Abstract
Compromised microcirculation and endothelial dysfunction are hallmarks of sickle cell disease (SCD). EAF PEG Haemoglobin (Hb) and EAF PEG Albumin (Alb) represent a novel class of semisynthetic colloidal supra plasma expanders that improve microcirculation. The therapeutic activity of supra plasma expanders to attenuate vaso-occlusion and restore the haemodynamic functions in patients with SCD has been investigated using NY1DD, a transgenic mouse model of mild SCD without anaemia. Vaso-occlusion and perturbation of haemodynamics are amplified in NY1DD by hypoxia-reoxygenation protocol. EAF P5K6 Alb and Alb T12 (Alb conjugated with 12 copies of antioxidant tempo) attenuate vaso-occlusion when infused at the start of reoxygenation. However, only EAF PEG Alb restores haemodynamics close to levels in control C57BL. EAF P5K6 Alb-T12, active plasma expander conjugated with antioxidant, completely clears vaso-occlusion and restores normal haemodynamics. EAF PEG Hb also completely clears vaso-occlusion and restores normal haemodynamics. Pretreating NY1DD with EAF PEG Hb protects it from hypoxia reoxygenation-induced damages. EAF P5K6 Alb T12 attenuates the endothelial dysfunction in S + S Antilles mice as reflected by the vasodilatory response of its arteries and arterioles to vaso-dilators. Active plasma expanders are novel therapeutics to restore normal haemodynamics in SCD patients to improve tissue oxygenation during episodes of painful vaso-occlusive crisis.
Abbreviations: 2-IT: 2-immothiolane; Mal-T: 4-Maleimido tempo; Alb: human serum albumin (HSA); Alb-T12: human albumin conjugated with 12 copies of tempo; EAF: extension arm facilitated; EAF PEG Hb: extension arm facilitated PEGylated haemoglobin; EAF PEG Alb: extension arm facilitated PEGylated albumin; EAF P3K6 Hb: extension arm facilitated PEGylated haemoglobin conjugated with 6 copies of PEG3K; EAF P5K6 Alb T12: extension arm facilitated PEGylated albumin conjugated with 6 copies of PEG5K and 12 copies of tempo; Hb: haemoglobin; HAS: human serum albumin (Alb); PEG: polyethylene glycol; MP4: MalPEG Hb, is formulated at 4.2 g/dL in lactated Ringer's solution, a product of Sangart; SCD: sickle cell disease; NO: nitric oxide; SEC: size exclusive chromatography; Vrbc: red cell velocity; Q: volumetric flow rates, Q; SNP: sodium nitroprusside
Introduction
Semisynthetic supra plasma expanders represent a new and novel class of colloidal plasma expanders formulated with a viscosity comparable to the conventional colloidal plasma expanders [Citation1–6]. EAF P5K6 Alb and EAF P5K6 Hb belong to this novel class of resuscitation fluids. Supra plasma expanders distinguish themselves from conventional colloidal plasma expanders by exhibiting therapeutic activity to counteract endothelial dysfunction by increasing shear thinning of RBC to increase endothelial NO production in vivo and by improved functional capillary density. Accordingly, these semisynthetic supra plasma expanders are excellent therapeutics to counteract the vaso-constrictive effects of blood loss as well as anaemia.
The semisynthetic supra plasma expanders exhibit an in vivo microvascular effect, previously seen with high viscosity colloidal plasma expanders generally with viscosities higher than blood. Very high molecular weight naturally occurring polymers like dextran or synthetic polymers like polyethylene glycol (PEG) with molecular weight in the region of 2 million and with viscosities higher than blood represent such materials. The supra plasma expansion activity of natural, synthetic as well as semisynthetic materials is a consequence of their intrinsic ability to improve the shear thinning of RBC and to thus increase the in situ production of NO, the intrinsic vasodilator by endothelium [Citation7]. In the case of semisynthetic supra plasma expanders, the covalent attachment of PEG chains to proteins, apparently, increase the intrinsic disorder of unconjugated PEG chains. The differences in the packing densities of the semisynthetic hybrid biopolymers, with regions of central protein core with high packing density vs. the lower packing density of the outer PEG shell induces a certain degree of pseudoplasticity to these semisynthetic supra plasma expanders. Accordingly, the new active plasma expanders (resuscitation fluids) serve dual roles when infused (in vivo), i.e. improve blood flow besides their intrinsic activity of replacing the blood volume resulting from blood loss and thereby neutralize the hypovolemic effects. The active plasma expanders could serve as therapeutics to improve microvascular blood flow thereby increase the perfusion and efficacy of oxygen delivery to the tissues without having to increase Hb/protein concentration. EAF PEG Hb has been designed for achieving a targeted oxygen delivery activity to the hypoxic regions besides it being an active plasma expander. Accordingly, EAF PEG Hb should be considered as anti-anaemia therapeutic.
In recent years, many therapies are being developed to treat sickle cell disease (SCD) that improve the blood flow by reducing the vaso-occlusion, that is expected to improve the perfusion and thereby the tissue oxygenation. These approaches are very distinct from the conventional anti-sickling approaches that are essentially deoxy HbS polymerization inhibitors. This advance has been the consequence of the recognition that SCD is co-morbidity disease, even though it is primarily a disease of polymerization of deoxy HbS and formation of sickled RBC. It should be noted that generally SCD is also associated with severe anaemia. The pathophysiology of SCD mimic many aspects of ischemia-reperfusion injury mediated pathology and situations where there is a decrease in the bioavailability of nitric oxide (NO). Both lead to endothelial dysfunction and result in an impairment of the blood flow (perfusion) and thus amplify the anaemia effect that is intrinsic to SCD patients. [Citation8–10]. The haemodynamics parameters altered in transgenic mouse models of SCD in terms of the pathophysiology is amplified by the decrease in the bioavailability of NO, in part because of increased oxidative stress intrinsic to SCD. These pathophysiological aspects of the disease eventually contribute to endothelial dysfunction that is reflected as decreased arterial vasodilatory activity in response to exposure to NO. The vaso-occlusive episodes further compromise the circulation, tissue oxygenation and induce severe pains. Antioxidant therapies and anti-inflammatory therapies, together referred to as anti-adhesive therapies have been advanced to attenuate vaso-occlusive episodes in circulation. These have been shown to result in an improvement in blood flow in transgenic sickle mouse. These anti-adhesives therapies are now considered as some of the most encouraging therapies for SCD.
Microcirculation represents the primary region of circulatory system that dictates the efficiency of oxygen delivery, and hence plays the dominant role in adequate supply of oxygen to organs. In sickle cell anaemia, the anaemia effect is compounded by the compromise of the microcirculation due to the clogging of the capillaries by sickled RBC. This increases the susceptibility of these patients/mouse models of the disease to ischemia reperfusion injury driven pathologies as compared to healthy person/mice with comparable anaemia levels. Antioxidants have shown to attenuate ischemic reperfusion injury experimental models of such injury. Active plasma expanders could serve as novel class of therapeutics for SCD by serving dual function: improve the microcirculation and thus perfusion with increased oxygenation of tissues. This should be accomplished without significantly increasing the overall oxygen-carrying capacity of system. In fact, it may be noted that Tsai and her colleagues have shown that in transgenic model of mild sickle disease with a mild pathophysiology of SCD, MP4 [Citation11], a prototype of EAF P5K6 Hb, increased functional capillary density when these animals are exposed to hypoxia for a short period of time. But this effect is not observed under normoxia conditions in these transgenic sickle mice. These aspects of the disease and the therapeutic benefit of oxygen carrying active plasma expanders has prompted us investigate the therapeutic benefits of EAF PEG Hb and EAF PEG-Alb in transgenic mouse models of SCD with NY1DD that exhibit mild pathophysiology of the disease and with limited anaemic, NY1DD. Hypoxia-reoxygenation platform that essentially represents an experimentally induced anaemia without reducing the haematocrit in these transgenic mice has been chosen to induce vaso-occlusive crisis in these mice. The activity of active plasma expanders to attenuate experimentally induced vaso-occlusive events has been followed as a measure of anti-ischemia reperfusion injury therapeutic activity.
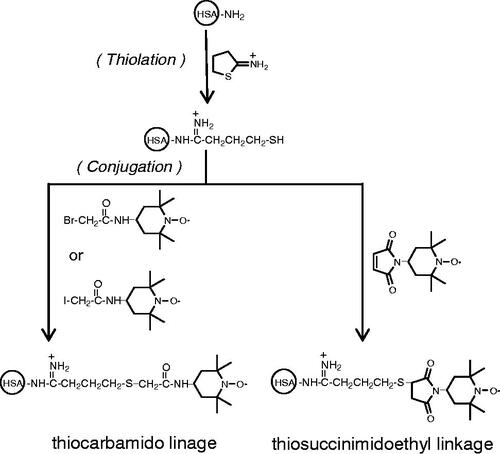
As noted above, SCD is a multifactorial, comorbidity disease and therapies addressing multiple physiological aspects of the disease have been advanced to treat the disease. Even though polymerization of deoxy Hb is the primary molecular basis of the disease, such therapies appear to modify the disease from severe to milder states. Sickle cell patients exhibit a high oxidative stress state and are in some pro-inflammatory state and therapeutics that attenuate these conditions have been shown to attenuate the painful vaso-occlusive crisis. Previous studies of Hsai [Citation12] have shown tempo conjugated to albumin, a macromolecular antioxidant, has therapeutic benefit in attenuating the vaso-occlusion in transgenic mouse models of disease.
Conjugation tempo to albumin has been accomplished in the present study using a combination of extension arm chemistry and maleimide chemistry, a milder platform that the previously used alkylation chemistry-based platform using Bromo-tempol. The number of tempo conjugated to albumin has been reduced from 35 that was used previously to 12 in the present study. A new tempo conjugated active plasma expander, P5K6 Alb T12, has been developed. The SCD therapeutic activity of the new active plasma expanders has been compared with that of EAF PEG Alb and EAF PEG Hb. The latter represents the combination of active plasma expansion activity with an increase in oxygen carrying capacity as well as targeted delivery activity to hypoxic areas in the body.
EAF P5K6 Alb, EAF P5K6 Alb T12 and EAF PEG Hb attenuate the hypoxia reoxygenation-induced increase in vaso-occlusion in NY1DD; besides these active plasma expanders also improve the haemodynamics. The base level vaso-occlusion under normoxia in NY1DD represents the disease induced endothelial dysfunction and in severe disease models of the disease like Berk, vaso-occlusion is significantly increased. With severe anaemia as in BERK or with models of the disease with mutant HbS with stronger polymerization potential as in S + S Antilles, the transgenic sickle mice have limited tolerance to hypoxia reoxygenation. The endothelial dysfunction in the transgenic mouse models of the disease can be assessed by measuring the vaso-dilatory response to external NO donors like sodium nitroprusside. Transgenic mouse model of disease, S + S Antilles, another mild model of disease pathophysiology with intrinsic base level vaso-occlusion under normoxia and limited anaemia just as with NY1DD, however, these mice does not tolerate hypoxia reoxygenation as well as NY1DD. The decreased tolerance of these mice to hypoxia is due to the presence of HbS Antilles, solubility of this protein in deoxy form is significantly reduced as compared to HbS. The endothelium of these regenerate a significant level of vaso-dilatory response to sodium nitropruside under normoxia when measured 8 h after infusion of EAF P5K6 Alb T12. Accordingly, we conclude that active plasma expenders, EAF PEG Alb, EAF PEG Hb T12 as well as EAF PEG Hb could serve as novel alternatives to the conventional therapies of transfusion or hydration for painful vaso-occlusive crisis with sickle cell patients in hospital settings.
Materials and methods
Chemicals
Human serum albumin (Alb) essentially fatty acids free, 2-imnothiolane (2-IT), 4-Maleimido tempo (Mal-T), from Sigma (St. Louis, MO). HbA was purified from the human erythrocyte lysate by DE-52 chromatography [Citation13]. Maleimidophenyl PEG5K (Mal PEG5K) was custom synthesized by BioAffinity Inc., Rockford, IL [Citation14]. Ingredients for PBS and other analytical chemicals were of HPLC grade from Sigma-Aldrich or Fisher Scientific, Lenexa, KS.
PEGylation of HSA
PEGylation of Alb was carried out by a thiolation-mediated maleimide chemistry-based protocol as detailed in Manjula et al. [Citation15].
Conjugation of tempo to albumin and PEG Alb
For conjugation of tempo to Alb as well as PEG Alb we have used a recently developed EAF conjugation approach. For EAF conjugation: 0.5 mM of PEG Alb is incubated with 10 mM of 2-IT and 10 mM of 4-Maleimido tempo in PBS (pH 7.4) at 4 °C for 16 h (). After the reactions, all the small chemical reagents in excess (unreacted) were removed using centricon (molecular weight cut off 50 K from Millipore, Billerica, MA) spinning at 5000 rpm for 30 min. The washings were repeated three times. Alb T12 was prepared using the EAF conjugation approach but using Alb instead of PEG albumin.
Analytical methods
Size SEC was performed on a Pharmacia FPLC system, at 25 °C using two HR10/30 Superose-12 columns connected in series with a 100 μL loop [Citation16].
Intravital microscopy
Intravital studies were approved by the Einstein Animal Care and Use Committee and were consistent with PHS recommendations. Male C57BL/6J control (n = 4) and transgenic sickle (NY1DD) (n = 12) mice weighing approximately 25–30 g (4–6 months old) were used. The mice were maintained on a standard diet and water ad libitum. Mice were anaesthetized i.p. with 10% urethane and 2% α-chloralose in saline (5 mL/kg). The animals were tracheostomized. In vivo microcirculatory observations were made in the open cremaster muscle preparation, prepared according to the method of Baez [Citation17]. The suffusion and maintenance of the mouse cremaster preparation were done as described [Citation18]. Microscopic observations were carried out using a Nikon microscope (model E400; Morrell Instrument Company Inc., Melville, NY) equipped with a Dage-MTI CCD television camera (model CCD-300T-RC, Dage-MTI Inc., Michigan City, IN) and a Sony U-matic video recorder (model VO5800; Sony, Teaneck, NJ).
Intravital measurements were initiated within 15 min of the surgical exteriorization of the tissue and completed within next 30 min. Red cell velocity (Vrbc) and leukocyte adhesive behaviour were determined in randomly chosen post capillary venules. Vessel luminal diameter (D) was measured on-line using an image-shearing device (model 907, Instruments for Physiology and Medicine, San Diego, CA). Vrbc was measured along the vessel centreline using the “dual-slit” photodiode and a velocity cross-correlator (model 102 BF, Instruments for Physiology and Medicine, San Diego, CA) [Citation19,Citation20]. The centreline Vrbc was converted to the mean Vrbc across the vessel diameter using a conversion factor of 1.6 (Vrbc/Vmean=1.6) as described [Citation21]. Volumetric flow rates (Q) were determined from Vmean and the vessel cross-sectional area (πD2/4) as described [Citation21,Citation22]. Shear rates along the wall of microvessel of a given luminal diameter (D) were calculated using the relationship = 8 Vmean/D [Citation22].
Adherent leukocytes were counted along the length of a given venule, and expressed as average number of cells per 100 μm length of the vessel. A leukocyte was considered adherent if it remained stationary for longer than 30 s. Trans-endothelial emigration (extravasation) of leukocytes was determined on-line as the number of interstitial leukocytes in the field of view adjacent (within 30 μm) to venules as described [Citation23].
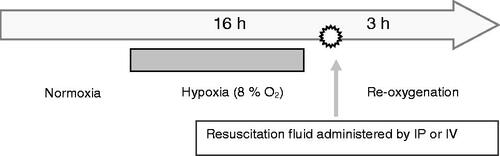
We subjected transgenic sickle (NY1DD) mice to 18 h of hypoxia (8% O2, 0.5% CO2, balance N2) followed by 3 h of reoxygenation at ambient air (). We selected NY1DD mice based on their highly exaggerated response to hypoxia-reoxygenation compared with C57BL (control) mice as reported by us [Citation23,Citation24]. Microhaemodynamic parameters (i.e. red cell velocity (Vrbc), wall shear rates and volumetric flow (Q)), leukocyte adhesion and leukocyte emigration were determined in the following groups of mice: 1) Normoxic wild type (C57BL) mice; 2) Normoxic sickle (NY1DD) mice; 3) NY1DD transgenic mice subjected to hypoxia-reoxygenation (untreated); 4) NY1DD mice subjected to hypoxia-reoxygenation and toploaded with PEG-Alb and 5) NY1DD mice subjected to hypoxia-reoxygenation and similarly top loaded with PEG-Alb-T12. In each group, mice were top loaded via tail vein with 4% of a given test substance at 10% of mouse blood volume. After 3 h of reoxygenation, the cremaster muscle microvasculature was exposed and the tissue adjacent (within ∼30 μm) to postcapillary venules (the sites of inflammatory response) was examined for leukocyte adhesion and emigration as described [Citation23].
Results
Colloidal plasma expander (resuscitation fluid) like properties of active plasma expanders
The plasma expander like properties of EAF P5K6 Alb conjugated with 12 copies of tempo is compared with the Alb T12, EAF P5K6 Alb and EAF P3K6 Hb in . Dodeca-nitroxylation of albumin has very little influence on the plasma expander like properties of Alb. Similarly, dodeca-nitroxylation of EAF P5K6 Alb has little influence on the plasma expander like properties of the molecule. The plasma expander like properties of EAF P5K6 Hb, MP4 is shown for comparison. MP4 of Sangart is a prototype of EAF P5K6 Hb of Einstein generated by EAF PEGylation.
Table 1. Solution properties of semisynthetic active plasma expanders.
Inhibition of hypoxia-reoxygenation induced vaso-occlusion in transgenic sickle mouse NY1DD by semisynthetic active plasma expanders without oxygen carrying activity, EAF P5K6 Alb and EAF P5K6 Alb T12
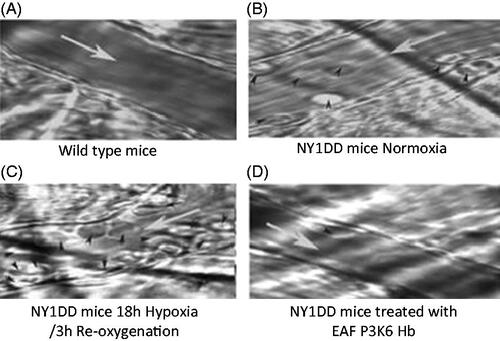
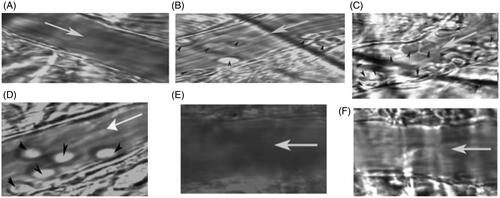
The intravital microscopic images of venules from transgenic sickle cell mice (NYIDD) subjected to hypoxia-reoxygenation and treated with therapeutic agents after hypoxia are illustrated in . Image of the venule of the wild-type mouse (C57 BL) was very clear and endothelial walls are smooth (). The NY1DD mouse also gave reasonably clear picture of venule as well (), but for the fact there were a few leukocytes adhering to the endothelium of the venule. On the other hand, when the NY1DD mice were subjected to the hypoxia for 18 h and then reoxygenated without treatment with test any therapeutic materials significant blockage of the venules for circulation ensues (), and many leukocytes (and deformed erythrocytes) were observed adhering to the endothelial walls of the venules. However, on top load of Alb T12 at the time of reoxygenation, the leukocyte adhesion is noticeably reduced (). After top load with P5K6 Alb T12 () at the time of reoxygenation and evaluated after 3 h, full clarity of the venules is essentially restored back to the compared to that in the venules of the untreated sickle cell mice (). There were essentially no visible differences between . On top load of P5K6 Alb () at the time of reoxygenation and evaluated after 3 h, a significant clearing of the vaso-occlusion is seen and the overall appearance seems to be comparable to that of Alb-T12. The clearing of the venules in NY1DD subjected hypoxia reoxygenation is a function of PEGylation as well as the antioxidant agent Temp. Control experiments with Alb alone did not show any therapeutic activity (data not shown). PEGylation induced supra perfusion resuscitation properties with Alb exhibits good SCD therapeutic activity in terms of clearing the leukocyte adhesion, it is slightly better than the macromolecular antioxidant Alb T12. Accordingly, when the macromolecular antioxidant is PEGylated, the SCD therapeutic activity of tempo and of active plasma expansion integrates to achieve a complete attenuation of experimentally induced vaso-occlusion.
Figure 3 Representative images of venules from transgenic sickle cell mice (NY1DD) treated with Alb T12, EAF P5K6 Alb T12 and PEG Alb in hypoxia/reoyxgenation. (A) Wild type (B) NY1DD mice (C) NY1DD – untreated H/R (D) NY1DD mice treated with Alb T12 (E) NY1DD mice treated with EAF P5K6 Alb T12 (F) NY1DD mice treated with EAF P5K6 Alb T12.White arrow (→) indicate the blood flow direction and black arrow head indicates leukocytes.
A quantitative analysis of attenuation of vaso-occulsion by macromolecular antioxidant infused at the start of the reoxygenation, PEGylated macromolecular antioxidant (anti-oxidant conjugated supra plasma expander) and active plasma expander without antioxidant is given in . Even without experimentally induced hypoxia, i.e. under normoxic conditions NY1DD mice show small increase in leukocyte adhesion in venules compared with wild type mice (p<.04). Hypoxia-reoxygenation in NY1DD mice caused a marked inflammatory response as evidenced by a significant increase in leukocyte adhesion compared with normoxic NY1DD mice (p<.00001). Infusion of Alb T12 at the onset of reoxygenation accomplishes some but noticeable reduction in leukocyte adhesion in comparison to untreated NY1DD. Infusion of EAF P5K6 Alb T12 at the onset of reoxygenation resulted in nearly complete attenuation in leukocyte adhesion to a level comparable to that with untreated control mice (p<.0001–.00001). Interestingly, leukocyte adhesion in the PEG Alb T12 treated group was not significantly different from the normoxic controls. However, P5K6 Alb control showed only a partial attenuation in the leukocyte adhesion comparable to that of EAF P5K6 Alb T12.
Figure 4 (A) NY1DD sickle mice receiving Alb T12, P5K6 Alb T12 and P5K6 Alb at the onset of reoxygenation showed marked reduction in leukocyte adhesion, with P5K6 Alb T12 having a normalizing effect. (B) NY1DD mice receiving P5K6 Alb and P5K6 Alb T12 at the onset of reoxygenation show marked reduction in leukocyte emigration compared with untreated NY1DD mice. *p< .03 vs. wild type; +p< .00001 vs. normoxic NY1DD mice; #p< .0001 vs. untreated NY1DD mice subjected to hypoxia-reoxygenation.
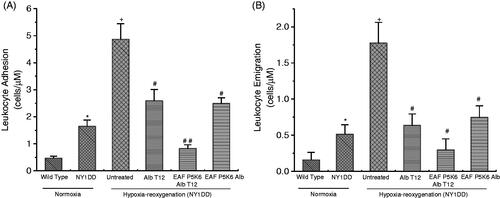
Under normoxic conditions (without experimentally induced hypoxia) NY1DD mice exhibits an increased leukocyte emigration as compared to wild-type mice (p<.03, ). Hypoxia-reoxygenation in NY1DD mice resulted in a marked increase in inflammatory response as reflected by leukocyte extravasation. Top loading P5K6 Alb T12 at the onset of reoxygenation resulted in a pronounced reduction in emigrated leukocytes compared with untreated NY1DD mice subjected to hypoxia-reoxygenation (p<.0001). PEG albumin control also showed a significant improvement. In fact, leukocyte emigration in the EAF P5K6 Alb T12 treated group was not significantly different from normoxic control mice.
Blood flow impairment of SCD seen in NY1DD amplified by hypoxia-reoxygenation are normalized by supra plasma expander, PEG Alb
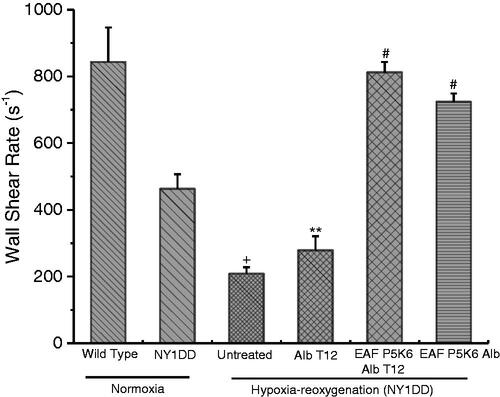
NY1DD transgenic sickle mouse has nearly a normal haematocrit, but under normoxic conditions exhibit an almost 60% decrease in blood flow. This reflects a significant reduction in wall shear and blood flow, Q () as compared with wild-type (C57BL) mice. Hypoxia-reoxygenation causes a significant reduction in flow parameters. These parameters showed a nearly complete reversal when treated with EAF P5K6 Alb T12 at the onset of reoxygenation, close the values with control C 57BL mice. These values are indeed significantly better than the base line values seen with NY1DD under normoxic condition, i.e. clears mild disease state present under normoxic conditions. On the other hand, haemodynamic properties of NY1DD treated with Alb T12 after hypoxia improved only marginally as compared to the untreated NY1DD. The haemodynamic properties of the NY1DD treated with P5K6 Alb show a significant improvement and are only slightly lower as compared to that of EAF P5K6 Alb T12. This reflects the unique role of active plasma expansion in nearly restoring the haemodynamic properties, even though the antioxidant and active plasma expansion appears to have comparable levels of activity in attenuating leucocyte adhesion.
Table 2. The influence of PEG Alb and PEG Alb T12 on haemodynamic parameters of sickle (NY1DD) mice subjected active plasma expansion therapy using Hypoxia-Reoxygenation system.
Lack of correlation of between SCD therapeutic activity as reflected by the attenuation of vaso-occlusion and improvements in haemodynamics in NY1DD when treated with therapeutic agents
shows the improvement in the haemodynamics as reflected by shear stress in NY1DD challenged with hypoxia reoxygenation protocol and with the infusion of the test material at the beginning of reoxygenation. The improvement in wall shear is considered for the improvement in haemodynamics and hence improving perfusion and of tissue oxygenation. Alb T12 exhibits very little therapeutic activity in improving the wall shear even though it exhibits significant therapeutic activity in attenuating the vaso-occlusion in induced in NY1DD by hypoxia reoxygenation. The wall shear is lower as compared to the starting level before hypoxia. This represents the presence of significant clogging of the capillaries due to the deoxygenation that occurred under hypoxia conditions. EAF hexa PEGylation of the drug Alb T12, engineering active plasma expansion activity, EAF P5K6 Alb T12 attenuates the vaso-occlusion completely and the restores the wall shear values to a level comparable to that of C57 BL. It is interesting that the supra perfusion resuscitation fluid, EAF P5K6 Alb, also restores the wall shear in NY1DD to a level far better than in the normoxic NY1DD, i.e. prior to challenging with hypoxia-reoxygenation protocol. However, it is not as good as that with the antioxidant conjugated EAF PEG albumin. Thus, in terms of re-establishing the haemodynamics, the active plasma expansion has a better therapeutic activity than the antioxidant conjugated to albumin, even though they seem to have nearly comparable therapeutic activity in terms of attenuation of vaso-occlusion or immigration of leucocytes.
Figure 5 Effect of Alb T12, P5K6 Alb T12, and P5K6 Alb on wall shear rate in transgenic sickle cell mice (NY1DD) and wild type (C57BL/6J). Values are mean ± SE. Four to six venules were examined for each mouse (n = 3–4 each experimental group); +p< .005–.0001 vs. normoxic wild type controls; **p< .05–.023 vs. respective normoxic values for NY1DD mice; #p< .004–.00001 vs. untreated NY1DD mice subjected to hypoxia-reoxygenation.
Inhibition of hypoxia-reoxygenation induced vaso-occlusion in transgenic sickle mouse NY1DD by EAF P3K6 Hb: active plasma expander with a targeted oxygen delivery activity
The intravital microscopic images of venules from transgenic sickle cell mice (NYIDD) subjected to hypoxia-reoxygenation protocol and treated with EAF P3K6 Hb at the start of reoxygenation are illustrated in . Image of the venule of the wild type mouse (C57 BL) was very clear and endothelial walls are smooth (). As seen earlier, NY1DD mouse exhibits limited vaso-occlusion (), there are a few leukocytes adhering to the endothelium. But, when the NY1DD mice were subjected to the hypoxia-reoxygenation significant blockage of the venules is seen (), and many leukocytes (and deformed erythrocytes) are observed adhering to the endothelial walls of the venules. After the top load of EAF P3K6 Hb, the leukocyte adhesion is completely cleared and is essentially comparable to that of control C57 mice (). Animals pretreated with EAF P3K6 Hb and then subjected to hypoxia re-oxygenation protocol did not induce vaso-occlusion. EAF P3K6 Hb afforded complete protection to NY1DD against experimentally induced vaso-occlusion. The quantitation of the vaso-occlusion suggests that the therapeutic efficacy of EAF P3K6 Hb is very good as seen by attenuation of vaso-occlusion but however it is better with EAF P5K6 Alb and EAF P5K6 Alb T12. Further studies will be needed to explain the differences between the two EAF P5K6 Alb and EAF P3K6 Hb.
EAF P5K6 Alb T12 facilitates the regeneration of vaso-dilatory response of endothelium to NO donor, sodium nitroprusside (SNP)
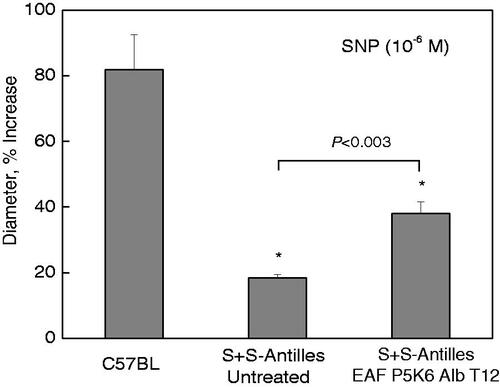
Earlier studies have established that the response to NO-mediated vasodilation is attenuated in sickle cell patients and transgenic mouse models. The transgenic mouse models S + S Antilles is also mild model of the disease without significant anaemia. But this mouse model is more sensitive to hypoxia than NY1DD. This is a consequence of the fact polymerization potential of HbS Antilles is significantly higher than that of HbS. These do not survive long periods of hypoxia re-oxygenation protocol. Nath and Katusic [Citation24] have shown that in SCD there is an extensive endothelial dysfunction as reflected resistance to NO and associated with marked oxidative stress in these mice [Citation27]. We have addressed question whether active plasma expander EAF P5K6 Alb T12 that shows excellent therapeutic activity against experimentally induced vaso-occlusion in NY1DD could restore/improve endothelial function in S + S Antilles as reflected by the response of arteries to NO-stimulated vasodilatation. We have examined the response of cremaster arterioles (A3) to topical application of SNP (10−6 M) nearly after 18 h (overnight) of pretreatment with 150 μL of 4 g % EAF P5K6 Alb T12. Illustrated in are the results of the vascular response to SNP in control C57BL, untreated S + S Antilles and active plasma expander treated mice. Control C57BL mice exhibited approximately 80% increase in its arteriolar (A3) vascular diameter (p<.01), while there was only an 18% increase in untreated transgenic SCD mouse. In contrast, following EAF P5K6 Alb T12 treatment for overnight, application of SNP induced a more than two-fold increase in A3 diameters, exhibiting an increased vascular response of NO in sickle mice on treatment supra perfusion fluid in transgenic sickle mouse S + S Antilles. Accordingly, we conclude that the active plasma expanders reverse the endothelial dysfunction, damage intrinsic to SCD patients.
Discussion
All therapeutic tested here, Alb T12, EAF P5K6 Alb T12 and EAF P5K6 Alb, attenuate the vaso-occlusion in NY1DD transgenic sickle mice with experimentally induced vaso-occlusion in NY1DD. However, therapeutic efficacy of Alb T12, the macromolecular antioxidant and of EAF P5K6 Alb is noticeably lower than EAF P5K6 Alb T12. The interesting aspect of this study is the observation that in terms of restoring the haemodynamic function, the therapeutic activity of Alb T12 is very poor, essentially non-existent and whereas both EAF PEG Hb and EAF PEG Alb T12 are excellent in restoring microcirculation. PEGylation induced active plasma expansion properties Alb by itself adequate to restore the haemodynamics and which in turn clears the vaso-occlusion with the dosage used. Synergizing the active plasma expansion with either antioxidant activity significantly increases the vaso-occlusion clearing activity. The macromolecular antioxidant, Alb T12, at the concentration used here, exhibits only vaso-occlusion clearing agent with limited activity in restoring the microcirculation. The clogging of the microcirculation by sickled RBC generated during hypoxia are not readily oxygenated by the clearance of the vaso-occlusion by the antioxidant. On the other hand, the active plasma expansion activity of EAF P5K6 Alb and EAF P5K6 Alb T12, which is known to increase the functional capillaries with some vasodilatory activity facilitates the improved the oxygenation of tissues and RBC as well as unclogging of the microcirculation by sickled RBC. Improving the microcirculation that can facilitate the oxygenation of deoxy Hb and improve the tissue oxygenation appears to be very effective as compared to the antioxidant therapy. None the less, a combination the active plasma expansion activity with antioxidant activity has noticeable impact on clearing the vaso-occlusion and synergizing with active plasma expansion.
EAF P3K6 Hb and EAF P5K6 Alb T12 exhibit essentially comparable therapeutic activities in terms of clearing the vaso-occlusion, but EAF P5K6 Alb and EAF P5K6 Alb T12 are better than EAF P3K6 Hb in restoring the normal haemodynamics. However, this conclusion is based on the results of a 3 h window study. A kinetic study of the therapeutic activity will be needed to establish and fully appreciate this difference. A targeted oxygen delivery activity to the hypoxic areas of body endowed to EAF P3K6 Hb by its high oxygen affinity appears to function as well as the antioxidant activity of tempol conjugated to active plasma expanders in terms of the overall therapeutic activity. It may be noted that NY1DD is a mild model of SCD with very little anaemia. But SCD is invariably associated with anaemia. Therefore, the two the supra plasma expanders fluid may exhibit very different level therapeutic activity in transgenic mouse models of SCD like BERK, which is a severe model od disease and has lower levels of haematocrit as compared to NY1DD. These exhibit very high vaso-occlusion under normoxia.
Accordingly, we conclude that the molecular basis of SCD therapeutic activity of supra plasma expanders is distinct from that accomplished by antioxidant tempo conjugated to Alb or EAF PEG Alb. The therapeutic efficacy of EAF PEG Alb is expected to come primarily from it potential to induce a degree of vasodilation and to improve the functional capillary density and therby improve the oxygen extraction. Both supra plasma expanders contribute to the improved tissue oxygenation and thus are expected to facilitate unclogging of the capillaries. Our previous studies have shown that vasodilation seen in extreme haemodilution in hamster is a result of an increase in endothelial NO production due to increased shear thinning of red blood cells. In the case of Alb T12, the therapeutic activity, both in terms of attenuation of the vaso-occlusion and improvement in blood flow is essentially a consequence of the antioxidant activity of tempo and the carrier of tempo. The carrier, albumin, has little or/no role in terms of SCD therapeutic activity. Neither the volumetric flow nor the RBC flow is improved with Alb T12. The deoxygenated cells are being oxygenated to accomplish the unclogging. The active plasma expansion activity of EAF P5K6 Alb is induced by the PEG-shell engineered around Alb T12 and this has endowed new SCD therapeutic activity to the molecule.
The reversal of the haemodynamics in NY1DD to a level of control C57BL by active plasma expansion activity is very significant and exposes a new therapeutic approach for treating the pathophysiological effects of SCD. In SCD mice lowering of the systemic blood flow is a function of severity of the disease. Severity of the disease as reflected by the blood flow correlates with the level of anaemia. Accordingly, it is conceivable that EAF P3K6 Hb may be a better supra plasma expander than EAF P5K6 Alb or EAF P5K6 Alb T12, in patients with severe disease with intrinsic severe anaemia as EAF P3K6 Hb has an intrinsic anti-anaemia activity besides being supra plasma expander.
One of the important pathological aspects of SCD is the desensitization of the endothelium to NO besides the lowering of the bioavailability of NO. This amplifies the overall pathophysiology of the disease. The studies with S + S Antilles establish that a single dose of active plasma expansion resuscitation fluid achieves a significant reversal of endothelial dysfunction. Preliminary studies with BERK mice have established that active plasma expansion resuscitation fluids attenuate the intrinsic vaso-occlusion intrinsic under normoxia and improve the haemodynamics blood flow.
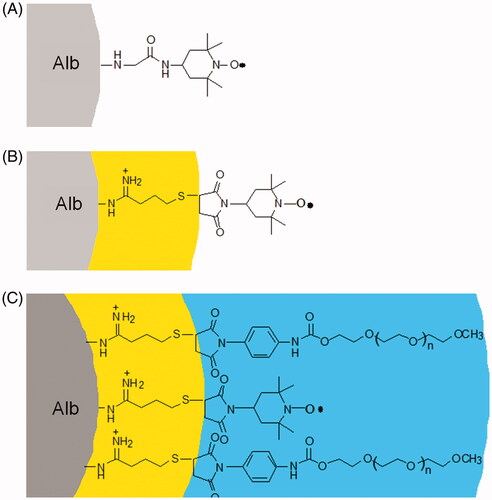
The studies discussed here establish that active plasma expanders have an intrinsic antianaemia therapeutic activity, as the anaemia induces a degree of vaso-constriction and reduction in functional capillary density. In sickle cell anaemia, the blood flow and microcirculation impairment are further amplified by sickling of RBC. It may be noted that EAF P5K6 Alb and EAF P3K6 Hb are semisynthetic hybrid biopolymers of unique molecular structures. These have a central protein core of high packing density and an outer PEG shell of lower packing density. The low packing density PEG shell is engineered onto the surface protein with a buffering zone of extension arms (). This endows the molecule with unique rheological properties, i.e. these semisynthetic supra perfusion resuscitation fluids are pseudo-plastic materials and increase their viscosity as an inverse correlate of wall shear [Citation28,Citation29]. Besides, these also increase functional capillary density, thus open connectivity between the arterial and venous systems. High viscosity plasma expanders (colloidal plasma expanders with viscosities higher than blood) like dextran 500 and PEG 2 M are active plasma expanders and have been shown to increase the shear thinning of RBCs and this ability to increase their viscosity is also an inverse correlate of shear. This inverse correlation aspect of low viscosity active plasma expander EAF P5K6 albumin is very distinct from other high viscosity supra perfusion resuscitation fluids. The EAF PEG Alb and EAF PEG Hb exhibit a significantly higher shear thinning when mixed with blood at lower shear rates as compared to other high viscosity plasma expanders [Citation4]. In this connection, it is also to be noted that MP4, a prototype of EAF P5K6 Hb, improves functional capillary density in the transgenic sickle mouse top-loaded with a 10% load only under hypoxia, however, not under normoxia [Citation30]. This is consistent with the mechanism proposed above, wherein the SCD therapeutic activity of the active plasma expansion resuscitation fluid is activated by hypoxia regions as perfusion mediated increase in tissue oxygenation is achieved. The observation in this study that pretreatment with EAF P3K6 Hb protects NY1DD from hypoxia reoxygenation induces damages is consistent with the earlier results. Accordingly, we suggest that EAF P5K6 Alb and EAF P3K6 Hb act as molecular sensors of lowered blood flow (shear) in a region-specific fashion and change their molecular shape to increase the viscosity and thus wall shear in these regions and improvement in blood flow.
Figure 8 Exploded view of schematic representation of the structure of (A) Alb tempo, (B) EAF-Alb-tempo and (C) EAF PEG Alb-tempo.
It may be noted here that a synthetic non-ionic linear block copolymer of polyoxyethylene and polyoxypropylene with a mean molecular weight of 8.5 K, known as proloxmer 188, has shown SCD therapeutic activity. A different shear rate dependent mechanism has been proposed for this therapeutic agent, MST-188 [Citation31], a rheological and cytoprotective agent that increased micro vascular blood flow. Hydrophobic interactions of this polymer with endothelium and/or RBC are the proposed mechanism for this therapeutic agent, molecular adhesive and sealant technology. However, it is not clear whether the PEG chains of PEG Alb or PEG Hb exert some amount of this activity.
In recent years, PEG-Hbs have emerged as vehicle to deliver SCD therapeutic agents, antioxidant molecules as well as anti-inflammatory molecules into circulatory system in much the same way as EAF P5K6 Alb has been used here to deliver tempo. However, such studies have not attempted to map the relative roles of active plasma expansion induced by the PEG shell of delivery molecule and by the antioxidant and/or anti-inflammatory therapeutics. It is conceivable that a significant degree of therapeutic effects in SCD in such molecules might have emerged from the active plasma expansion activity. MP4CO and Sanguinate are the examples of this class of molecules [Citation32–36]. In vivo, the carbon monoxide bound to PEG Hb is slowly released and acts as anti-inflammatory therapeutic agent [Citation32].
In short, the SCD therapeutic activity of supra plasma expanders intrinsic to EAF PEG Alb and EAF PEG Hb to clear the vaso-occlusion is excellent. Influence of hypoxia is amplified in these sickle cell mice due the sickling of the RBC and clogging of the microcirculation as compared to wild-type C57Bl. The targeted influence of these supra plasma expanders in clearing the vaso-occlusion and improving the microcirculation makes these therapeutics unique to treat sickle cell patients, particularly to protect the organs from blood flow impairment damages. Ischemic reperfusion injury is a common phenomenon that occurs in the body when the blood supplied to an area of tissue is reduced or cut off and as the resupply oxygen is initiated. This is further amplified in anaemia. The incidence of ischemic injury is of common occurrence; myocardial infarction, stroke and other thrombotic events affect millions of individuals each year. Accordingly, we speculate that the supra plasma expansion resuscitation fluids will be very valuable therapeutic to treat ischemic reperfusion injury induced by mild anaemia besides in the treatment of SCD during vaso-occlusive crisis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dieterich HJ. Recent developments in European colloid solutions. J Trauma. 2003;54:S26–S30.
- Schortgen F, Deye N, Brochard L. Preferred plasma volume expanders for critically ill patients: results of an international survey. Intensive Care Med. 2004;30:2222–2229.
- Winslow RM, Lohman J, Malavalli A, et al. Comparison of PEG-modified albumin and hemoglobin in extreme hemodilution in the rat. J Appl Physiol. 2004;97:1527–1534.
- Assaly RA, Azizi M, Kennedy DJ, et al. Plasma expansion by polyethylene-glycol-modified albumin. Clin Sci. 2004;107:263–272.
- Cabrales P, Tsai AG, Winslow RM, et al. Extreme hemodilution with PEG-hemoglobin vs. PEG-albumin. Am J Physiol Heart Circ Physiol. 2005;289:H2392–2H2400.
- Martini J, Cabrales P, Ananda K, et al. Survival time in severe hemorrhagic shock after perioperative hemodilution is longer with PEG-conjugated human serum albumin than with HES 130/0.4: a microvascular perspective. Crit Care. 2008;12:R54.
- Sriram K, Tsai AG, Cabrales P, et al. PEG-albumin supra plasma expansion is due to increased vessel wall shear stress induced by blood viscosity shear thinning. Am J Physiol Heart Circ Physiol. 2012;302:H2489–H2497.
- Dos Santos JL, Chin CM. Recent insights on the medicinal chemistry of sickle cell disease. Curr Med Chem. 2011;18:2339–2358.
- Wang WC. The pharmacotherapy of sickle cell disease. Expert Opin Pharmacother. 2008;9:3069–3082.
- Wood KC, Granger DN. Sickle cell disease: role of reactive oxygen and nitrogen metabolites. Clin Exp Pharmacol Physiol. 2007;34:926–932.
- Winslow RM. MP4, a new nonvasoactive polyethylene glycol-hemoglobin conjugate. Artif Organs. 2004;28:800–806.
- Hsai CJ. Compositions and methods utilizing nitroxides in combination with biocompatible macromolecules. United State patent 5,840,701, 1998.
- Manjula BN, Acharya AS. In: Nagel, R. L. editors. Methods in molecular medicine: hemoglobin disorders: molecular methods and protocols. Vol. 82, Totowa (NJ): Humana Press; 2003. p. 31–47.
- Manjula BN, Tsai A, Upadhya R, et al. Site-specific PEGylation of hemoglobin at Cys-93(beta): correlation between the colligative properties of the PEGylated protein and the length of the conjugated PEG chain. Bioconjugate Chem. 2003;14:464–472.
- Manjula BN, Tsai AG, Intaglietta M, et al. Conjugation of multiple copies of polyethylene glycol to hemoglobin facilitated through thiolation: influence on hemoglobin structure and function. Protein J. 2005;24:133–463.
- Meng F, Manjula BN, Smith PK, et al. PEGylation of human serum albumin: reaction of PEG-phenyl-isothiocyanate with protein. Bioconjugate Chem. 2008;19:1352–1360.
- Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvas Res. 1973;5:384–394.
- Kaul DK, Fabry ME, Costantini F, et al. In vivo demonstration of red cell-endothelial interaction, sickling and altered microvascular response to oxygen in the sickle transgenic mouse. J Clin Invest. 1995;96:2845–2853.
- Silva J, Intaglietta M. The correlation of photometric signals derived from in vivo red blood cell flow in microvessels. Microvasc Res. 1974;7:156–169.
- Wayland H, Johnson PC. Erythrocyte velocity measurement in microvessels by a two-slit photometric method. J Appl Physiol. 1967;22:333–337.
- Baker M, Wayland H. On-line volume flow rate and velocity profile measurement for blood in microvessels. Microvasc Res. 1974;7:131–143.
- Lipowsky HH, Usami S, Chien S. In vivo measurements of “apparent viscosity” and microvessel hematocrit in the mesentery of the cat. Microvasc Res. 1980;19:297–319.
- Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice [see comments]. J Clin Invest. 2000;106:411–420.
- Kaul DK, Liu XD, Choong S, et al. Anti-inflammatory therapy ameliorates leukocyte adhesion and microvascular flow abnormalities in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2004;287:H293–H301.
- Vandegriff KD, McCarthy M, Rohlfs RJ, et al. Colloid osmotic properties of modified hemoglobins: chemically cross-linked versus polyethylene glycol surface-conjugated. Biophys Chem. 1997;69:23–30.
- Migita R, Gonzales A, Gonzales ML, et al. Blood volume and cardiac index in rats after exchange transfusion with hemoglobin-based oxygen carriers. J Appl Physiol (1985). 1997;82:1995–2002.
- Nath KA, Katusic ZS. Vasculature and kidney complications in sickle cell disease. J Am Soc Nephrol. 2012;23:781–784.
- Acharya SA, Intaglietta M, Tsai AG, et al. In: Kim HW, Greenburg AG, editors. Hemoglobin-based oxygen carriers as red cell substitutes and oxygen therapeutics. Heidelberg, Germany: Springer-Verlag Berlin Heidelberg; 2013. Chapter 11, Design of non-hypertensive conjugated hemoglobins as novel resuscitation fluids. p. 185–234.
- Salazar Vázquez BY, Hightower CM, Yalcin O, et al. Semisynthetic hybrid biopolymers for non-pharmacological intervention of the microcirculation. Curr Drug Metab. 2013;14:540–546.
- Tasi AG, Cabrales P, Young MK, et al. Microvascular response to cell free Hb infusion in transgenic mouse model of sickle cell anemia. FASEB J. 2009;23(Suppl. 1).
- Moloughney JG, Weisleder N. Poloxamer 188 (P188) as a membrane resealing reagent in biomedical applications. Recent Pat Biotechnol. 2012;6:200–211.
- Belcher JD, Young M, Chen C, et al. MP4CO, a pegylated hemoglobin saturated with carbon monoxide, is a modulator of HO-1, inflammation, and vaso-occlusion in transgenic sickle mice. Blood. 2013;122:2757–2764.
- Vandegriff KD, Young MA, Lohman J, et al. CO-MP4, a polyethylene glycol-conjugated haemoglobin derivative and carbon monoxide carrier that reduces myocardial infarct size in rats. Br J Pharmacol. 2009;154:1649–1661.
- Klaus JA, Kibler KK, Abuchowski A, et al. Early treatment of transient focal cerebral ischemia with bovine PEGylated carboxy hemoglobin transfusion. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:223–229.
- Ananthakrishnan R, Li Q, O’Shea KM, et al. Carbon monoxide form of PEGylated hemoglobin protects myocardium against ischemia/reperfusion injury in diabetic and normal mice. Artif Cells Nanomed Biotechnol. 2013;41:428–436.
- Zhang J, Cao S, Kwansa H, et al. Transfusion of hemoglobin-based oxygen carriers in the carboxy state is beneficial during transient focal cerebral ischemia. J Appl Physiol (1985). 2012;113:1709–1717.