Abstract
Curcumin, an active principle of Curcuma longa, is extracted from the rhizome. Its therapeutic efficiency has been proved using various in vitro and in vivo models. Inflammatory, neoplastic and preneoplastic diseases are the major targets using curcumin as therapeutic agent. Feasible clinical formulations could not be obtained because of its lack of solubility, stability and higher degradation rate. Recently, many techniques have been evolved to improve the physicochemical properties of pharmacological compounds, thereby increasing their biological activity. Curcumin has been developed using various techniques, particularly micro and nanotechnology to improve its stability and bioavailability. This review focuses on the studies pertaining to the delivery of curcumin in the form of micro and nanosize formulations for the treatment of a variety of diseases.
Keywords:
Introduction
Curcumin is a polyphenolic and active curcuminoid present in the rhizome of Curcuma longa. Therapeutic efficiency of curcumin has long been known and its merits and demerits have been extensively reviewed [Citation1,Citation2]. Manach et al. have reviewed the oral bioavailability of 97 polyphenolic compounds and they have shown that curcumin is one among the least absorbed polyphenols [Citation3]. Despite curcumin’s multiple therapeutic benefits, limited oral bioavailability of curcumin continues to be a major challenge in developing formulations for clinical applications [Citation4]. Low serum and tissue levels of curcumin are observed irrespective of the route of administration due to extensive intestinal and hepatic metabolism and rapid elimination thus restraining curcumin’s bioavailability [Citation5–7]. The role of serum proteins, such as human serum albumin (HSA), fibrinogen, immunoglobulin and transferrin in stabilizing curcumin has been investigated [Citation8]. Curcumin continues to undergo rapid hydrolysis but this reaction is suppressed by the presence of either HSA or fibrinogen with an impressive yield of approximately 95%. This indicates that the structural integrity of HSA could have contributed to the stabilization [Citation8].
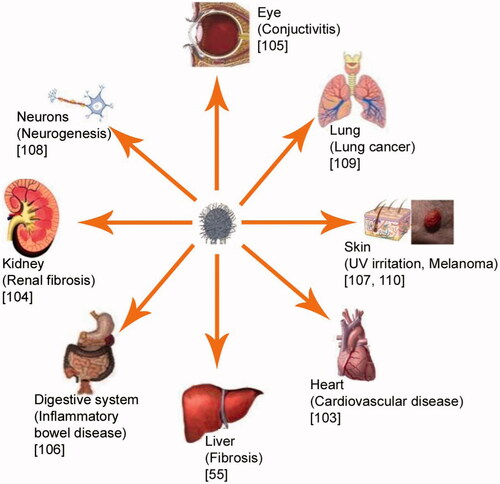
Microencapsulation and entrapment of small molecules and proteins in matrix materials have emerged as important devices in the drug delivery domain. Especially, nanotechnology is an enabling technology that has the potential to revolutionize agriculture and food systems. The unusual properties of materials that are on the nanometre (10−9 m) length scale and the development of technology to manipulate or self-assemble such materials, molecule by molecule, provide potentially world-changing scientific, technological and commercial opportunities [Citation9]. Nanoscale control over food molecules may lead to the modification of many macroscale characteristics, such as texture, taste, other sensory attributes, processability and stability during shelf life. Given that the sizes of functional elements in biology are in the nanometre scale range, it is not surprising that nanomaterials interact with biological system at the molecular level [Citation10]. Nanotechnology revolutionizes biomedical fields, such as drug delivery [Citation11], biomolecules delivery [Citation12], artificial tissue constructs [Citation13] and imaging [Citation14]. Encapsulated delivery of curcumin is suggested to overcome the limitations associated with the dissolution of curcumin in the systemic circulation [Citation15–17]. Treatment efficiency of curcumin-loaded nanoparticles to various diseases is shown in . The role of nanotechnology in increasing stability, solubility, bioavailability and activity of potent pleotropic curcumin for therapeutic application is critically discussed in this review.
Solubility and stability of curcumin
One of the major limitations to use curcumin as a therapeutic agent from bench to bed is the solubility and stability and subsequent bioavailability in in vivo. Curcumin is photosensitive and its solubility mainly depends upon the pH of the dissolving medium [Citation18]. Various solubilization techniques have been developed to increase the drug solubility and dissolution properties, including use of surfactants, water-soluble carriers, polymeric conjugates and solid dispersions [Citation19,Citation20]. Incorporation of therapeutic proteins and drugs into other polymers via simple complexation or micelle formation and nanoconversion with polymers and microspheres are prime techniques being developed in the current decade [Citation21]. Biopolymer micelles are now a fast-growing area in the delivery of nutraceuticals or drugs since they prolong their blood circulation time. Recently, it has been shown that hydrophobically modified starch (HMS) forms micelles in aqueous solution [Citation22]. Curcumin is complexed with cyclodextrin and consequently solubility of curcumin is increased in the acidic condition by at least 104. Influence of cavity size, charge and bulkiness of the cyclodextrin sidechains on the stability factor are the reasons for slower degradation and higher solubility [Citation23]. Additionally, solubility of curcumin in HMS micelles is increased almost 1700-fold when compared with that in pure water. Small molecular-weight surfactants, such as cetyltrimethylammonium bromide (CTAB), have been shown to improve the stability of curcumin [Citation24].
Shoba et al. have proved that stabilizing agent could improve the bioavailability of curcumin by administering piperine followed by curcumin injection in rats. The serum concentration is increased after piperine administration and the same trend is observed in healthy human volunteers [Citation25]. However, how concomitant administration helps to improve the serum concentration and mechanistic actions over stabilization of drug in in vivo is not understood. In addition, piperine has adverse effects on the metabolism of a wide range of drugs [Citation26,Citation27]. Graft-vinyl acetate copolymers are synthesized by free radical polymerization and curcumin nanoparticles are prepared by ultrasonic irradiation. The nanoparticles are discrete and uniform spheres, covered with positive charges. The encapsulation efficiency of nanoparticles is up to 91.6% and a slower release rate of curcumin is achieved [Citation28]. Because of highly hydrophobic nature, curcumin cannot be administered systemically. Liposomal encapsulation of curcumin makes this agent amenable to intravenous dosing and circumvents the problem of poor oral availability that limits the utility of free curcumin. The composition shows increased resistance to cancer cell growth; however, in vitro release is not explained [Citation29].
Curcumin-encapsulated oil in water (O/W) microemulsion system is constructed using food-acceptable components, lecithin and Tween 80 as the surfactants and ethyl oleate (EO) as the oil phase. Composition of curcumin microemulsion (made up of distilled water: surfactants; the mole ratio of lecithin/Tween 80 is 0.3; EO = 10:1.7:0.4 in wt ratio) is stable for 2 months with an average diameter of 71.8 ± 2.45 nm. The microemulsion could be diluted with aqueous buffer without destroying its structure for 48 h [Citation30]. In another study, curcumin-phospholipid complex is shown to demonstrate an appropriate 2-fold increase in plasma concentration after oral intake in rat [Citation31].
Curcumin is formulated into various biodegradable nanoparticles with a view to improve its oral bioavailability. The curcumin encapsulated nanoparticles withstand the test conditions [International Conference on Harmonization (ICH) accelerated stability test conditions for refrigerated products] for the studied duration of 3 months. The in vitro release is predominantly by diffusion phenomenon and follows Higuchi’s release pattern. The in vivo pharmacokinetics reveals that curcumin entrapped nanoparticles demonstrate at least a 9-fold increase in oral bioavailability when compared to curcumin administered with piperine as absorption enhancer [Citation32]. The pH and long-term stability of polyphenols including epigallocatechin gallate (EGCG) and curcumin encapsulated in nanoemulsions are evaluated and nanoemulsions could improve stability and oral bioavailability of EGCG and curcumin [Citation33]. To improve curcumin solubility further, Lopes-Rodrigues et al. encapsulated curcumin into polyethylene glycol-pentacosadiynoic acid (PEG-PCDA) complex and found that 500 times more soluble than free curcumin with significant anti-cancer activity [Citation34]. Various formulations of curcumin and their effect on therapeutic improvements are summarized in .
Table 1. Different formulations of curcumin.
Influence of processes on physicochemical and biological activities
The physicochemical as well as biological activities highly dependent upon the methods of preparation, conditions, such as pH, temperature and the types of polymers. The influence of the aqueous monomer (ethylene diamine, hexamethylene diamine and 1,4-diaminobutane), the effect of stirring rate and impact of the nanoencapsulation method on the encapsulation efficiency was investigated. The encapsulation yield depends not only on the encapsulation process but also on the chemical structure of the diamine, whereas, the size and its distribution vary according to the process choice and the emulsification stirring rate [Citation58].
A system that integrates electrophoretic deposition (EPD) technology with the polymeric nanoparticles in drug-eluting stents (DES) for local drug delivery and a controlled release system has been investigated [Citation59]. The surface morphology and drug loading amount of DES by EPD have been investigated under different operational conditions, such as operation time, voltage and composition of media. Poly-D, L-lactide-co-glycolic acid (PLGA) nanoparticles embedded with curcumin are prepared by a modified spontaneous emulsification method, using polyacrylic acid (PAA) as a surfactant because its carboxylic group contributes negative charge to the surface of nanoparticles (−53.5 ± 5.8 mV). In the process of “trial and error” endeavours, it has been observed that it is easy to control the drug loading amount deposited onto the stent while keeping uniform surface morphology.
Recently, Tsai et al. [Citation60] prepared curcumin nanoformulation with PLGA in the presence of PVA using high-pressure emulsification-solvent-evaporation system and excellent physic–chemical properties were obtained. Increased bioavailability of curcumin nanoparticles by intravenous injection and oral routes were 15% and 21-fold, respectively. Excretion study also reflected these results. The authors claimed that such system could effectively be used to prepare nanoparticels with polyphenolic compounds with the increased bioavailability. Chitosan and polycaprolactone acidic and pH-sensitive benzylidene acetal based polymer were used to prepare nanoparticles incorporating curcumin. This system helped to intracellular solubilize NP-curcumin than free curcumin as per confocal microscopy and showed significant growth reduction of EC-109 and hepG-2 cells [Citation61] and in another study, similar acetic environment helped to release curcumin from NPs prepared by ploy (L-lysine) [Citation62]. Dual-purpose nanoparticles using Fe3O4 and chitosan were prepared and its cell-intake ability and in vivo targeting were evaluated. Fe3O4–Curcumin nanoparticles showed higher cellular uptake and accumulation of these nanoparticles was displayed by the MRI results. So these nanoparticles could be used for the therapeutic applications with multiple applications [Citation48].
Role of curcumin in different diseased states
Presence of active functional groups such as two methoxy groups, two phenolic hydroxyl groups and two ketoenol groups makes curcumin as a potential chemotherapeutic agent. Hence, it plays multifunctional roles in mediating cellular actions during the pathological conditions of various organs.
Toxicity study of curcumin nanocomplex
Extraordinary characteristics, for example increased surface area, of nanomaterials offer immaculate potentials for therapeutic applications. On the other hand, it also poses threats because of overwhelming cellular uptake, possible long-term toxicity due to cumulative retention inside the cells [Citation63]. Curious to answer the question of curcumin toxicity, the authors established the indispensable toxicological profiles, genotoxicity assays, of curcumin nanoparticles using advanced techniques at various injury levels. Dandekar et al. developed the chitosan nanoparticles, named Eudragit S100 (Evonik Industries, Essen, Germany) and its toxicity was evaluated through acute-toxicity study, sub-acute-toxicity study (28 d) and various genotoxicity studies like in vivo micronucleus assay, in vivo chromosomal aberration assay and in vivo comet assay. It was reported that prepared formulation was tolerable and safer than the therapeutic dose level in both in vitro and in vivo conditions [Citation64]. Solubility of curcumin limits its applications for therapeutics. Curcumin nanosuspension was prepared with solvents of PVPK30 and SDS and curcumin released 84% within 34 h and it showed higher toxicity as well. Pharmacokinetic studies showed that CU-NS quickly reached the tissue organs, suggesting that the formulation is effective; however, toxicity of SDS remains questionable when translated [Citation65]. No other study showed about the toxicity of curcumin or its derivative molecules with or without other compounds and hence further studies is needed to confirm the previous findings.
Anti-inflammatory properties of nanoemulsified curcumin
Curcumin has a preventive role in various diseases through multiple mechanisms and it has been reported that the effect of curcumin on the prevention of collagen synthesis is also reported in isoproterenol induced myocardial necrosis [Citation66]. Isoproterenol (ISO)-induced ischemic insult in rat is protected by curcumin treatment, as shown by the increase of the levels of antioxidants [Citation67,Citation68]. Curcumin is also known to prevent the inflammation and its cascade actions in various diseases viz. atherosclerosis, inflammatory disease in myocardium [Citation69], wound healing through suppression of inflammatory cytokines [Citation70], mainly by increasing the level of peroxisome proliferators-activator receptors [Citation71]. Recently, Wang et al. [Citation33] have used high-pressure homogenization method to successfully generate nanoemulsions with a formulation using medium chain triglyceride (MCT), Tween 20 and water at a ratio of 10:10:80. It is observed that multiple cycles of high-pressure homogenization generate smaller sized nanoemulsions with less polydispersity and the finest oil droplets obtained are about 79.5 nm in diameter. Furthermore, this nanoemulsion encapsulates 1% curcumin. Tested in a 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse ear inflammation model, curcumin nanoemulsions have been shown to have much better bioactivity than curcumin solution, and the curcumin nanoemulsion of 79.5 nm inhibits 85% mouse ear inflammation when compared to 43% inhibition for 618.6 nm curcumin nanoemulsion. This study has suggested that i) formulated MCT lipids have a positive impact on the anti-inflammation properties of curcumin and (ii) smaller curcumin emulsion droplets have better biological efficacy than larger curcumin emulsions. Recently, Jiang [Citation72] has improved the nanoemulsion formulation prepared from the mixtures of water, Tween 40/Span 20 and MCT. Oral administration of these new curcumin nanoemulsions could inhibit TPA-induced inflammation in mouse ears by 100%. ELISA and immunoblotting results also show dose-responsive and significant inhibition of proinflammatory factors interleukin-1β (IL-1β), interleukin (IL-6), matrix metalloproteinase-9 (MMP-9) and cyclin D1. It is clearly indicated that oral administration of two doses (1 and 1.5%) of nano-emulsified curcumin could inhibit the expression of cyclin D1 and the higher dose (1.5%) of curcumin could suppress the expression to a larger degree than the lower dose (1.0%). It is difficult to determine the plasma concentration of curcumin because it may metabolize within half an hour. The enhanced anti-inflammatory activity of curcumin encapsulated in O/W emulsions was demonstrated in the mouse ear inflammation model. In addition to dispersibility of curcumin in the emulsion, emulsion droplets contain lipid, which increases the adsorption on the intestinal tract.
Curcumin is soluble in lipid and hence, to increase its solubility, it is dissolved in lipid derivatives. Phospholipid vesicles or lipid-nanospheres embedding curcumin (CmVe or CmLn) are formulated. Curcumin could be solubilized in hydrophobic regions of these particles to form nanoparticle dispersions, and these formulations show ability to scavenge reactive oxygen species in dispersions. CmVe or CmLn are delivered to tissue macrophages through intravenous injection. At 6 h after intravenous injection in rats via the tail vein (2 mg Cm/kg bw), confocal microscopic observations of tissue sections show that curcumin is massively distributed in cells. These results indicate that the lipid-based nanoparticulates when given intravenously provide improved delivery of curcumin to tissue macrophages, specifically in bone marrow and spleen [Citation73].
Recently, a novel self-emulsifying drug delivery system (SEDDS) composed of oil, surfactant and co-surfactant is developed. The physicochemical properties viz. antiangiogenic and anti-inflammatory activities of curcumin using SEDDS are evaluated in colitis induced rat model. SEDDS formulation 2 (SF-2) (curcumin with 2-fold of tween 20) has shown maximum solubility as well as optimal in vitro release. In addition, SF-2 is found to have angioinhibitory activity, as demonstrated by inhibition of angiogenesis in chorioallantoic membrane assay (CAM) [Citation74]. Anti-inflammatory activity of SEDDS is evaluated using dextran sulphate sodium (DSS)-induced colitis rat model. Curcumin and SF-2 treated rats show rapid weight gain and the whole colon length appears to be significantly longer than that in pure curcumin and DSS-treated controls. An additional finding in the DSS-treated rats is the predominance of eosinophils in the chronic cell infiltrate. Decreased mast cell numbers in the mucosa of the colon of SF-2 and pure curcumin treated rats are observed. This study has conclusively demonstrated that the degree of colitis caused by administration of DSS is significantly attenuated by SEDDS formulation (SF-2). Being a nontoxic natural dietary product, curcumin could be useful in the therapeutic strategy for patients having inflammatory bowel diseases [Citation74]. To further evaluate the curcumin on the anti-inflammatory property, the gold nanoparticle was used as carrier for curcumin and studied against lymphocytes. When they are treated with nanoparticles, condensed chromatin, membrane damage and apoptotic bodies were found and the study suggested that this nanoparticle compound can be used as drug in non-toxic range [Citation75]. Curcumin also showed interesting inflammatory suppressive characteristics against infectious diseases. Leishmaniasis is the inflammatory disease followed by the attack of leishmania parasite. Curcumin-loaded mannosylated CNP was tested in mice model of leishmaniasis and the study showed the improved endocytosis of macrophages within reticuloendothelial system [Citation76] suggesting the possible application of such system against macrophage-based inflammatory diseases. Activation of proinflammatory cascade molecules and cells results in multiple diseases and targeting them using nanotechnology with the plant-derived molecule could be translated without major concern.
Hepatic protection of stabilized curcumin
Curcumin has shown hepatic protective effect in vitro [Citation74] and in vivo [Citation28]. Taking advantage of the lipophilic nature of curcumin, it was complexed with phospholipids [Citation51,Citation77]. Curcumin-phospholipid complex was evaluated in a rat model of carbon tetrachloride (CCl4)-induced acute liver damage. The complex circulated longer period than its purified form and the authors found the hepatic protective effect of the complex is due mainly to anti-oxidant properties and helped to balance the redox enzymes. Thus, the bioavailability of curcumin could be enhanced by curcumin-phospholipid complex. Later, a research group demonstrated its possible direct chemical interaction with acetaldehyde. The group dispersed curcumin in the polysaccharide based gel, named theracurmin and the blood level of acetaldehyde was measured after consumption in the drinkers. Surprisingly, the level of acetaldehyde, in vivo activity of compound of alcohol, was less and the curcumin was able to interact with the active compound and suppress its level to ameliorate the alcohol toxicity [Citation78].
Neuroprotective properties
Curcumin also has the capability to prevent neurogenic diseases, one of the fatal and challenging diseases causing death. Apolipoprotein E3 mediated poly(butyl) cyanoacrylate nanoparticles containing curcumin (ApoE3-C-PBCA) are formulated to provide photostability and enhanced cell uptake of curcumin by targeting. In vitro cell culture study shows enhanced therapeutic efficacy of ApoE3-C-PBCA against beta-amyloid-induced cytotoxicity in SH-SY5Y neuroblastoma cells compared to plain curcumin solution. It is noted that ApoE3-C-PBCA reduces the ROS level induced by amyloid beta to a considerable extent. ApoE3 Caspase 3 level is effectively reduced with the treatment of ApoE3-C-PBCA and the subG1 fraction (hypodiploid peak) is also reduced to 3.4% demonstrating increased antiapoptotic activity of curcumin with ApoE3-C-PBCA [Citation79]. Recently, Tiwari et al. showed that curcumin prevented BPA (bisphenol)-induced hippocampal toxicity by activating Wnt/beta-catenin signalling pathway [Citation80]. In line with the mechanism, recently, the interventional mechanism of curcumin for neuronal protection in the focal ischemia-reperfusion model was investigated. The authors found the increase of Notch singling in addition to doublecortin suggesting the pathway that curcumin induces the neurogenesis [Citation81]. Liposomal nanoparticles decorated with curcumin targeted the amyloid protein aggregates. In vitro study showed that, as per results of surface resonance experiments, the nanoparticles possessed high affinity towards Ab1–42 fibril likely because of occurrence of multivalent interactions. The study further suggested that the nanoparticles could be used as vectors for targeted delivery as well as diagnostic purposes [Citation82]. Trimethyl-modified CNP was synthesized to evaluate the oral bioavailability and brain distribution. Pharmacokinetic studies showed enrichment of TMCNPs with curcumin in the brain than its free form and the authors put forward the possibility of using curcumin encapsulated with TMCNP for effective treatment of neuronal diseases [Citation83]. To make available of curcumin for repair Parkinson disease, curcumin along with piperine was encapsulated in the nanoparticles made with glyceryl monooleate. Both in vitro and in vivo studies showed that glyceryl monooleate NPs were able to cross BBB efficiently to deliver curcumin, which protected the neurons against rotenone-induced injury [Citation84]. Wang et al. incorporated curcumin into silica nanoparticles modified with folic acid. Folic acid modified silica nanoparticles released curcumin efficiently and pH sensitive and increased intracellular ROS production reduced mitochondrial membrane potential and enhanced G2/M cell cycle retardation of human breast cancer cells. When intraperitoneally injected, curcumin retention in the serum and tumour tissues are higher in the treatment of nanoparticles group than free form [Citation85].
Anticancer property of encapsulated curcumin
Anticancer property of curcumin in solution form has already been reported [Citation86,Citation87]; however, lower bioavailability in soluble form has been a major setback for the inefficiency of curcumin in anticancer treatment. The bioactivity of encapsulated curcumin is tested in an in vitro anticancer model and it is found that encapsulated curcumin demonstrates a significant higher anticancer activity than free curcumin [Citation22]. It is noteworthy that not all micellar formulation could enhance bioactivities. Taking curcumin-encapsulation formulations as an example, micelles formed by poly(ethylene oxide)-b-poly(epsilon caprolactone) and methoxy poly(ethylene glycol)-palmitate do not increase the delivery of curcumin to tested cancer cells [Citation88,Citation89]. On the contrary, curcumin encapsulated in hydrophobically modified starch micelles and in casein micelles display enhanced bioactivity, giving rise to a hypothesis that natural components may facilitate the cellular uptake of micelles [Citation22,Citation90]. Curcumin incorporated into PLGA nanoparticle formulated by O/W emulsion technique is evaluated for anticancer properties using prostate cancer cell lines, LNCaP, PC3 and DU-145. LNCaP cells treated with curcumin-loaded PLGA nanoparticles show a distinct decrease in NF-κB activity. This indicates that PLGA nanospheres are capable of delivering curcumin over a prolonged period achieving a sustained delivery of curcumin, thus making it a potential candidate for cancer therapy [Citation91]. The objective of this study is to enhance the bioavailability of curcumin, simultaneously reducing the required dose through selective targeting to colon. Eudragit S100 is chosen to aid targeting since the polymer dissolves at cationic pH to result in selective colonic release of the entrapped drug. Solvent emulsion-evaporation technique is employed to formulate the nanoparticles. Anticancer potential of the formulation is demonstrated by 3–(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in HT-29 cell line. Nanometric, homogeneous, spherical particles are obtained with encapsulation efficiency of 72%. Freeze-dried nanoparticles exhibit a negative surface charge, drug content of >99% and presence of drug in amorphous form which may result in possible enhanced absorption. MTT assay has demonstrated almost a 2-fold inhibition of the cancerous cells by nanoparticles, as compared to curcumin alone, at the concentrations tested [Citation92]. PLGA nanoparticle formulation of curcumin (nano-curcumin) is developed by a modified nano-precipitation method [Citation93]. Physicochemical characterization of nano-curcumin indicates an average particle size of ∼70 nm, a steady and prolonged release of curcumin, antibody conjugation capability and an effective inhibition of ovarian cancer cell growth.
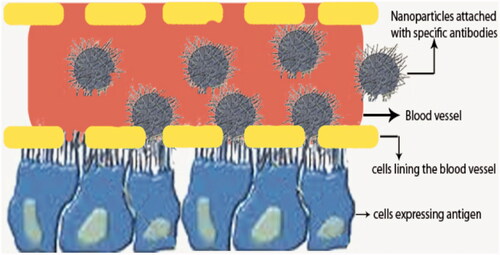
Curcumin-loaded nanoparticles do not have any off-target effect since they can reach multiple parts of the body and hence the amount of nanoparticles available to the target site may be low. Recently, antigen–antibody receptor mechanisms have been demonstrated to target specific cells [Citation94]. The antibodies specific for any one of the antigens expressed by cells are incorporated into nanosystem, which moves and reach target cells more specifically. To enhance targeted delivery of an anticancer agent for prostate cancer treatment, curcumin is incorporated into the liposomes coated with antibodies against prostate membrane specific antigenic protein. Curcumin-loaded liposomes of various lipid compositions are prepared by sonication at an average size of 100–150 nm. Nanoparticles are evaluated with two human prostate cancer cell lines (LNCaP and C42B) by MTT assay. Treatment of cells with liposomal curcumin (5–10 μM) for 24–48 h at 37 °C has resulted in at least 70–80% inhibition of cellular proliferation without affecting their viability. On the other hand, free curcumin exhibits similar inhibition only at 10-fold higher doses (>50 μM). It has been observed that LNCaP cells are relatively more sensitive to liposomal curcumin-mediated blocking of cellular proliferation than C42B cells [Citation44]. However, the reasons for the different activities between these two cell lines are not known.
Curcumin is incorporated into liposomal delivery system taking into advantage the lipophilic nature of curcumin. In vitro and in vivo effects of this complex on proliferation, apoptosis, signalling and angiogenesis using human pancreatic carcinoma cells have been critically studied. Liposomal curcumin has consistently suppressed NF-kB binding (electrophoretic mobility gel shift assay) and decreased the expression of NF-kB regulated gene products, including cyclooxygenase-2 and interleukin-8, both of which have been implicated in tumour growth/invasiveness [Citation29]. Paclitaxel (PTX) and curcumin have been encapsulated in flaxseed oil containing nanoemulsion formulations. Augmentation of therapeutic efficacy upon co-administration of PTX and curcumin is observed in human ovarian adenocarcinoma cells. PTX and curcumin combination therapy, especially when administered in the nanoemulsion formulations, is very effective in enhancing the cytotoxicity in wild-type and resistant cells by promoting the apoptotic response [Citation95]. Polymeric nanoparticle encapsulated with curcumin called “nanocurcumin” is synthesized utilizing the micellar aggregates of cross-linked and random copolymers of N-isopropylacrylamide (NIPAAM), with N-vinyl-2-pyrrolidone (VP) and poly(ethyleneglycol)monoacrylate (PEG-A). Size distribution is in the 50 nm range and unlike free curcumin; it is readily dispersed in aqueous media. Nanocurcumin demonstrates comparable in vitro therapeutic efficacy to free curcumin against a panel of human pancreatic cancer cell lines. Its mechanism is the same as that of free curcumin, including induction of cellular apoptosis, blockade of NF-κB activation and downregulation of steady-state levels of multiple proinflammatory cytokines (IL-6, IL-8 and TNFα) [Citation96].
Studies on the effects of herbal medicine on nanostructural chemical assemblies have been directed on human hepatocellular carcinoma. This study demonstrates the effect of curcumin nanoassemblies on human hepatocellular carcinoma growth [Citation63]. Biologically derived nanoparticles using silk fibroin (SF) and chitosan having sizes around 100 nm are fabricated by blending non-covalently to encapsulate curcumin using the devised capillary-microdot technique [Citation97]. Curcumin-polymer conjugates are frozen, lyophilized, crystallized, suspended in phosphate-buffered saline for characterization, and tested for efficacy against breast cancer cells. The entrapment and release of curcumin over 8 d are very high for SF-derived nanoparticles as compared to all SF and chitosan (SFCS) blends. The uptake and efficacy of SF-coated curcumin are significantly higher (p < .001) than SFCS-coated curcumin in Her2/neu expressing breast cancer cells. SF-derived curcumin nanoparticles show higher efficacy against breast cancer cells and have the potential to treat in vivo breast tumours by local, sustained and long-term therapeutic delivery as a biodegradable system.
Due to the specific properties of blood–brain barrier (BBB), only trace amount of curcumin in solution form is transported across BBB. The study is designed to load curcumin into polybutylcyanoacrylate nanoparticles (PBCN) coated with polysorbate 80, and to evaluate the effect of PBCN as a delivery system for carrying curcumin across BBB. With an average diameter of 152.0 nm, the in vitro release behaviour of drug from the nanoparticles is fitted to a double phase kinetics model. The distribution of PBCN into brain is evaluated after intravenous administration. In plasma, the area under concentration–time curve (AUC0–∞) for curcumin-loaded nanoparticles is greater than that for the control solution; moreover, the mean residence time of curcumin loaded nanoparticles is 14-fold more than that of the control solution. In brain, AUC0–∞ for curcumin-loaded nanoparticles is 2.53-fold that for the control solution. This study has demonstrated that PBCN could enhance the transport of curcumin to brain and has a potential as a delivery system to cross the BBB [Citation98]. Recently, curcumin was able to inhibit the metabolic activity of OE33 oesophageal cancer cells in a dose-dependent way, while metabolic activity decreased with prolonged incubation times [Citation99]. Curcumin was encapsulated with PLGA in the presence of poly(vinyl alcohol) and poly(L-lysine) stabilizers and good solid–solid compatibility and dispersion in the PLGA core of the nanoparticles was observed. Cellular uptake was also observed 2- and 6-fold increase in cisplatin-resistant A2780CP ovarian and metastatic MDA-MB-231 breast cancer cells compared to free curcumin. Effective apoptosis against these cell lines was induced by Nano-CUR6 formulation as indicated by the cell proliferation and clonogenic assays. Tumour-specific targeted delivery of curcumin was made feasible by coupling of anti-cancer antibody to the NPs [Citation93]. Klippstein et al. [Citation100] developed the nanocapsules in the form of shell to efficiently deliver the curcumin and examine its ability to ameliorate the colon cancer. The authors used PLGA and PEG to prepare the nanoshell and load the curcumin. The shell was stable in vitro for longer period compared to the other reported forms. Additionally, the authors found exclusive enhanced distribution of drugs in the major organs followed by intravenous injection in addition to reducing the colon tumour size efficiently. Curcumin and 5-flurourasil were released from CNPs and authors fund that compared to free form of compounds, NP associated release of compounds showed increased anti-cancer activity against colon cancer (HT29) cells and when injected, serum levels of curcumin and 5-fluorosil was higher in the NP treatment group than their free form [Citation101]. Popat et al. [Citation102] developed CNP incorporated with curcumin-gamma hydroxypropyl cyclodextrin. Nanoparticle system increased the solubility and cellular uptake. It showed 100% apoptosis against SCC25 cells. The results suggest that curcumin needed effective delivery mode to improve its performance.
Performance of curcumin was further improved by applying heat followed by delivery of curcumin encapsulated nanoparticles. Rao et al. [Citation103] encapsulated curcumin in CNPs. They tested their efficiency in vitro. Heat treatment increased the cellular uptake and as well as induced apoptosis in the prostate cancer cells. The authors further found that HSP70 and HSP27 elevated during the combination treatment and it could cause the enhanced apoptotic death in the cancer cells. Feline McDonough Sarcoma (FMS)-like tyrosine kinase 3 (FLT3) protein targeted delivery of curcumin was prepared using poloxamer 407. FLT3 specific peptide with poloxamer formed micelle containing curcumin. In vitro studies using flow cytometry and fluorescence microscopy showed that FLT3-curcumin killed leukaemic cells by enriched internalization [Citation104]. Further studies in detail about the mechanism can quickly transform curcumin from benchside to bedside and clinical applications since it has an impressive performance against various diseases including cancers.
Analogues of curcumin
Several pharmacological activities and therapeutic applications of curcumin in a variety of diseased conditions have been demonstrated [Citation105]. Poor solubility, very low gastrointestinal tract (GIT) dissolution rate, low absorption and extensive systemic metabolism are the reasons for its delivery problems and lack of clinical success. In order to improve the delivery of curcumin, phosphatidylcholine formulations, lipid complexes, solid-dispersions, prodrugs, microspheres, analogues, derivatives and nanoscale formulations are among those that have been investigated [Citation36,Citation44,Citation106]. To improve its stability and activity, many derivatives have been synthesized and studied, among which bis-demethoxycurcumin (bDMC) and diacetylcurcumin (DAC) are important [Citation107]. This study has shown that both bDMC and DAC are more stable than curcumin in physiological medium. To explore the mechanism of their chemotherapeutic effect, their role in proliferation in the HCT116 human colon cancer cells is studied and kinetic stability and cellular uptake data are correlated to their biological effects. Both bDMC and DAC impair correct spindle formation and induce a p53- and p21CIP1/WAF1-independent mitotic arrest, which is more stable and long-lasting for bDMC. A subsequent p53/p21CIP1/WAF1-dependent inhibition of G1 to S transition is triggered by curcumin and DAC as a consequence of the mitotic slippage, preventing post-mitotic cells from re-entering the cell cycle. Conversely, the G1/S arrest induced by bDMC is a direct effect of the drug and concomitant to the mitotic block. It is also demonstrated that bDMC induces rapid DNA double-strand breaks, indicating its possible development in anti-cancer clinical applications. Analogues of curcumin are more effective than curcumin in the soluble form for a variety of diseases including carcinomas [Citation108–110]. The therapeutic potential of analogues of curcumin can be improved by making it into microspheres or nanospheres, which enhance the release as well as biological activities. To prove this point, bis-demethoxy curcumin analogue (BDMCA), a novel curcumin analogue, was prepared as nanoparticles and evaluated for its in vitro and in vivo characteristics [Citation111]. The nanoparticle formulations effectively sustain the release of the drug for more than 10 d both in vitro and in vivo. Also, in vivo study has demonstrated that intravenous nanoparticular administration is shown to reverse serum liver enzyme levels by 90%, compared to 52% for repeated intravenous administration of the solution form. In another study, chitosan and starch were used to prepare NP and curcumin analogue was entrapped in them. Such nanoparticles were evaluated its anti-cancer activity against breast cancer cell lines. Nanoparticle treatment induced membrane breakage, aggregated and rounded the cells [Citation112]. 3,4-Difluorobenzylinine curcumin (CDF) is another derivative of natural curcumin showing potential anticancer activity [Citation37], however, its solubility was challenging escalating higher dose. Kesharwani et al. [Citation113] incorporated CDF into an amphiphilic styrene-maleic acid copolymer (SMA) to make curcumin micelles. SMA-CDF circulated longer time in the blood and it killed pancreatic cancer cells. Recently, allylated mono-carbonyl curcumin was prepared and evaluated its anti-cancer activity in vitro and such analogue showed improved performance against cancer cells meditating BAX and BCL2 proteins. Thus, curcumin analogues have enormous potentials for therapeutic applications [Citation114].
Dual drug delivery approach
Cancer treatment needs to be approached with multiple targets. Tumours often develop multidrug resistance (MDR). Investigators developed combination therapy combining well-known drugs such as doxorubicin and curcumin. Misra and Sahoo [Citation115] evaluated the dual delivery system against chronic myelogenous leukaemia (CML). Curcumin and DOX were encapsulated in PLGA nanoparticles and sustainable release rate was achieved for both drugs. Dual drug loading NPs enriched higher amount of DOX in the nucleus compared to individually loaded NPs suggesting assistance of curcumin against multidrug resistance. Apoptotic rate was significantly higher in the dual drug NPs treatment than single drug loaded NP and free curcumin based on Bcl2, c-JUN and p21 expression. Advantages of dual drug delivery system were further portrayed in vitro in breast cancer cells using PBCA NPs [Citation116]. In another study, anti-inflammatory effect against LPS-induced inflammation was observed due to synergistic effect when puerarin and curcumin were incorporated into the gold nanoparticle and delivered into rat. Both organ and circulation levels of inflammatory molecules were suppressed followed by combination of drugs than individual drug of nanoparticles. Dual delivery system was further evaluated in irreversible HCC model induced my diethylnitroamine. Synergic effect on the apoptosis, proliferation and angiogenesis was observed with the CUR-DOX-NPs. Effective anti-cancer treatment was due to reduced expression of P-glycoprotein (MDR protein), Bcl2 and HIF-1a proteins and further confirming MDR effect of curcumin [Citation117]. These results indicate the necessary consideration of multidrug administration for development of effective therapeutics.
Limitations of curcumin
The chelator activity of curcumin [Citation118] is sufficient to induce iron deficiency in vivo. Curcumin represses synthesis of hepcidin, a peptide that plays a central role in regulation of systemic iron balance. These results demonstrate that curcumin has the potential to affect systemic iron metabolism, particularly in a setting of subclinical iron deficiency. This may affect the use of curcumin in patients with marginal iron stores or those exhibiting the anaemia of cancer and chronic disease [Citation119]. Low bioavailability of curcumin could be the reason for the inefficient therapeutic effect. However, at certain load levels, 8 g per day [Citation120,Citation121] toxicity is observed. At higher concentration of curcumin with nanocarriers, the expectation of toxicity is increased. Though different studies have confirmed the beneficial effects of nanotechnology to overcome the shortcomings of curcumin bioavailability, a recent few studies have expressed some adverse effects over nanoparticles applications for curcumin delivery [Citation121]. Recently, degradation of curcumin in the presence of silver (Ag) nanoparticles has been reported [Citation122]. The degradation of curcumin is catalytically enhanced in the presence of Ag nanoparticles mainly at extreme conditions, such as alkaline pH and upon laser irradiation. Particularly, the laser irradiation of curcumin in the presence of Ag nanoparticles and alkaline pH induces the formation of polymerization photoproducts similar to those formed by polymerization of catechol rendering polycatechol [Citation122].
Future perspectives
One of the greatest problems in the conversion of herbal medicine into commercial products is the lack of fastness in the healing process, side effects due to impurity and lack of understanding at molecular level interactions with cellular proteins and consequent elevation and suppression of genes responsible for curing. There have been a number of studies for the improvement of bioavailability with various techniques and carrier compounds (1); however, it is difficult to determine the concentration of curcumin in vivo. Elaborate studies with curcumin nanoparticles are required in the preclinical and clinical levels. Quantitative estimation of clinical parameters both in biochemical and molecular levels are required for comparison with different models, mode of injection and various nanomaterials which will give a direction on the amelioration of disease conditions. As well, the toxicity of nanocarriers and degradable compounds must also be considered for safety. Site-targeted delivery of nanoparticles coated with curcumin and antibodies could enhance treatment efficiency and it can also alleviate the toxicity of nanocarriers as well as higher concentration. Site-targeted delivery of curcumin using nanoparticles is shown in . Different analogues of curcumin have been synthesized and therapeutic properties have been explored. Recently, biodegradable nanoparticle formulation of bis-demethoxy curcumin analogue (BDMCA) is fabricated by double emulsion method [Citation111]. Sustained release of the drug for more than 10 d both in vitro and in vivo is obtained. Intravenous nanoparticular administration reverses serum liver enzyme levels by 90%, compared to 52% for repeated i.v. administration in solution form. However, analogues of curcumin and their safety have not been found out thoroughly. At lower concentration of curcumin analogues, no toxicity is found in studies using small animal experiments. However, it is unknown whether high concentration of curcumin’s analogues could pose any health complications.
Conclusions
Nanoparticles loaded with curcumin are transformed as chemotherapeutic agents for curing multiple diseases ranging from inflammatory to cancer and viral diseases. In addition to development of bioavailability by oral administration, intravenous administration could benefit more since it can directly reach the site of action earlier. Development of analogues of curcumin and nanocarrier with receptor-mediated assistance would be much useful. Though a few studies have demonstrated biochemical pathways of pharmacological actions of curcumin, comprehensive understanding of mechanistic actions of curcumin-loaded nanoparticles at molecular level and their impact and possible toxic/adverse effects need to be thoroughly studied before its acceptance as commercial drug.
Acknowledgements
Dr. T. Hemalatha [No. SR/WOS-A/LS-64/2016(G)] gratefully acknowledge Department of Science and Technology, India for the DST-Women Scientist (A) fellowship.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin: from kitchen to clinic”. Biochem Pharmacol. 2008;75:787–809.
- Hatcher H, Planalp R, Cho J, et al. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652.
- Manach C, Williamson G, Morand C, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230s–242s.
- Garcea G, Jones DJL, Singh R, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:1011–1015.
- Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–494.
- Anand P, Kunnumakkara AB, Newman RA, et al. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818.
- Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol. 2007;595:453–470.
- Leung MHM, Kee TW. Effective stabilization of curcumin by association to plasma proteins: human serum albumin and fibrinogen. Langmuir. 2009;25:5773–5777.
- Ernest H, Shetty R. Impact of nanotechnology on biomedical sciences: review of current concepts on convergence of nanotechnology with biology. Tissue Eng. 2005;18:19.
- Bogunia-Kubik K, Sugisaka M. From molecular biology to nanotechnology and nanomedicine. Biosystems. 2002;65:123–138.
- Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20.
- Pulavendran S, Rajam M, Rose C, et al. Hepatocyte growth factor incorporated chitosan nanoparticles differentiate murine bone marrow mesenchymal stem cell into hepatocytes in vitro. IET Nanobiotechnol. 2010;4:51–60.
- Murugan R, Ramakrishna S. Nano-featured scaffolds for tissue engineering: a review of spinning methodologies. Tissue Eng. 2006;12:435–447.
- Gunasekera UA, Pankhurst QA, Douek M. Imaging applications of nanotechnology in cancer. Targ Oncol. 2009;4:169–181.
- Kumar V, Lewis SA, Mutalik S, et al. Biodegradable microspheres of curcumin for treatment of inflammation. Indian J Physiol Pharmacol. 2002;46:209–217.
- Ma Z, Shayeganpour A, Brocks DR, et al. High-performance liquid chromatography analysis of curcumin in rat plasma: application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomed Chromatogr. 2007;21:546–552.
- Narayanan NK, Nargi D, Randolph C, et al. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125:1–8.
- Rahman SMH, Telny TC, Ravi TK, et al. Role of surfactant and pH in dissolution of curcumin. Indian J Pharm Sci. 2009;71:139–142.
- Yoo SD, Lee SH, Kang E, et al. Bioavailability of itraconazole in rats and rabbits after administration of tablets containing solid dispersion particles. Drug Dev Ind Pharm. 2000;26:27–34.
- Kaewnopparat N, Kaewnopparat S, Jangwang A, et al. Increased solubility, dissolution and physicochemical studies of curcumin-polyvinylpyrrolidone K-30 solid dispersions. World Acad Sci Eng Technol. 2009;31:229.
- Reddy PD, Swarnalatha D. Recent advances in novel drug delivery systems. Int J PharmTech Res. 2010;2:2025–2027.
- Yu H, Huang Q. Enhanced in vitro anti-cancer activity of curcumin encapsulated in hydrophobically modified starch. Food Chem. 2010;119:669–674.
- Tonnesen HH, Masson M, Loftsson T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int J Pharm. 2002;244:127–135.
- Iwunze MO. Binding and distribution characteristics of curcumin solubilized in CTAB micelle. J Mole Liq. 2004;111:161–165.
- Shoba G, Joy D, Joseph T, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356.
- Bano G, Raina RK, Zutshi U, et al. Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. Eur J Clin Pharmacol. 1991;41:615–617.
- Velpandian T, Jasuja R, Bhardwaj RK, et al. Piperine in food: interference in the pharmacokinetics of phenytoin. Eur J Drug Metab Pharmacokinet. 2001;26:241–247.
- Liu ZJ, Han G, Yu JG, et al. Preparation and drug releasing property of curcumin nanoparticles. Zhong Yao Cai. 2009;32:277–279.
- Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104:1322–1331.
- Lin CC, Lin HY, Chen HC, et al. Stability and characterisation of phospholipid-based curcumin-encapsulated microemulsions. Food Chem. 2009;116:923–928.
- Liu A, Lou H, Zhao L, et al. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal. 2006;40:720–727.
- Shaikh J, Ankola DD, Beniwal V, et al. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37:223–230.
- Wang X, Wang YW, Huang Q. Enhancing stability and oral bioavailability of polyphenols using nanoemulsions, in micro/nanoencapsulation of active food ingredients. Washington (DC): American Chemical Society; 2009. p. 198–212.
- Lopes-Rodrigues V, Sousa E, Vasconcelos MH. Curcumin as a modulator of p-glycoprotein in cancer: challenges and perspectives. Pharmaceuticals. 2016;9:71.
- Song IS, Cha JS, Choi MK. Characterization, in vivo and in vitro evaluation of solid dispersion of curcumin containing d-alpha-tocopheryl polyethylene glycol 1000 succinate and mannitol. Molecules. 2016;21:1386.
- Paradkar A, Ambike AA, Jadhav BK, et al. Characterization of curcumin-PVP solid dispersion obtained by spray drying. Int J Pharm. 2004;271:281–286.
- Wang X, Jiang Y, Wang YW, et al. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008;108:419–424.
- Hu L, Jia Y, Niu F, et al. Preparation and enhancement of oral bioavailability of curcumin using microemulsions vehicle. J Agric Food Chem. 2012;60:7137–7141.
- Lin CC, Lin HY, Chi MH, et al. Preparation of curcumin microemulsions with food-grade soybean oil/lecithin and their cytotoxicity on the HepG2 cell line. Food Chem. 2014;154:282–290.
- Zou L, Zheng B, Liu W, et al. Enhancing nutraceutical bioavailability using excipient emulsions: influence of lipid droplet size on solubility and bioaccessibility of powdered curcumin. J Funct Foods. 2015;15:72–83.
- Krausz AE, Adler BL, Cabral V, et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine. 2015;11:195–206.
- Reeves A, Vinogradov SV, Morrissey P, et al. Curcumin-encapsulating nanogels as an effective anticancer formulation for intracellular uptake. Mol Cell Pharmacol. 2015;7:25–40.
- Purpura M, Lowery RP, Wilson JM, et al. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur J Nutr. 2017 [Feb 16]. DOI:https://doi.org/10.1007/s00394-016-1376-9
- Thangapazham RL, Puri A, Tele S, et al. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int J Oncol. 2008;32:1119–1123.
- Saengkrit N, Saesoo S, Srinuanchai W, et al. Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloids Surf B Biointerfaces. 2014;114:349–356.
- Dai F, Zhang X, Shen W, et al. Liposomal curcumin inhibits hypoxia-induced angiogenesis after transcatheter arterial embolization in VX2 rabbit liver tumors. Onco Targets Ther. 2015;8:2601–2611.
- Nair KL, Thulasidasan AKT, Deepa G, et al. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int J Pharm. 2012;425:44–52.
- Tran LD, Hoang NMT, Mai TT, et al. Nanosized magnetofluorescent Fe3O4–curcumin conjugate for multimodal monitoring and drug targeting. Colloids Surf A Physicochem Eng Asp. 2010;371:104–112.
- Mancarella S, Greco V, Baldassarre F, et al. Polymer-coated magnetic nanoparticles for curcumin delivery to cancer cells. Macromol Biosci. 2015;15:1365–1374.
- Yallapu MM, Othman SF, Curtis ET, et al. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int J Nanomedicine. 2012;7:1761–1779.
- Lv L, Shen Y, Liu J, et al. Enhancing curcumin anticancer efficacy through di-block copolymer micelle encapsulation. J Biomed Nanotechnol. 2014;10:179–193.
- Leung MHM, Colangelo H, Kee TW. Encapsulation of curcumin in cationic micelles suppresses alkaline hydrolysis. Langmuir. 2008;24:5672–5675.
- Song Z, Zhu W, Liu N, et al. Linolenic acid-modified PEG-PCL micelles for curcumin delivery. Int J Pharm. 2014;471:312–321.
- Manju S, Sreenivasan K. Hollow microcapsules built by layer by layer assembly for the encapsulation and sustained release of curcumin. Colloids Surf B Biointerfaces. 2011;82:588–593.
- Paşcalău V, Soritau O, Popa F, et al. Curcumin delivered through bovine serum albumin/polysaccharides multilayered microcapsules. J Biomater Appl. 2016;30:857–872.
- Ghosh M, Singh ATK, Xu W, et al. Curcumin nanodisks: formulation and characterization. Nanomedicine. 2011;7:162–167.
- Paramera EI, Konteles SJ, Karathanos VT. Stability and release properties of curcumin encapsulated in Saccharomyces cerevisiae, β-cyclodextrin and modified starch. Food Chem. 2011;125:913–922.
- Salaün F, Vroman I. Curcumin-loaded nanocapsules: formulation and influence of the nanoencapsulation processes variables on the physico-chemical characteristics of the particles. Int J Chem React Eng. 2009;7:11–08.
- Nam SH, Nam HY, Joo JR, et al. Curcumin-loaded PLGA nanoparticles coating onto metal stent by electrophoretic deposition techniques. Bull Korean Chem Soc. 2007;28:397.
- Tsai YM, Chien CF, Lin LC, et al. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int J Pharm. 2011;416:331–338.
- Zhao J, Liu J, Xu S, et al. Graft copolymer nanoparticles with pH and reduction dual-induced disassemblable property for enhanced intracellular curcumin release. ACS Appl Mater Interfaces. 2013;5:13216–13226.
- Patra D, Sleem F. A new method for pH triggered curcumin release by applying poly(L-lysine) mediated nanoparticle-congregation. Anal Chim Acta. 2013;795:60–68.
- Ma N, Ma C, Li C, et al. Influence of nanoparticle shape, size, and surface functionalization on cellular uptake. J Nanosci Nanotech. 2013;13:6485–6498.
- Dandekar P, Dhumal R, Jain R, et al. Toxicological evaluation of pH-sensitive nanoparticles of curcumin: acute, sub-acute and genotoxicity studies. Food Chem Toxicol. 2010;48:2073–2089.
- Li X, Yuan H, Zhang C, et al. Preparation and in-vitro/in-vivo evaluation of curcumin nanosuspension with solubility enhancement. J Pharm Pharmacol. 2016;68:980–988.
- Nirmala C, Anand S, Puvanakrishnan R. Curcumin treatment modulates collagen metabolism in isoproterenol induced myocardial necrosis in rats. Mol Cell Biochem. 1999;197:31–37.
- Nirmala C, Puvanakrishnan R. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mole Cell Biochem. 1996;159:85–93.
- Manikandan P, Sumitra M, Aishwarya S, et al. Curcumin modulates free radical quenching in myocardial ischaemia in rats. Int J Biochem Cell Biol. 2004;36:1967–1980.
- Quiles JL, Aguilera C, Mesa MD, et al. An ethanolic-aqueous extract of Curcuma longa decreases the susceptibility of liver microsomes and mitochondria to lipid peroxidation in atherosclerotic rabbits. Biofactors. 1998;8:51–57.
- Sidhu GS, Singh AK, Thaloor D, et al. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998;6:167–177.
- Jacob A, Wu R, Zhou M, et al. Mechanism of the anti-inflammatory effect of curcumin: PPAR-gamma activation. PPAR Res. 2007;2007:89369.
- Jiang Y. Micro-and nano-encapsulation and controlled-release of phenolic compounds and other food ingredients. New Brunswick (NJ): Rutgers University-Graduate School-New Brunswick; 2009.
- Sou K, Inenaga S, Takeoka S, et al. Loading of curcumin into macrophages using lipid-based nanoparticles. Int J Pharm. 2008;352:287–293.
- Ramshankar YV, Suresh S, Devi K. Novel self-emulsifying formulation of curcumin with improved dissolution, antiangiogenic and anti-inflammatory activity. Clin Res Regul Aff. 2008;25:213–234.
- Sindhu K, Indra R, Rajaram A, et al. Investigations on the interaction of gold-curcumin nanoparticles with human peripheral blood lymphocytes. J Biomed Nanotechnol. 2011;7:56.
- Chaubey P, Patel RR, Mishra B. Development and optimization of curcumin-loaded mannosylated chitosan nanoparticles using response surface methodology in the treatment of visceral leishmaniasis. Expert Opin Drug Deliv. 2014;11:1163–1181.
- Maiti K, Mukherjee K, Gantait A, et al. Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330:155–163.
- Sasaki H, Sunagawa Y, Takahashi K, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 2011;34:660–665.
- Mulik RS, Mo¨nkko¨nen J, Juvonen RO, et al. ApoE3 mediated poly(butyl) cyanoacrylate nanoparticles containing curcumin: study of enhanced activity of curcumin against beta amyloid induced cytotoxicity using in vitro cell culture model. Mol Pharmaceutics. 2010;7:815–825.
- Tiwari SK, Agarwal S, Tripathi A, et al. Bisphenol-A mediated inhibition of hippocampal neurogenesis attenuated by curcumin via canonical Wnt pathway. Mol Neurobiol. 2016;53:3010–3029.
- Liu S, Cao Y, Qu M, et al. Curcumin protects against stroke and increases levels of Notch intracellular domain. Neurol Res. 2016;38:553–559.
- Mourtas S, Canovi M, Zona C, et al. Curcumin-decorated nanoliposomes with very high affinity for amyloid-β1–42 peptide. Biomaterials. 2011;32:1635–1645.
- Ramalingam P, Ko YT. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: pharmacokinetic and brain distribution evaluations. Pharm Res. 2015;32:389–402.
- Kundu P, Das M, Tripathy K, et al. Delivery of dual drug loaded lipid-based nanoparticles across the blood–brain barrier impart enhanced neuroprotection in a rotenone-induced mouse model of Parkinson’s disease. ACS Chem Neurosci. 2016;7:1658–1670.
- Wang J, Wang Y, Liu Q, et al. Rational design of multifunctional dendritic mesoporous silica nanoparticles to load curcumin and enhance efficacy for breast cancer therapy. ACS Appl Mater Interfaces. 2016;8:26511–26523.
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398.
- López‐Lázaro M. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol Nutr Food Res. 2008;52:S103–127.
- Ma Z, Haddadi A, Molavi O, et al. Micelles of poly(ethylene oxide)-b-poly(ε-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J Biomed Mater Res. 2008;86A:300–310.
- Sahu A, Kasoju N, Bora U. Fluorescence study of the curcumin-casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules. 2008;9:2905–2912.
- Sahu A, Bora U, Kasoju N, et al. Synthesis of novel biodegradable and self-assembling methoxy poly(ethylene glycol)-palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008;4:1752–1761.
- Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867–3875.
- Prajakta D, Ratnesh J, Chandan K, et al. Curcumin loaded pH-sensitive nanoparticles for the treatment of colon cancer. J Biomed Nanotechnol. 2009;5:445–455.
- Yallapu MM, Maher DM, Sundram V, et al. Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. J Ovarian Res. 2010;3:11.
- Rossi JJ. Receptor-targeted siRNAs. Nat Biotechnol. 2005;23:682–684.
- Ganta S, Amiji M. Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharmaceutics. 2009;6:928–939.
- Bisht S, Feldmann G, Soni S, et al. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol. 2007;5:3.
- Gupta V, Aseh A, Ríos CN, et al. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int J Nanomedicine. 2009;4:115–122.
- Sun M, Gao Y, Guo C, et al. Enhancement of transport of curcumin to brain in mice by poly(n-butylcyanoacrylate) nanoparticle. J Nanopart Res. 2010;12:3111–3122.
- van de Luijtgaarden W, Guha S, Milano F, et al. Nano-curcumin as a promising new therapy for treatment of esophageal adenocarcinoma. Gastroenterology. 2011;140:S–223–S-224.
- Klippstein R, Wang JTW, El-Gogary RI, et al. Passively targeted curcumin-loaded PEGylated PLGA nanocapsules for colon cancer therapy in vivo. Small. 2015;11:4704–4722.
- Anitha A, Sreeranganathan M, Chennazhi KP, et al. In vitro combinatorial anticancer effects of 5-fluorouracil and curcumin loaded N,O-carboxymethyl chitosan nanoparticles toward colon cancer and in vivo pharmacokinetic studies. Eur J Pharm Biopharm. 2014;88:238–251.
- Popat A, Karmakar S, Jambhrunkar S, et al. Curcumin-cyclodextrin encapsulated chitosan nanoconjugates with enhanced solubility and cell cytotoxicity. Colloids Surf B Biointerfaces. 2014;117:520–527.
- Rao W, Zhang W, Poventud-Fuentes I, et al. Thermally responsive nanoparticle-encapsulated curcumin and its combination with mild hyperthermia for enhanced cancer cell destruction. Acta Biomater. 2014;10:831–842.
- Tima S, Okonogi S, Ampasavate C, et al. Development and characterization of FLT3-specific curcumin-loaded polymeric micelles as a drug delivery system for treating FLT3-overexpressing leukemic cells. J Pharm Sci. 2016;105:3645–3657.
- Anand P, Thomas SG, Kunnumakkara AB, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–1611.
- Markatou E, Gionis V, Chryssikos GD, et al. Molecular interactions between dimethoxycurcumin and Pamam dendrimer carriers. Int J Pharm. 2007;339:231–236.
- Basile V, Ferrari E, Lazzari S, et al. Curcumin derivatives: molecular basis of their anti-cancer activity. Biochem Pharmacol. 2009;78:1305–1315.
- Adams BK, Ferstl EM, Davis MC, et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12:3871–3883.
- Furness MS, Robinson TP, Ehlers T, et al. Antiangiogenic agents: studies on fumagillin and curcumin analogs. Curr Pharm Des. 2005;11:357–373.
- Ohori H, Yamakoshi H, Tomizawa M, et al. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther. 2006;5:2563–2571.
- Anuradha C, Aukunuru J. Preparation, characterisation and in vivo evaluation of bis-demethoxy curcumin analogue (BDMCA) nanoparticles. Trop J Pharm Res. 2010;9:51–58.
- Subramanian SB, Francis AP, Devasena T. Chitosan-starch nanocomposite particles as a drug carrier for the delivery of bis-desmethoxy curcumin analog. Carbohydr Polym. 2014;114:170–178.
- Kesharwani P, Banerjee S, Padhye S, et al. Parenterally administrable nano-micelles of 3,4-difluorobenzylidene curcumin for treating pancreatic cancer. Colloids Surf B Biointerfaces. 2015;132:138–145.
- Qiu C, Hu Y, Wu K, et al. Synthesis and biological evaluation of allylated mono-carbonyl analogues of curcumin (MACs) as anti-cancer agents for cholangiocarcinoma. Bioorg Med Chem Lett. 2016;26:5971–5976.
- Misra R, Sahoo SK. Coformulation of doxorubicin and curcumin in poly(d,l-lactide-co-glycolide) nanoparticles suppresses the development of multidrug resistance in K562 cells. Mol Pharmaceutics. 2011;8:852–866.
- Duan J, Mansour HM, Zhang Y, et al. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm. 2012;426:193–201.
- Zhao X, Chen Q, Li Y, et al. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur J Pharm Biopharm. 2015;93:27–36.
- Jiao Y, Wilkinson J, Christine Pietsch E, et al. Iron chelation in the biological activity of curcumin. Free Radic Biol Med. 2006;40:1152–1160.
- Jiao Y, Wilkinson J, Di X, et al. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood. 2008;113:462–469.
- Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900.
- Burgos-Moron, E, Calderón-Montaño JM, Salvador J, et al. The dark side of curcumin. Int J Cancer. 2010;126(7):1771–1775.
- Canamares MV, Garcia-Ramos JV, Sanchez-Cortes S. Degradation of curcumin dye in aqueous solution and on ag nanoparticles studied by ultraviolet-visible absorption and surface-enhanced Raman spectroscopy. Appl Spectrosc. 2006;60:1386–1391.