?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of the study is to screen the effective shRNA sequence which can silence the human COX-2 expression level in synovial cells of rheumatoid arthritis (RA) patient transfected by the lentivirus. Four pairs of hCOX-2 shRNA were designed and inserted into lentivirus to form pGPHI/GFP/Neo-shRNA vector. The reconstructed virus was transfected into synovial cells derived from RA patients, and then the expression level of hCOX-2 mRNA and the protein of the inflammatory factors including prostaglandin E2 (PGE2), vascular endothelial growth factor (VEGF), interleukin-1β (IL-1β) and tumour necrosis factor alpha (TNF-α) in the supernatants were examined with real-time PCR and ELISA, respectively. There was no obvious negative influence on cell growth and morphology after hCOX-2 shRNA gene transfection mediated by lentivirus. The hCOX-2 mRNA expression level, as well as the concentration of PGE2, VEGF, IL-1β and TNF-α, decreased significantly (p < .05). RNAi mediated by lentivirus can significantly inhibit hCOX-2 mRNA expression level in synovial cells of RA patients, so as to reduce the expression of inflammatory cytokines.
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease with unknown aetiology that causes the immune system to attack the synovium, leading to chronic inflammation. A crucial factor which contributes to chronic inflammatory responses in the pathogenesis of RA is the activation of members of NF-κB transcription family [Citation1,Citation2]. The signal transduction proteins of RA are usually affected by the activation of NF-κB, including iNOS, cyclooxygenase-2 (COX-2) and components of the mitogen-activated protein kinase (MAPK) [Citation3,Citation4]. The expression of COX-2, instead of COX-1, was found to be elevated in a disease-related pattern in the synovial tissue from patients with RA [Citation5]. Growing evidence suggests that activated rheumatoid synovial fibroblasts (RASFs) actively play a role in RA synovitis. The inflammatory mediators, such as cytokines, matrix metalloproteinases (MMPs) and COX-2, help RASFs to form the pannus in RA joints that eventually destroy bone and cartilage [Citation6].
In arthritic patients, COX-2 expresses in chondrocytes and synovial tissue [Citation7] and promotes the production of prostaglandins, in particular prostaglandin E2 (PGE2), which regulate the anabolic/catabolic processes and are important mediators of inflammation and pain [Citation8,Citation9]. In the last decade, RNA interference (RNAi) has rapidly become an innovative and elective tool for gene silencing. Small interfering RNAs (siRNAs), 21–23 nt dsRNAs molecules with 2–3 nt overhangs on their 3′-ends, are capable of binding to homologous target messenger RNA (mRNA), leading to cleavage of the transcript near the centre of the pairing sequence, are defined as the effectors of the RNAi pathway [Citation10]. The synthetic siRNA molecules introduced into cells result in target mRNA degradation. Efficacy, target specificity and preclinical safety have been demonstrated for several distinct RNAi technologies, such as siRNA, shRNA and bi-shRNA [Citation11].
In this study, hCOX-2 was selected to be silenced to testify the possibility of suppressing the expression of COX-2 and reducing the inflammation of RA. Lentivirus containing difference hCOX-2 shRNA sequences were transfected into synovial cells derived from RA patients individually, and then the inhibition efficiency and related inflammatory factors were detected to testify the value of the hCOX-2 gene silencing.
Materials and methods
Culture of the RASFs
Thirteen patients diagnosed as RA (six men and seven women) were selected after the approval of ethics committee of our hospital and written informed consents were signed. Synovial tissue was taken out from the knee joint during surgery like arthroscopy or arthroplasty. After washed with PBS mixture containing 400 u/ml of penicillin, 0.4 mg/ml of streptomycin, the synovial tissue was cut into pieces of 1 mm3, and then 4% triploid type II collagen enzyme was added. Foetal bovine serum in 10% concentration (Hyclone, Logan, UT) was introduced after 4 h of digestion in 37 °C water bath, and then the cells were collected by centrifugation and plated into 25 cm2 culture flasks at a density of 2 × 104/cm2. After trypan blue staining, 5 ml 1640 culture medium (Invitrogen, Carlsbad, CA) containing 10% foetal bovine serum was added into the medium, the cells were incubated at 37 °C in 5% CO2 and 100% humidity. When cells grew into 70–80% confluency, they were trypsinized and reseeded into new flasks. Cells in second generation were used for the follow-up experiments.
Informed consent
This research was carried out in compliance with the Helsinki Declaration of 1975, as revised in 1983, and was approved by the Research Ethics Committee of Qingdao University. Patients were informed before any procedure and only after the informed consent was signed that the material can be used for scientific research.
Construction of hCOX-2 shRNA lentivirus vectors
Four pairs of hCOX-2 shRNA ( and ) were designed with Designer3.0 (GenePharma, Shanghai, China) software according to the human COX-2 mRNA (NM_000963.2) sequence obtained from GenBank (http://www.ncbi.nlm.nih.gov/genbank), and the shRNA design principles. Double-stranded DNA fragments, with cohesive termini of the BhsI and BamHI restriction enzymes and hairpin sequence, were synthesized in vitro. The fragments were ligated into pGPHI/GFP/Neo vectors and transformed into E. coli DH5α for propagation, and then were amplified and screened. Lentivirus vector pGPHI/GFP/Neo-shRNA was synthesized and packed by Shanghai GenePharma Co., Ltd. (Shanghai, China).
Table 1. Sequence of DNA oligo.
Table 2. The target sequence and location.
Lentivirus transfection
Lentivirus liquid was added into the medium of the second generation synovial cells in six-hole plate at a density of 2 × 105 cells/hole. The cells were divided into five groups, and lentivirus at 3 × 108 virus titer carrying hCOX-2shRNA1, hCOX-2shRNA2, hCOX-2shRNA3, hCOX-2shRNA4 and negative control group for each group, respectively. Three concentrations of lentivirus, 200 µl/hole (Group A), 100 µl/hole (Group B) and 50 µl/hole (Group C), were added into each group. Polybrene (6 μg/ml) (Sigma, St. Louis, MO) was also introduced into each hole. Culture medium instead of the virus was served as blank control group. HeLa cell, instead of synovial cell, was set up as the positive control group, and the rest of the procedures were the same. After 24 h, phorbol esters were added into each group (except for HeLa cell group) at the final concentration of 100 nmol/l to stimulate the hCOX-2 expression. Forty-eight hours after transfection, the cells were screened by G418 with the concentration of 400 mg/l. The efficiency was calculated at 48 h, 72 h, 96 h after transfection, and the cell growth curve was drawn.
Fluorescent quantitative reverse transcription polymerase chain reaction (RT-PCR)
About 1 × 106 cells were collected of each group at 2, 5 and 10 days after transfection. After cells were fully cracked by Trizol reagent, chloroform was added into the supernatants. Blended by concussion, the supernatants were added isopropyl alcohol and centrifuged, and then the mRNA was diluted with ultrapure water after being washed with 75% ethanol. Optical density (OD) value and concentration of the RNA were measured with the spectrophotometer, and the purity was detected by RNA electrophoresis. Total RNA was extracted and cDNA was synthesized with reverse transcription following the instruction of the RT-PCR kit (Takara, Tokyo, Japan). Then real-time fluorescence quantitative PCR was carried out with RTFQ PCR kit (Takara, Tokyo, Japan) following the instruction. The reaction conditions were as follows: pre-denaturation at 95 °C for 15 s, denaturation at 90 °C for 10 s, annealing at 60 °C for 30 s, and for 40 cycles. Then the mixtures were denatured at 95 °C for 60 s at the end of the PCR process, and cooled to 55 °C. Sequence of the primer refers to . Reaction products were examined with 1% agarose gel electrophoresis. OD values of the electrophoresis bands were analysed with gel imaging system to verify the stripes of target gene. Quantitative analysis was performed using the ratio of the target gene to hGAPDH.
Table 3. The primer sequence for hCOX-2 and hGAPDH.
Expression level of TNF-α, PGE2, VEGF, IL-1β
Culture supernatants of synovial cells of each group at 2, 5 and 10 days after transfection were collected, and the concentration of TNF-α, PGE2, vascular endothelial growth factor (VEGF), interleukin-1β (IL-1β) was examined by ELISA kit (R&D Co., Minneapolis, MN) according to the instructions.
Statistical analysis
All data were collected as means ± standard deviation and analysed using SPSS 19.0 software (SPSS Inc., Chicago, IL). Group mean values were compared using Student’s t-test for two different groups, or ANOVA for multiple groups. Statistical significance was set at p values less than .05.
Results
Phenotype of RASFs
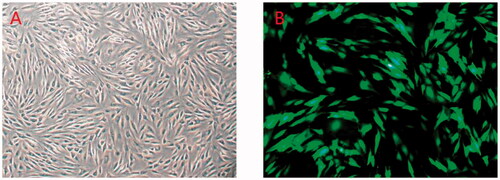
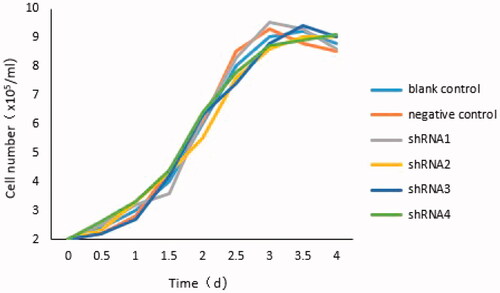
The viability of synovial cells counted by trypan blue staining was 96%±2% (). Most cells adhered onto the bottom at 6 h, began to grow branches in 12 h, and became long fusiform shape in 24 h. Compared with the blank control group, the cells showed no obvious abnormalities after transfection (), except for the group with the higher multiplicity of infection (MOI) value which was more than 100, of which toxic reaction in the cells was present, even lead to cell death. Growth curves of all groups were roughly parallel, indicated that the transfection had no obvious effect on cell growth ().
Transfection efficiency measurements
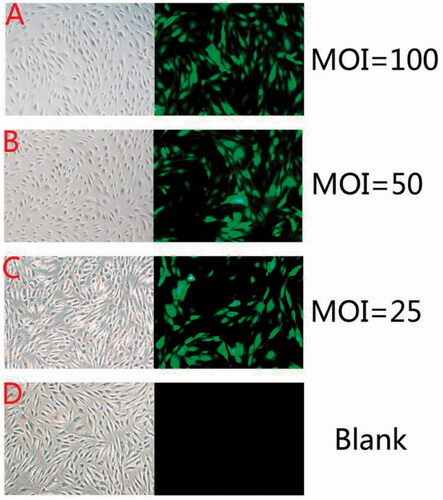
Transfection efficiency was calculated after 72 h and the optimum MOI was determined by the formula: MOI = virus titer × volume of virus (ml)/amount of cell. Transfection efficiency=(amount of cell under the fluorescent/amount of cell under white light) %. The MOI of the group which added 200 µl, 100 µl, 50 µl lentivirus was 100, 50, 25, respectively, and the corresponding transfection efficiency was 92.5% ± 2.5%, 63.5% ± 2% and 31.5% ± 1.5% ().
Expression level of hCOX-2 mRNA
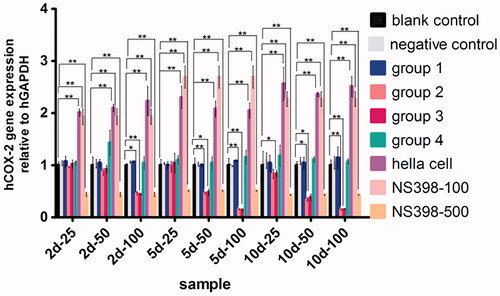
All values of quantitative RT-PCR were normalized with GAPDH mRNA concentration so as to take relative COX-2 mRNA concentration into account. Compared with the blank control group, COX-2 mRNA expression in group 2 and group 3 was significantly reduced after transfection (p < .05) ( and ). PCR products were shown expected size of strips by electropherogram.
Table 4. hCOX-2 mRNA expression level.
Expression level of TNF-α, PGE2, VEGF, IL-1β
Compared with the blank control groups, the expression level (ng/l) of TNF-α, PGE2, VEGF, IL-1β decreased significantly in group 2 and group 3, especially on day 5 and 10 after transfection (p < .01).
Concentration of TNF-α
Ten days after transfection, the concentration of TNF-α decreased from 31.52 ± 0.33 ng/l to 14.55 ± 0.59 ng/l in group 2 (p < .01) and decreased from 30.06 ± 0.57 ng/l to 15.12 ± 0.78 ng/l in group 3 (p < .01), while no statistical significance existed between group 1 and group 4 (p>.05) ( and .
Figure 5. (A) Concentration of TNF-α. (B) Concentration of IL-1β. (C) Concentration of VEGF. (D) Concentration of PGE2. **p < .01.
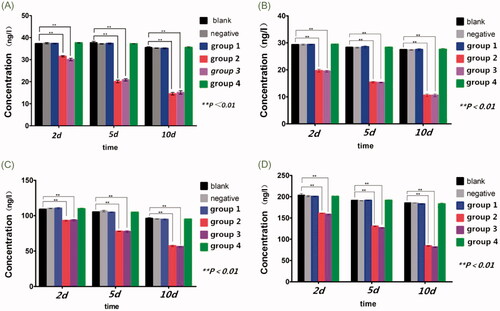
Table 5. Concentration of TNF-α.
Concentration of IL-1β
Concentration of IL-1β decreased from 19.69 ± 0.57 ng/l to 10.60 ± 0.53 ng/l in group 2 (p < .01), and decreased from 19.48 ± 0.33 ng/l to 10.59 ± 0.55 ng/l in group 3 (p < .01). No statistical significance existed between group 1 and group 4 (p>.05) ( and ).
Table 6. Concentration of IL-1β.
Concentration of VEGF
Ten days after transfection, the concentration of VEGF decreased from 93.10 ± 0.84 ng/l to 57.35 ± 1.04 ng/l in group 2 (p < .01) and decreased from 93.71 ± 1.06 ng/l to 56.10 ± 0.77 ng/l in group 3 (p < .01), while no statistical significance was found between group 1 and group 4 (p>.05) ( and ).
Table 7. Concentration of VEGF.
Concentration of PGE2
In group 2, the concentration of PGE2 decreased from 160.96 ± 0.23 ng/l to 84.44 ± 1.02 ng/l (p < .01), and in group 3, decreased from 158.44 ± 1.27 ng/l to 81.48 ± 1.10 ng/l (p < .01). No statistical significance detected between group 1 and group 4 (p>.05) ( and ).
Table 8. Concentration of PGE2.
Discussion
Rheumatoid arthritis is a chronic inflammatory systemic disease of unknown etiology. The most typical characteristic of it is chronic synovitis that subsequently causes bone and cartilage destruction. When activated RASFs play a key role in the pathogenesis of RA synovitis through proliferation and resultant pannus formation [Citation6,Citation12,Citation13]. Inflammatory cytokines, like the MMPs and COX-2 released by RASFs, can lead to the destruction of bone and articular cartilage [Citation14]. In many situations, inflammation and angiogenesis are linked tightly to create the vicious cycle, which lead to up-regulation of pro-angiogenic factors and pro-inflammatory cytokines, such as tumour necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) [Citation15]. Inflammation is the result of a variety of pro-inflammatory cytokines including TNF-α and IL-6, also, PGE2 and NO, which are synthesized by COX-2 and inducible nitric oxide synthase (iNOS) [Citation16]. It was revealed that TNF-α can increase the expression of COX-2 and induce PGE2 production via PI3K-Akt and NF-κB pathways in synovial fibroblasts, and the effects can be inhibited by honokiol [Citation17]. So, in this topic, human COX-2 (NM_000963.2) was selected as a target gene to be inhibited through COX-2 shRNA transfection mediated by lentivirus, in order to reduce the expression of downstream inflammatory factors.
RNAi technique has been introduced to silence the COX-2 protein mainly in tumor cells, which is beneficial for realizing the molecular and phenotypical cut down the function of COX-2 gene by selecting techniques based on stronger COX-2 silencing deprived of aspecific effects [Citation18]. Compared with NSAIDs, the RNAi technology is a more powerful and specific tool for researching the functional role and the effection of COX-2 gene blocking. Nevertheless, limited reports can be searched out based on human synoviocytes derived from RA patients, and the changes of inflammatory factors after gene silencing. Our results showed that lentivirus transfection had no obvious negative impact on synoviocyte growth. Limited studies were focused on the lentivirus transfection to synoviocyte, and similar results were made out that no obvious negative impact of lentivirus transfection on chondrocytes [Citation19]. Through screening, the COX-2 mRNA expression was down-regulated successfully, and we tested the most effective sequence COX-2 shRNA-2 and 3. PGE2 as one of the inflammatory factors which contribute to inflammation, fever and pain in pathological processes, is affected significantly by COX-2, a key enzyme in the processes of synthesis [Citation20]. Angiogenesis is an important characteristics in chronic inflammatory diseases such as RA [Citation21]. During the progression of RA, VEGF is a critical pro-angiogenic factor in angiogenesis [Citation22]. Clinical study has been focused on TNF blockade in RA patients [Citation23]. It is well known that cell signalling induced by IL-1 takes part in the activation of NF-κB, which plays a key role via regulating the transcriptional of proinflammatory genes during inflammation [Citation24,Citation25].
Conclusions
After inhibition of COX-2, the inflammation factors such as PGE2, VEGF, TNF-α could be down-regulated. The reduction of IL-1β may be ascribed to complex signalling pathway, and the inhibition efficiency was similar with NS398 in 500 nmol/l concentration on synovial cells. In a study about retrovirus infection, transducing efficiency at 60–70% was obtained in primary human synovial fibroblasts under the conditions of MOI ranged between 120 and 12 T.U./cell, when 2000 or 20,000 cell/cm2 were seeded. In the current research, transfection efficiency at 70% was achieved when the MOI was 70–100, while obvious toxicity on the cells occurred when the MOI was higher than 100. Higher transfection efficiency and inhibition ratio appeared 5–10 days later after transfection. Therefore, COX-2 siRNA transfection mediated by lentivirus is a theoretically effective way to control the inflammatory reaction in synovium of RA patients. Since multiple target sequences according to the COX-2 mRNA can be designed for gene silencing, more efficient COX-2 shRNA can be screened for further clinical trial.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Blüml S, Redlich K, Smolen JS. Mechanisms of tissue damage in arthritis. Semin Immunopathol. 2014;36:531–540.
- Alghasham A, Rasheed Z. Therapeutic targets for rheumatoid arthritis: progress and promises. Autoimmunity. 2014;47:77–94.
- Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford). 2008;47:409–414.
- Thummuri D, Jeengar MK, Shrivastava S, et al. Thymoquinone prevents RANKL-induced osteoclastogenesis activation and osteolysis in an in vivo model of inflammation by suppressing NF-KB and MAPK signalling. Pharmacol Res. 2015;99:63–73.
- Laveti D, Kumar M, Hemalatha R, et al. Anti-inflammatory treatments for chronic diseases: a review. IADT. 2013;12:349–361.
- Lefevre S, Meier FM, Neumann E, et al. Role of synovial fibroblasts in rheumatoid arthritis. Curr Pharm Des. 2015;21:130–141.
- Choi YJ, Lee WS, Lee EG, et al. Sulforaphane inhibits IL-1β-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of MMPs, COX-2, and PGE2. Inflammation. 2014;37:1496–1503.
- Yoon HY, Lee EG, Lee H, et al. Kaempferol inhibits IL-1β-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. Int J Mol Med. 2013;32:971–977.
- Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res. 2004;427:S37–S46.
- Dyawanapelly S, Ghodke SB, Vishwanathan R, et al. RNA interference-based therapeutics: molecular platforms for infectious diseases. J Biomed Nanotechnol. 2014;10:1998–2037.
- Baigude H, Rana TM. Strategies to antagonize miRNA functions in vitro and in vivo. Nanomedicine (Lond). 2014;9:2545–2555.
- Leech MT, Morand EF. Fibroblasts and synovial immunity. Curr Opin Pharmacol. 2013;13:565–569.
- Dimitroulas T, Nikas SN, Trontzas P, et al. Biologic therapies and systemic bone loss in rheumatoid arthritis. Autoimmun Rev. 2013;12:958–966.
- Filer A. The fibroblast as a therapeutic target in rheumatoid arthritis. Curr Opin Pharmacol. 2013;13:413–419.
- Semerano L, Clavel G, Assier E, et al. Blood vessels, a potential therapeutic target in rheumatoid arthritis? Joint Bone Spine. 2011;78:118–123.
- Lee AS, Ellman MB, Yan D, et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527:440–447.
- Li J, Shao X, Wu L, et al. Honokiol: an effective inhibitor of tumor necrosis factor-alpha-induced up-regulation of inflammatory cytokine and chemokine production in human synovial fibroblasts. Acta Biochim Biophys Sin (Shanghai). 2011;43:380–386.
- Mollaie HR, Monavari SH, Arabzadeh SA, et al. RNAi and miRNA in viral infections and cancers. Asian Pac J Cancer Prev. 2013;14:7045–7056.
- Wang ZH, Yang ZQ, He XJ, et al. Lentivirus-mediated knockdown of aggrecanase-1 and -2 promotes chondrocyte-engineered cartilage formation in vitro. Biotechnol Bioeng. 2010;107:730–736.
- Siebert S, Tsoukas A, Robertson J, et al. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol Rev. 2015;67:280–309.
- Selmi C, Generali E, Massarotti M, et al. New treatments for inflammatory rheumatic disease. Immunol Res. 2014;60:277–288.
- Moon SJ, Park MK, Oh HJ, et al. Engagement of toll-like receptor 3 induces vascular endothelial growth factor and interleukin-8 in human rheumatoid synovial fibroblasts. Korean J Intern Med. 2010;25:429–435.
- Nanau RM, Neuman MG. Safety of anti-tumor necrosis factor therapies in arthritis patients. J Pharm Pharm Sci. 2014;17:324–361.
- Lie PP, Cheng CY, Mruk DD. The biology of interleukin-1: emerging concepts in the regulation of the actin cytoskeleton and cell junction dynamics. Cell Mol Life Sci. 2012;69:487–500.
- Yoon JS, Lee HJ, Choi SH, et al. Quercetin inhibits IL-1beta-induced inflammation, hyaluronan production and adipogenesis in orbital fibroblasts from Graves' orbitopathy. PLoS One. 2011;6:e26261.