Abstract
In this study, three-dimensional (3D) hydrogels were used for human hepatocellular carcinoma (HepG2) cells culture systems in vitro and establishment of an in vivo xenografted tumor model. Based on our previous work on the biotin-conjugated pullulan acetate nanoparticles (Bio-PA NPs) as anticancer drug carriers, we further studied the anti-tumor effect of the NPs in two-dimensional (2D) and 3D cell culture system. When embedded in 3D hydrogels, HepG2 cells formed tumor spheroids and the cytoplasmic actin microfilamentrates were rearranged over a period of 7 days. In vitro cytotoxicity results indicated that HepG2 cells in 3D hydrogels were more resistant to Bio-PA NPs treatments compared to the 2D system. The tumor formation rate of in vivo xenografted tumor model using 3D culture systems method was 98.2%, which was significantly higher than that using of 2D cultured cells (76.4%). Then we injected the 3D HepG2 cells systems in the right anterior axillary of female Balb/c nude mice, and evaluate the in vivo anti-tumor efficacy of Bio-PA NPs. In summary, these results suggested that HepG2 cells in 3D hydrogel system has shown the potential to provide an in vitro and in vivo model and for the evaluation of Bio-PA NPs.
Graphical Abstract
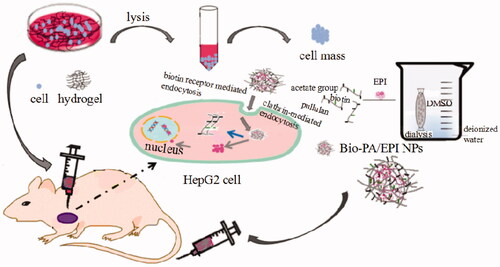
Schematic illustration for the preparation of Bio-PA/EPI NPs using dialysis method and establishment of tumor model in vivo and in vitro.
Introduction
Cancer cells have been increasingly grown in pharmaceutical research to explore tumorigenesis and develop new therapeutic drugs. Currently, cells are typically grown using standard culture (two-dimensional, 2D) systems, where the native tumor microenvironment is difficult to recapitulate.Citation1–Citation3 Thus, one of the main obstacles in oncology is the lack of proper models that recount main features present in tumors. For example, monolayers of cultured cells are considered to be more susceptible to anti-tumor therapeutic agents.Citation4–6 Moreover, cells cultured on planar rigid surface could enhance cell proliferation but inhibit cell differentiation due to the limited cell–cell interactions.Citation7,Citation8
With the support of natural or synthetic polymers which were safety and stability, 3D innovative culture systems were developed to enhance the adhesion, proliferation and increase the release of secreted factors by cultured cells.Citation9–11 Hydrogels are a 3D network made of natural or synthetic polymers crosslinked that can entrap large volume of water or biological fluid.Citation12–14 In recent years, hydrogels have been employed to generate three-dimensional (3D) models that better mimic the specific native microenvironment compared to 2D cell cultures. Due to the higher stability and safety and higher cell fixation, hydrogels not only protect but may also promote over a long period of time for tumor cell growth.Citation15,Citation16 Owing to its mechanical properties, well-deserved biocompatibility and susceptibility degradation, hydrogels constitutes a suitable matrix for cells normally residing in soft tissues.Citation17,Citation18 In addition, tumor cells tend to form large cell clumps in 3D culture systems,Citation19–21 and in this sense, 3D culture tumor cells in hydrogels could be used for establish in vivo subcutaneous tumor mice models.
Herein, in the present study, we established nude mice tumor model by two strategies, 2D cultured HepG2 cells and 3D cultured cells system method. The tumor volume, weight, tumor formation rate and histology of the subcutaneous tumor tissue were evaluated. In our previous reports, we prepared biotin grafted pullulan acetate nanoparticles (Bio-PA NPs) to be used as drug carriers, and also confirmed that in HepG2 tumor-bearing nude mice, Bio-PA NPs were mainly distributed in the tumor after 24 h post administration. EPI encapsulated in Bio-PA NPs resulted in the accumulation and retention of drugs at the tumor site. In HepG2 tumor-bearing nude mice, Bio-PA/EPI NPs could increased distributions and prolonged the retention time in the tumor, and also inhibited the tumor growth at low EPI dose.Citation22 In this study, we further measured the in vitro cytotoxicity and EPI-loaded Bio-PA NPs on HepG 2 cells with 2D and 3D cultured cells system. We also established a xenograft model by implanting HepG2 3D cultured cells system subcutaneously into nude mice and further evaluated the in vivo antitumor activity of Bio-PA NPs.
Materials and methods
Materials
3D cell culture hydrogel (ShakeGel 3D) was obtained from Biomaterials LLC, Richmond, VA, USA. Pullulan (Mw 200,000) was purchased from Hayashibara Biochemical Laboratories, Inc. (Okayama, Japan). Biotin was purchased from Sigma-Aldrich (St. Loius, MO, USA). Acetonitrile and methanol used for high performance liquid chromatography (HPLC) analysis were of HPLC grade and provided by Thermofisher Scientific (Waltham, MA, USA). Cell Counting Kit (CCK-8) was obtained from Promega Systems Ltd. (Beijing, China). All other chemical reagents were analytical grade and obtained from commercial sources. Female nude mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China), and operated in accordance with protocols approved by Xinxiang Medical University Laboratory Animal Research Center.
Preparation and characterization of Bio-PA NPs
Preparation of Bio-PA NPs
EPI loaded Bio-PA NPs were prepared by the dialysis method according to our recently reported paper.Citation22,Citation23 Briefly, 100 mg of Bio-PA and 5 mg EPI were dissolved in 4 ml dimethyl sulfoxide (DMSO). To form NPs, the solution was injected in the dialysis tubing to against 1000 ml deionized water and the dialyzed liquids were exchanged six times followed by sonication step using ultrasonic probe (Scientz JY92-II, Ningbo, China) at 100 W for 2 min. The ice water bath and pulse (pulse on 2.0 s, pulse off 2.0 s) function were indispensable to protect the sample solution against heating built-up during the sonication. The solution of self-aggregated NPs was filtered through a membrane filter (pore size: 0.8 µm, Millipore, Burlington, MA, USA) to remove dust and then stored at 4 °C.
Surface morphology
To observe the morphology, Bio-PA NPs dispersion was dropped on the carbon support film and dried under an infrared lamp, afterward imaged using a transmission electron microscope (TEM, Hitachi High-Technologies Corp. HT7700, TokyoJapan).
Particle size analysis and zeta potential measurement
The particle size and size distribution of the freshly prepared Bio-PA NPs were measured by a dynamic light scattering (90 Plus Particles Size Analyzer, Malvern nanoZS, Malvern, England) at room temperature.
Drug loading content and encapsulation efficiency determination
Known amount of Bio-PA NPs formulation was dissolved in DMSO solution and then the amount of EPI was determined using the HPLC method.Citation22 The determination was carried out on a HPLC system (715, Gilson, Inc., Middleton, WI, USA). The mobile phase consisted of 0.02 M KH2PO4, CH3CN and CH3OH (49:17:34 v/v/v) and the flow rate was 1 ml/min. The detection wavelength of EPI was set at 485 nm. Bio-PA NPs without any drug were used as a blank test.
Loading Content (LC) = Weight of encapsulated EPI in the NPs/Weight of NPs × 100%
Encapsulation Efficiency (EE) = EPI loaded in the NPs/Total weight of EPI × 100%
In vitro drug release
The in vitro releases of EPI from the NPs were studied using dynamic dialysis method in phosphate buffer media (PBS, pH 7.4). Briefly, 20 mg of EPI-loaded NPs was placed in 5 ml of PBS and gently shaken in an incubator at 37 °C and stirred at 75 rpm. The samples were taken at specific time intervals and measured with HPLC to assess the drug release profile of Bio-PA NPs.
To simulate the released drugs penetrating into gels, Firstly, we mixed the hydrogel solution with EPI or the hydrogel solution with EPI-loaded NPs. Then, we placed them into a dialysis bag. And followed experimental procedures are the same as above.
Cells culture
HepG2 cells in 2D culture
HepG2 cell lines were obtained from Cell Center of Chinese Academy of Medical Sciences. All the cells were grown in Dulbecco's modification of Eagle medium (DMEM) high medium with 10% fetal bovine serum supplemented with antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin), and incubated in a Thermo CO2 incubator (Thermo Forma 4111, ThermoFisher Scientific) at 37 °C in a humidified environment of 5% CO2.
HepG2 cells in 3D culture
For the 3D system method, we gently mixed the hydrogel solution and HepG2 cells suspension into centrifuged tube (V/V, 1:1), and votexed for 1 s. Then added the hydrogel-cell mixture (cells concentration 1 × 106 cells/ml) to the well and gently rocked the culture-ware to spread the mixture evenly over the surface of the well. After that, incubated at 37 °C in a humidified environment of 5% CO2.
HepG2 cell sphere formation assay
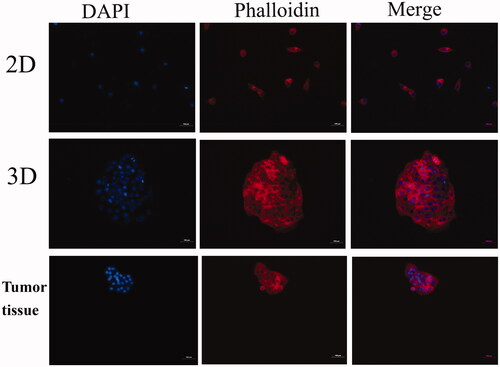
HepG2 cells were seeded in six-well (monolayer) cell culture plates and cultured for 7 days using hanging drop method. After that, 2D cultured cells and 3D spheroids were fixed with 4% (v/v) paraformaldehyde on a rocking platform at room temperature for 20 min respectively. The frozen section of mice tumor tissue were used as the control. Fixed samples were permeabilized in 1% (v/v) Triton X-100 (in PBS) for 10 min and then incubated with TRITC labelled phalloidin which was configured by 1% (w/v) bovine serum albumin (in PBS). Nuclei were stained with DAPI for 5 min at room temperature. The fixed cytoskeletal morphology was then observed under a fluorescent microscope.
Cytotoxicity assay
Cell growth and cell viability were quantified using the CCK-8 assay kit. Briefly, the cells in atlogarithmic growth phase were seeded in a 96-well plate at a density of 2.0 × 104 cells/well which were cultured by 2D and 3D method, and cultured for 24 h. After that, the culture media were replaced with 200 µl of DMEM containing different concentrations (0–500 µg/ml) of Bio-PA NPs and EPI and EPI-loaded NPs. The cells were further incubated for 48 h. At designated time intervals, the medium was removed, and the wells were washed with PBS for two times. Then 10 µl of CCK-8 solution was added to each well of the plate and the plate was incubated at 37 °C for an additional 1 h in the incubator. The absorbance was measured at 450 nm using a microplate reader (Thermo Multiscan MK3, Thermo Fisher Scientific) with the plain cell culture media as the control. Cell viability was expressed by the ratio between the absorbance of the cells incubated with EPI-loaded NPs and free drugs to that of the cells incubated with blank culture media only. The survival curves were plotted and the IC50, defined as the EPI concentrations required for 50% inhibition of cell growth, were calculated based on the survival curves.
Establishment of an in vivo subcutaneous tumor model
HeG2 cells (1 × 106 cells) in 0.1 ml of normal saline were inoculated subcutaneously in the right anterior axillary of female Balb/c nude mice to model the mice bearing hepatic tumor.
At the same time, we established tumor models using 3D cultured cells. Briefly, HepG2 cells were mixed with equal volume of hydrogel, then 0.1 ml hydrogel embedded with cells (1 × 106 cells) were inoculated subcutaneously in the right anterior axillary of female Balb/c nude mice.
The length and width of the tumor were measured every three days for a total of 21 days. The tumor volume was calculated according to the following formula: volume = width2 × (length/2). Relative tumor volumes were normalized to their initial sizes. At 21 days post-treatment, all mice were sacrificed and subcutaneous tumors were excised for weighting and histological analysis.
Anti-tumor effect of Bio-PA/EPI NPs in vivo
In vivo anti-tumor efficacy was conducted on the subcutaneously implanted 3D cultured HepG2 tumor model. When the tumors grew up to 200 ∼ 300 mm3 in average, the mice were randomly divided into three groups (n = 6), respectively treated with physiological saline (control), free EPI, Bio-PA/EPI NPs. The treatments with EPI dose of 3 mg/kg were injected through the tail vein every three days for a total of 5 times. The tumor volume was calculated according to the following formula: volume = widthCitation2 × (length/2). Relative tumor volumes were normalized to their initial sizes. At 21 days post-treatment, these mice were sacrificed and tumors were excised for weightings.
Statistical analysis
Data were analyzed using the F test with subsequent t tests (equal variance) for the comparison between two different groups. For three or more groups, ANOVA test was used followed by a Least Significant Differences method. GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA) was used to obtain graphs and statistics. Significant values were designated as follows: *p < 0.05 and **p < 0.01. All data were shown as the mean ± standard deviation (SD).
Results and discussion
Preparation and characterization of Bio-PA NPs
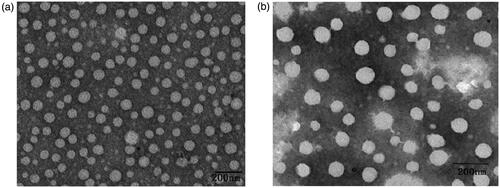
Bio-PA NPs were prepared by the dialysis method according to our previous study.Citation22,Citation23 TEM shows that both Bio-PA NPs and EPI-loaded NPs fabricated by dialysis method are spherical in shape (). However, the mean diameter of the particle was increased from (130.9 ± 10.36) nm (Bio-PA NPs) to (169.6 ± 7.94) nm (EPI-loaded NPs) with the polydipersity index lower than 0.5, suggesting mono-dispersion, determined by dynamic light scattering. In addition, the zeta potentials were − (28.8 ± 2.02) mv and − (15.2 ± 2.76) mv, respectively. The EPI-loading content and encapsulation efficiency in Bio-PA NPs were 0.73 ± 0.038 µmol/100 mg and 79.8% ± 3.0%, respectively.
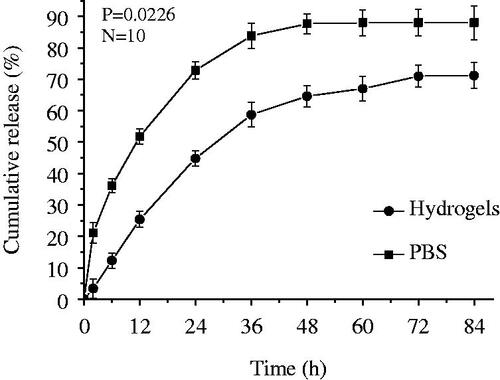
The cumulative release profile of EPI from Bio-PA NPs is presented in . Clearly, EPI exhibited a rapid initial drug release during the first 12 h, and then followed by a significant sustained release from these NPs. A sustained drug release pattern was observed, and the amount of EPI released from the NPs was higher than Bio-PA NPs mixed with 3D hydrogel. For example, EPI release amount was only 24.1% from Bio-PA NPs over the 12 h period, but reached up to 51.6% from the Bio-PA NPs mixed with 3D hydrogel. As expected, the Bio-PA NPs mixed with 3D hydrogel caused an impressive decrease in release rate and released amount. This result was due to the fact that hydrogel decreases the drug diffusion rate.Citation24,Citation25
Cell growth curve and cellular morphology
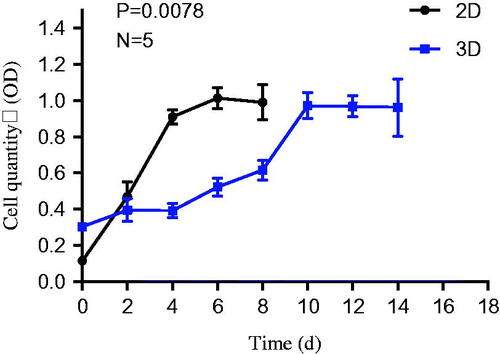
It can be seen from the cell growth curve (), The lag phase of HepG2 cell growth curve in 3D culture system was longer than that in the 2D culture medium. Since the survival advantages of HepG2 cells studies have been reported, it has been shown that more cells in the 3D accumulated in the G1 phase of the cell cycle as compared to the 2D cells.Citation26,Citation27
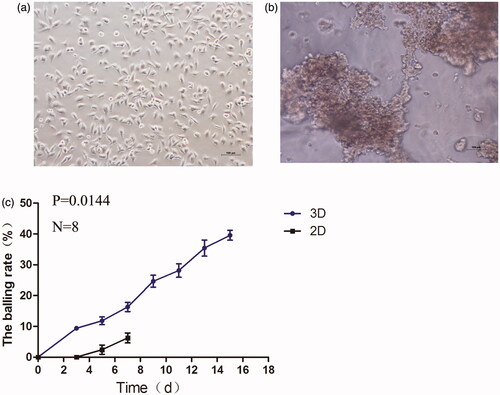
The adherent HepG2 cells in 2D culture environment were polygonal-shaped on planar rigid surface (), and in 3D growing into the sphericals and gathered to form homogenous multicellular spheroids (). We found that cells in 3D culture environment showed higher formation rate of spheroids. The HepG2 cells grew in a 3D manner within the hydrogel, which lead to forming an aggregated cell mass with many cells layers along with the prolongation of the culture time. Cultures of cells aggregated into spheroids have been shown to successfully mimic solid tumor heterogeneity. 3D cell culture is providing a suitable micro-environment for individual cells to maintain their normal 3D shape, structure, optimal cells growth, differentiation and function, and the ability to create tissue-like constructs. 3D cells culture method is a powerful technique to study in vivo, cell–cell interactions and cell–extracellular matrix (ECM) interactions, and are usually used for anticancer drug screening.Citation28 Because they mimic the pathophysiological conditions of solid tumors, such as the specific hypoxic areas in the center and proliferation gradients more properly.Citation29,Citation30 Several approaches reported that cells were tend to aggregated into spheroids in 3D system. One of the main reasons is adhesive forces between the cells were stronger than adhesive forces between the cells and the surface.Citation31,Citation32 Hydrogels are ideal materials for 3D tissue platforms as their easily controlled density and flexibility facilitate the mimicking of ECMs, Which encouraging cells to form complex interactions with adjacent cells and receive and transmit signals. For this reason, homogenous multicellular spheroids of HepG2 cells were generated by using 3D hydrogels.Citation33
Figure 4. Morphological changes of HepG2 cells in defferent culture model: 2D (a), 3D (b) imaged by invert microscope. The balling rate (%) of two cell culture methods (c).
We further observed the cytoskeleton of HepG2 cell in 3D cell culture system and 2D cell culture. As shown in , we found that the cytoplasmic actin microfilamentrates of HepG2 cells in 3D hydrogels rearranged and displayed structural similarity to in vivo tumor tissue. We think that the 3D cell culture hydrogel can fix cells and reduce the loss of cells, moreover, it provides a microenvironment which similar to the extracellular matrix in vivo.Citation34 The external stimuli such as tissue-specific effects or certain physical forces have a profound impact on cellular performance, cell shape and cytoskeletal architecture. HepG2 cells in 3D were tend to aggregated into multicellular spheroids. And the cell parameters including cell morphology, physical forces between cell membranes, the actin cytoskeleton, the nuclear are all different from 2D. The tighter cell arrangement and more volumetric 3D aggregates of cells characteristic of 3D models are examples of features that promote aggregating into multicellular spheroids, leading to similar to tumor tissue in vivo.Citation30
In vitro cell cytotoxicity study
The viabilities of the HepG2 cells grown on a 2D plastic surface and a 3D system have been evaluated using the CCK8 assay. From the result data (), it could be seen that blank Bio-PA NPs had no significant influence on the proliferation of HepG2 cells which were cultured by 2D or 3D cell culture method in NPs concentration range of 0–500 µg/ml at 24 and 48 h incubation, thus it could be deduced that blank Bio-PA NPs had no toxic properties for the cells in the concentration range used.
Figure 6. Cytotoxicity of Bio-PA NPs (a), EPI(b) and Bio-PA/EPI NPs (c) against HepG2 cells in 2D and 3D culture model.
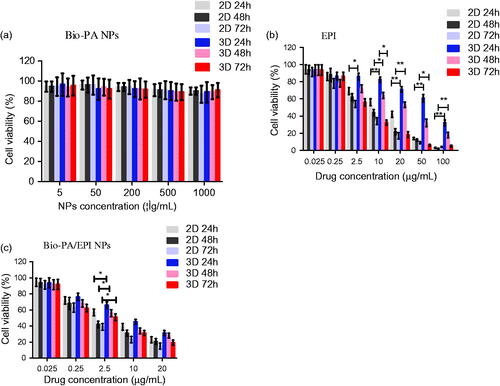
Next, we further measured the cytotoxicity of EPI and EPI-loaded NPs on HepG2 cells, along with 2D and 3D system were evaluated. As shown in , comparing the results of treated groups, cells in 2D and 3D culture exhibited a dose-dependent decline in viability. These results indicate that EPI and EPI-loaded NPs have growth-inhibitory effect on HepG2 cells monolayer while being decline drug sensitivity to HepG2 cells in 3D.
The in vitro therapeutic effects of a dosage form can be quantitatively evaluated by its IC50, which is defined as the drug concentration at which 50% of the cells in culture have been killed in a designated time period. gives the IC50 value of HepG2 cells in 2D and 3D culture after 24, 48 and 72 h incubation with EPI formulated in Bio-PA NPs at various drug concentrations, respectively. It can be found from that the IC50 value for HepG2 cells was increased from 12.63, 7.34 and 4.02 μg/ml for EPI in 2D to 63.34, 23.21 and 6.89 μg/ml for EPI in 3D. Similarly, IC50 value was increased from 2.42, 1.35 and 0.84 μg/ml to 12.27, 3.52 and 2.42 μg/ml for Bio-PA NPs after 24, 48 and 72 h incubation, respectively. Overall, the results indicated that all the cells exhibited higher viabilities after doxorubicin treatments in 3 D culture system compared to the 2D system. 3D cultures have been used previously to evaluate drug resistance and sensitivity and typically show more resistance to chemotherapy when compared with 2D monolayer cultures.Citation35,Citation36 The HepG2 cells in 3D incubated with Bio-PA/EPI NPs were induced to adapted the hypoxic and innutritious environment in the hydrogel compared with cells in 2D, which might be one of the reason of drug resistance. Furthermore, 3D cultured spheroidal cell may acts as a barrier for nanoparticle penetration.Citation37,Citation38 Cells in 3D culture may showed more closely mimic in vivo tumor behavior, and a number of 3D culture systems have been reported that more accurately predict the cellular response compared to 2D culture.Citation39 Thus, 3D culture model is necessary to more accurate clinical drug screening.
Table 1. IC50 of HepG2 Cells after 24, 48 and 72-h incubation with EPI, Bio-PA NPs at various drug concentrations in 2D and 3D culture method.
Establishment of the subcutaneous tumor model
We established the subcutaneous tumor model in nude mice through two cell culture methods. HeG2 cells in 2D and in 3D were respectively subcutaneously injected into nude mice to model the mice bearing hepatic tumor. The tumor formation rate injected with cells in 3D was 98.2%, which was significantly higher than that of 2D cultured cells (76.4%), and the time of tumor formation (tumor volume to 200 mm3) was significantly shortened (). Tumor formation and development are dynamic processes where cancer cells differentiate, proliferate and migrate interacting among each other and with the surrounding 3D environment in vivo.Citation40 Cancer cells in 3D culture system closely mimic pathologic events of tumors formation were reported.Citation34,Citation41,Citation42 HeG2 cells in 3D more easily self-assemble into spheroids, and then spheroids reproduce morphology and biologic properties of tumors tissue.
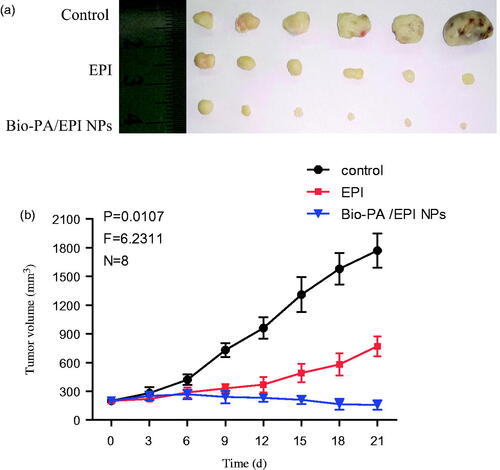
Figure 7. The changes of the tumor volumes in the tumor-bearing mice after 2D and 3D HepG2 cells subcutaneous injection (a). Average weights of different groups in the process of tumor growth (b). The picture of tumors/hydrogels removed from nude mice at specific time intervals after inoculated subcutaneously (c). Representative photomicrographs of the tumor sections (H&E staining) of mice of 2D and 3D (d).
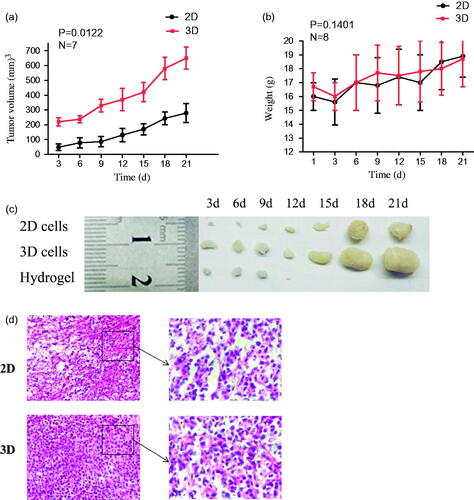
In addition, for body weight and daily food intake of nude mice, there were also no significant differences between the 2D and 3D group (). At 21 day post-injection, all animals were sacrificed and tumors were excised for weightings and histological analysis. shows microscopic examination of the representative histopathological of tumor tissue sections stained with haemotoxylin and eosin (H&E). No significant differences were observed between the 3D group and 2D group in histological analysis. The results indicated that the tumor tissue in vivo have no differences between 3D culture and 2D culture. Due to high tumor formation rate and tumor volume, the subcutaneous tumor model in nude mice with 3D cell culture appears to be an effective and simple method. And it could be used as the following in vivo anti-tumor efficacy research.
In vivo anti-tumor efficacy
We further evaluated the in vivo antitumor activity of Bio-PA/EPI NPs using free EPI as the controls. The mice were injected with free EPI and NPs at EPI doses of 3.0 mg/kg every 3 days for consecutive five times. After the injection, the tumor volume changes were detected every three days. Obviously, Bio-PA/EPI NPs significantly inhibited the tumor growth even at a low EPI dose of 3.0 mg/kg and the average tumor weight was markedly lower than those of the control group and the treatment groups of free EPI (). We believed that the effective activity of EPI-loaded NPs on the tumor growth was due to their long circulation and in vivo tumor-targeted delivery for EPI. These results are in accordance with the results of in vivo anti-tumor efficacy with 2 D cell subcutaneous tumor model.Citation22
Conclusion
In summary, we report the ability to culture HepG2 cells in 3D and evaluation anti-tumor efficacy of Bio-PA/EPI NPs with the 3D cultured cells in vitro and in vivo. The HepG2 cells grew in a 3D manner within the hydrogel, which lead to forming homogenous multicellular spheroids along with the prolongation of the culture time. Bio-PA/EPI NPs have growth-inhibitory effect on HepG2 cells monolayer while being decline drug sensitivity to HepG2 cells in 3D. The HepG2 cells grew in a 3D manner within the hydrogel, which lead to forming homogenous multicellular spheroids along with the prolongation of the culture time. In 3D HepG2 cell tumor-bearing nude mice, Bio-PA/EPI NPs could inhibited the tumor growth at low EPI dose. The 3D hydrogels culture model has the potential to improve cell culture strategies and be used for establishment the subcutaneous tumor model in nude mice. So, the data presented in this study can therefore be of great value in the evaluation of targeted anticancer drug NPs.
Disclosure Statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Khademhosseini A, Vacanti JP, Langer R. Progress in tissue engineering. Sci Am. 2009; 300:64–71.
- Antoni D, Burckel H, Josset E, et al. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015; 16:5517–5527.
- Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988; 240:177–184.
- Gurski LA, Petrelli NJ, Farach-Carson MC, et al. 3D matrices for anti-cancer drug testing and development. Oncol. 2010; 25:20–25.
- Vörsmann H, Groeber F, Walles H, et al. Development of a human three-dimensional organotypic skin-melanoma spheroid model for in vitro drug testing. Cell Death Dis. 2013; 4:719–729.
- Horning JL, Sahoo SK, Vijayaraghavalu S, et al. 3-D tumor model for in vitro evaluation of anticancer drugs. Mol Pharm. 2008; 5:849–862.
- Hara J, Tottori J, Anders M, et al. Trehalose effectiveness as a cryoprotectant in 2D and 3D cell cultures of human embryonic kidney cells. Artif Cells Nanomed Biotechnol. 2017; 45:609–616.
- Yu HS, Lee EJ, Seo SJ, et al. Feasibility of silica-hybridized collagen hydrogels as three-dimensional cell matrices for hard tissue engineering. J Biomater Appl. 2015; 30:338–350.
- Lee KY, Peters MC, Anderson KW, et al. Controlled growth factor release from synthetic extracellular matrices. Nature 2000; 408:998–1000.
- Pedron S, Becka E, Harley BA. Spatially gradated hydrogel platform as a 3D engineered tumor microenvironment. Adv Mater. 2015; 27:1567–1572.
- Das S, Kumar R, Jha NN, et al. Controlled exposure of bioactive growth factor in 3D amyloid hydrogel for stem cells differentiation. Adv Healthc Mater 2017;6(18):1-14. DOI:10.1002/adhm.201700368.
- Smith DK. Supramolecular gels: building bridges. Nat Chem. 2010; 2:162–163.
- Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002; 14:633–639.
- Jonker AM, Borrmann A, Van Eck ER, et al. A fast and activatable cross-linking strategy for hydrogel formation. Adv Mater Weinheim. 2015; 27:1235–1240.
- Peppas NA, Hilt JZ, Khademhosseini A, et al. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006; 18:1345–1360.
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Delivery Rev.2012; 64:18–23.
- Gurski LA, Jha AK, Zhang C, et al. Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials 2009; 30:6076–6085.
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007; 130:601–610.
- Feder-Mengus C, Ghosh S, Reschner A, et al. New dimensions in tumor immunology: what does 3D culture reveal? Trends Mol Med. 2008;14:333–340.
- Michele Z, Filippo P, Chiara A, et al. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep 2016;6:19103–19107.
- Froehlich K, Haeger JD, Heger J, et al. Generation of multicellular breast cancer tumor spheroids: comparison of different protocols. J Mammary Gland Biol 2016; 21:1–10.
- Achilli TM, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther. 2012; 12:1347–1360.
- Chen HL, Wei XJ, Qin JW, et al. Synthesis, characterization and in vivo efficacy of biotin-conjugated pullulan acetate nanoparticles as a novel anticancer drug carrier. J Biomed Nanotechnol. 2017; 13:1134–1146.
- Chen HL, Nan WenBin N, Wei XJ, et al. Toxicity, pharmacokinetics, and in vivo efficacy of biotinylated chitosan surface-modified PLGA nanoparticles for tumor therapy. Artif Cells Nanomed Biotechnol. 2017;45:1115–1122.
- Thambi T, Li Y, Lee DS. Injectable hydrogels for sustained release of therapeutic agents. J Control Release. 2017; 267:57–66.
- Wan J, Geng S, Zhao H, et al. Doxorubicin-induced co-assembling nanomedicines with temperature-sensitive acidic polymer and their in-situ-forming hydrogels for intratumoral administration. J Control Release.2016; 235:328–336.
- Li CL, Tian T, Nan KJ, et al. Survival advantages of multicellular spheroids vs. monolayers of HepG2 cells in vitro. Oncol Rep. 2008;20:1465–1471.
- Hurrell T, Ellero AA, Masso ZF, et al. Characterization and reproducibility of HepG2 hanging drop spheroids toxicology in vitro. Toxicol in Vitro. 2018; 50:86–94.
- Lan SF, Starly B. Alginate based 3D hydrogels as an in vitro co-culture model platform for the toxicity screening of new chemical entities. Toxicol Appl Pharm. 2011; 256:62–72.
- Yajima Y, Lee CN, Yamada M, et al. Development of a perfusable 3D liver cell cultivation system via bundling-up assembly of cell-laden microfibers. J Biosci Bioeng 2018;126(1):111-118. DOI:10.1016/j.jbiosc.2018.01.022.
- Meli L, Jordan ET, Clark DS, et al. Influence of a three-dimensional, microarray environment on human Cell culture in drug screening systems. Biomaterials 2012; 33:9087–9096.
- Akasov R, Zaytseva-Zotova D, Burov S, et al. Formation of multicellular tumor spheroids induced by cyclic RGD-peptides and use for anticancer drug testing in vitro. Int J Pharm. 2016; 506:148–157.
- Guan Y, Sheng F, Wei Y, et al. Hyaluronic acid nanogels prepared via ortho ester linkages show pH-triggered behavior, enhanced penetration and antitumor efficacy in 3-D tumor spheroids. J Colloid Interf Sci 2017; 504:25–38.
- Anada T, Fukuda J, Sai Y, et al. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials 2012; 33:8430–8441.
- Dubiak-Szepietowska M, Karczmarczyk A, Jönsson-Niedziółka M, et al. Development of complex-shaped liver multicellular spheroids as a human-based model for nanoparticle toxicity assessment in vitro. Toxicol Appl Pharm.2016; 294:78–85.
- Jaganathan H, Gage J, Leonard F, et al. Three-dimensional in vitro co-culture model of breast tumor using magnetic levitation. Sci Rep. 2014;4:6468–6476.
- Luo Y, Wang C, Hossain M, et al. Three-dimensional microtissue assay for high-throughput cytotoxicity of nanoparticles. Anal Chem. 2012;84:6731–6738.
- Mehta G, Hsiao AY, Ingram M, et al. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release. 2012;164:192–204.
- Priwitaningrum DL, Blondé J-BG, Sridhar A, et al. Tumor stroma-containing 3D spheroid arrays: A tool to study nanoparticle penetration. J Control Release.2016;244:257–268.
- Yue X, Nguyen TD, Zellmer V, et al. Stromal cell-laden 3D hydrogel microwell arrays as tumor microenvironment model for studying stiffness dependent stromal cell-cancer interactions. Biomaterials 2018; 170:37–48.
- Oladapo HO, Tarpley M, Sauer SJ, et al. Pharmacological targeting of GLI1 inhibits proliferation, tumor emboli formation and in vivo tumor growth of inflammatory breast cancer cells. Cancer Lett.2017; 411:136–149.
- Jo Y, Choi N, Kim HN, et al. Probing characteristics of cancer cells cultured on engineered platforms simulating different microenvironments. Artif Cells Nanomed Biotechnol. 2018;3:1-10. Published online: 08 Mar. DOI: https://doi.org/10.1080/21691401.2018.1446970.
- Fischbach C, Chen R, Matsumoto T, et al. Engineering tumors with 3D scaffolds. Nat Methods. 2007; 4:855–860.