cultured on chitosan was also significantly lower than those on PVA and pHEMA (p < .05). These results indicated, compared to PVA and pHEMA, both extracellular and intracellular TGF-β expression could be significantly decreased when senescent fibroblasts cultured on chitosan, which is consistent with the result of Figure S1 that the addition of in the medium could enhance the senescence of the foreskin fibroblasts [Citation33].
Since no cell senescence was obviously mediated by PVA and pHEMA, the formation of multicellular spheroids suspended on biomaterials is not a promising method to mediate cell senescence. Therefore, chitosan was directly added into the medium to investigate whether chitosan could interact with TGF-β to delay cell senescence. As shown in , no matter fibroblasts were cultured on TCPS, PVA or pHEMA, the added chitosan could decrease the tendency of SA β-gal-positive cells, increase the tendency of BrdU incorporation absorbance and downregulate the cell TGF-β expression, indicating chitosan-mediating cell senescence does not result from the formation of multicellular spheroids. On the other hand, PVA and pHEMA were also directly added into the medium to evaluate their effect of on delaying cell senescence. Similar to coating on TCPS surface, senescent fibroblasts exhibited high β-gal-positive ratio but low BrdU incorporation absorbance in the PVA- and pHEMA-containing medium (). Therefore, the delaying of the induction of cell senescence by biomaterials was chitosan-specific and the TGF-β pathway was involved in the chitosan-mediating fibroblast senescence process.
Hydrophilic biomaterial surface is usually unfavorable for cell attachment due to the weak interaction with proteins, especially for adsorption of extracellular proteins [Citation52–54]. Therefore, we tested whether chitosan was more able to bind to TGF-β than PVA and pHEMA and whether chitosan could more efficiently to bind to TGF-β by appropriate modification. Firstly, TGF-β protein from senescent cell lysis could be almost removed by the added chitosan (). Moreover, if cultured fibroblast spheroids used fresh chitosan-coated wells every day, more substantial effect on delaying cell senescence could be observed (). This result suggested that more TGF-β could be removed by interaction with fresh chitosan in the senescent fibroblast culture system. Therefore, we tried to further increase the chitosan binding ability with TGF-β to mediate cell senescence by increasing the amount of amino groups in chitosan and its ionization degree. and show both the effects of the DDA of chitosan and the ionization degree of amino groups in chitosan on mediating fibroblast senescence were significant (p < .01). These results indicate the ammonium cations of chitosan, different from the chemical structure of PVA and pHEMA, played an important role in binding with TGF-β to decrease the extracellular TGF-β concentration and to downregulate the intracellular TGF-β expression to inhibit the TGF-β induced pathway.
Conclusion
In summary, the exact regulatory mechanism of cell senescence by biomaterials needs more investigation. However, we demonstrated that the suppression of TGF-β pathway is one of the effects of chitosan-mediated fibroblast senescence. The results provide support for the potential applications of chitosan in cell therapy and regeneration medicine.
Supplementary_figures.docx
Download MS Word (550.6 KB)Acknowledgement
The authors thank the staff of the Third and Eighth Core Lab, Department of Medical Research, National Taiwan University Hospital, for technical support. The authors also thank the staff of the Imaging Core, and the Flow Cytometric Analyzing and Sorting Core at the First Core Labs, National Taiwan University College of Medicine, for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cristofalo VJ, Kritchevsky D. Cell size and nucleic acid content in the diploid human cell line WI-38 during aging. Med Exp Int J Exp Med. 1969;19:313–320.
- Greenberg SB, Grove GL, Cristofalo VJ. Cell size in aging monolayer cultures. In Vitro. 1977;13:297–300.
- Mitsui Y, Schneider EL. Relationship between cell replication and volume in senescent human diploid fibroblasts. Mech Ageing Dev. 1976;5:45–56.
- Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367.
- Lee BY, Han JA, Im JS, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187–195.
- Macieira-Coelho A, Azzarone B. Correlation between contractility and proliferation in human fibroblasts. J Cell Physiol. 1990;142:610–614.
- Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990;249:1129–1133.
- Fumagalli M, Rossiello F, Clerici M, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 2012;14:355–365.
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460.
- Chen Q, Ames BN. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci USA. 1994;91:4130–4134.
- Parrinello S, Samper E, Krtolica A, et al. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747.
- Passos JF, Miwa S, von Zglinicki T. Measuring reactive oxygen species in senescent cells. Methods Mol Biol. 2013;965:253–263.
- Tchkonia T, Zhu Y, van Deursen J, et al. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Investig. 2013;123:966–972.
- Zhu Y, Armstrong JL, Tchkonia T, et al. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17:324–328.
- Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31.
- Kiyono T. Molecular mechanisms of cellular senescence and immortalization of human cells. Expert Opin Ther Targets. 2007;11:1623–1637.
- Piegari E, De Angelis A, Cappetta D, et al. Doxorubicin induces senescence and impairs function of human cardiac progenitor cells. Basic Res Cardiol. 2013;108:334.
- Choi HR, Cho KA, Kang HT, et al. Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix. Aging Cell. 2011;10:148–157.
- Sato Y, Yoshida K, Nozawa S, et al. Establishment of adult mouse Sertoli cell lines by using the starvation method. Reproduction. 2013;145:505–516.
- Buttiglieri S, Ruella M, Risso A, et al. The aging effect of chemotherapy on cultured human mesenchymal stem cells. Exp Hematol. 2011;39:1171–1181.
- Tsai CC, Chen YJ, Yew TL, et al. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117:459–469.
- Tsai CW, Kao YT, Chiang IN, et al. Chitosan treatment delays the induction of senescence in human foreskin fibroblast strains. PLoS One. 2015;10:e0140747.
- Kas HS. Chitosan: properties, preparations and application to microparticulate systems. J Microencapsul. 1997;14:689–711.
- Shi C, Zhu Y, Ran X, et al. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133:185–192.
- Yang TL, Lin L, Hsiao YC, et al. Chitosan biomaterials induce branching morphogenesis in a model of tissue-engineered glandular organs in serum-free conditions. Tissue Eng Part A. 2012;18:2220–2230.
- Cheng NC, Wang S, Young TH. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. 2012;33:1748–1758.
- Chen YH, Chung YC, Wang IJ, et al. Control of cell attachment on pH-responsive chitosan surface by precise adjustment of medium pH. Biomaterials. 2012;33:1336–1342.
- Lou PJ, Chiu MY, Chou CC, et al. The effect of poly (ethylene-co-vinyl alcohol) on senescence-associated alterations of human dermal fibroblasts. Biomaterials. 2010;31:1568–1577.
- Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–5227.
- Hernando E, Nahle Z, Juan G, et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802.
- Ishihara M, Fujita M, Obara K, et al. Controlled releases of FGF-2 and paclitaxel from chitosan hydrogels and their subsequent effects on wound repair, angiogenesis, and tumor growth. Curr Drug Deliv. 2006;3:351–358.
- Masuoka K, Ishihara M, Asazuma T, et al. The interaction of chitosan with fibroblast growth factor-2 and its protection from inactivation. Biomaterials. 2005;26:3277–3284.
- Zerlanko BJ, Bartholin L, Melhuish TA, et al. Premature senescence and increased TGFbeta signaling in the absence of Tgif1. PLoS One. 2012;7:e35460.
- Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414.
- Pandamooz S, Hadipour A, Akhavan-Niaki H, et al. Short exposure to collagenase and coculture with mouse embryonic pancreas improve human dermal fibroblast culture. Biotechnol Appl Biochem. 2012;59:254–261.
- Tsai CW, Young TH. CD44 expression trends of mesenchymal stem-derived cell, cancer cell and fibroblast spheroids on chitosan-coated surfaces. Pure Appl Chem. 2016;88:843–852.
- Severino J, Allen RG, Balin S, et al. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000;257:162–171.
- Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496.
- Beausejour CM, Krtolica A, Galimi F, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222.
- Campisi J, di Fagagna FD. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740.
- Senturk S, Mumcuoglu M, Gursoy-Yuzugullu O, et al. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology. 2010;52:966–974.
- Bai H, Gao Y, Hoyle DL, et al. Suppression of transforming growth factor-beta signaling delays cellular senescence and preserves the function of endothelial cells derived from human pluripotent stem cells. Stem Cells Transl Med. 2017;6:589.
- Lasry A, Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 2015;36:217–228.
- Ahmadi F, Oveisi Z, Samani SM, et al. Chitosan based hydrogels: characteristics and pharmaceutical applications. Res Pharm Sci. 2015;10:1–16.
- Saether HV, Holme HK, Maurstald G, et al. Polyelectrolyte complex formation using alginate and chitosan. Carbohydr Polym. 2008;74:813–821.
- Chen MH, Chen YJ, Liao CC, et al. Formation of salivary acinar cell spheroids in vitro above a polyvinyl alcohol-coated surface. J Biomed Mater Res. 2009;90A:1066–1072.
- Kim JH, Lim IR, Joo HJ, et al. Sphere formation of adipose stem cell engineered by poly-2-hydroxyethyl methacrylate induces in vitro angiogenesis through fibroblast growth factor 2. Biochem Biophys Res Commun. 2015;468:372–379.
- Li CY, Suardet L, Little JB. Potential role of Waf1/Cip1/P21 as a mediator of Tgf-Beta cytoinhibitory effect. J Biol Chem. 1995;270:4971–4974.
- Wu JF, Niu J, Li XP, et al. TGF-beta 1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev Biol. 2014;14:21.
- Lin S, Yang JH, Elkahloun AG, et al. Attenuation of TGF-beta signaling suppresses premature senescence in a p21-dependent manner and promotes oncogenic Ras-mediated metastatic transformation in human mammary epithelial cells. Mol Biol Cell. 2012;23:1569–1581.
- Nicolas FJ, Hill CS. Attenuation of the TGF-beta-Smad signaling pathway in pancreatic tumor cells confers resistance to TGF-beta-induced growth arrest. Oncogene. 2003;22:3698–3711.
- Rabinow BE, Ding YS, Qin C, et al. Biomaterials with permanent hydrophilic surfaces and low-protein adsorption properties. J Biomater Sci Polym E. 1994;6:91–109.
- Liu SX, Kim JT, Kim S. Effect of polymer surface modification on polymer-protein interaction via hydrophilic polymer grafting. J Food Sci. 2008;73:E143–E150.
- Martins MCL, Wang D, Ji J, et al. Albumin and fibrinogen adsorption on PU-PHEMA surfaces. Biomaterials. 2003;24:2067–2076.