?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Glioblastoma is a heterogeneous disease with multiple genotypic origins. Despite treatment protocols such as surgery, radiotherapy and chemotherapy, the prognosis for patients remains poor. This study investigates the cytotoxic and radiation dose-enhancing and radiosensitizing ability of five rare earth oxide nanoparticles, in two different immortalized mammalian cell lines; U-87 MG and Mo59K. Significant cytotoxicity was observed in U-87 MG cells when exposed to Nd2O3 and La2O3. Autophagy was also detected in cells after incubation with Nd2O3. Radiosensitization was observed in U-87 MG when incubated with Gd2O3, CeO2-Gd and Nd2O3:Si. Importantly, these elements did not cause any intrinsic toxicity in the absence of irradiation and so could be considered biocompatible. The Gd2O3 and CeO2-Gd nanoparticles were also seen to generate ROS in U-87 MG cells after irradiation. Furthermore, the Mo59K and U-87 MG cells responded very differently to exposure to the rare earth nanoparticles. This may indicate the importance of the genotype of cells in the successful use of rare earth oxides for treatment.
Introduction
The prognosis for glioblastoma (GBM) patients remains poor with no significant progress over the last decade in prolonging the median survival of 14 months [Citation1]. GBM account for 15% of all primary brain cancers [Citation2,Citation3] with multiple genotypic origins resulting in a very heterogeneous disease [Citation4]. At present, conventional treatment protocols involve surgical resection, followed by concurrent radiotherapy and chemotherapy [Citation1]. In children under the age of three, however, treatment options are further limited since radiotherapy cannot be used due to the risk of neurological sequelae. Consequently, novel treatments which can improve the survival rates of GBM patients are desperately needed.
This study explores the use of rare earth oxide nanoparticles as cytotoxic and radioenhancement agents. The rare earth elements (REE) are a series of chemically similar metallic, nonradioactive, elements that include the fifteen lanthanide elements, plus scandium and yttrium. These can be further divided into the light REEs which include lanthanum, cerium, praseodymium, neodymium, samarium and europium and the heavy REEs gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, scandium and yttrium. The unique electronic configuration of the REEs means that they react vigorously with oxygen and produce stable oxides, generally as (REE)2O3, although one notable exception is CeO2. The rare earth oxides may then easily be formulated into nanoparticles as a consequence of their chemical stability. The advantage in using nanoparticles rather than free REEs is the potential to localize the particles. In vivo animal studies have shown that nanoparticles up to 160 nm in size are able to pass the blood brain barrier into brain matter [Citation5–7]. Furthermore, the enhanced permeation and retention (EPR) effect could be used to either administer the particles, or to retain the particles in the tumour for greater efficacy and to enable repeat radiosensitization without repeat administration.
There is considerable evidence to show that REEs inhibit proliferation and induced apoptosis in a number of different cancer cell lines [Citation8–11] and that they may also act as autophagy inducers [Citation12]. In addition to their cytotoxic activity, the REEs may also act as radiosensitizers or dose-enhancers. Radiation therapy is often used in the treatment of GBM, either to reduce the size of the tumour prior to surgery, or as an adjuvant therapy [Citation13]. All radiotherapy works by either direct interaction with DNA or the radiolysis of water and subsequent generation of highly reactive oxygen species (ROS). The ROS are unstable and interact with proteins, lipids and nucleotides within the cell, compromising cellular repair mechanisms, and may also trigger apoptosis which leads to cell death. Due to the differences in antioxidant capacity, threshold and growth rates, cancerous cells are more susceptible than healthy ones. The efficacy of radiotherapy can be enhanced further by the use of radiosensitizers at the tumour site, enhancing therapeutic ratio. Classical radiosensitizers are high atomic number (Z) materials, which work by increasing the photoelectric cross section resulting in dose enhancement due to an increase in photoelectron production in the nanoparticles. These photoelectrons are predominantly emitted from the inner shell and, therefore, will also be accompanied by the emission of Auger electrons. The Auger effect describes the release of an electron from an outer-shell to replenish an inner-shell ionized by radiation, which then leads to an energy emission [Citation14]. REEs also display the Auger effect in response to irradiation due to their electronic structure.
Herein, we investigate the efficacy of nanoparticles of three light REE and one heavy REE in causing cytotoxicity or radiosensitization in two different GBM cell lines. Cell death, proliferation, autophagy and ROS generation were assessed to establish whether these particles could be used to improve the treatment protocols of brain cancer.
Methods
Materials
Rare earth nanopowders were purchased from Sigma Aldrich (Poole, UK): Cerium (IV) oxide nanopowder (< 50 nm), Cerium oxide nanopowder doped with 10% gadolinium (< 100 nm), Neodymium oxide nanopowder (< 100 nm), Lanthanum (III) oxide nanopowder (< 100 nm), Gadolinium (III) oxide nanopowder (< 100 nm).
Silica coating
Silica coating can sometimes alter the way in which particles aggregate and interact with cells. To enable a pairwise comparison Nd2O3 nanoparticles were coated with silica. The Nd2O3 nanoparticles were resuspended in 200 ml distilled water (pH 4.5) [Solution I]. Next, 3-Maercaptoethnaol-trimethoxy-silane (1.89 ml; Sigma-Aldrich) was added to 50 ml distilled water [Solution II]. Twenty millilitres of solution II were then added to solution I and stirred. After 1 h, sodium silicate (40 ml; Sigma-Aldrich) was added. Samples were removed after 10 min, centrifuged immediately and washed with distilled water three times.
Zeta potential measurement
The zeta potential of the nanoparticles was measured using a Malvern Zetasizer Nano and folded capillary cells (DTS1060, Malvern). Samples were prepared at a concentration of 0.2–0.3 mg ml−1 in PBS
Immortalized cell culture
Two human GBM tumour cell lines (U-87 MG, Mo59K) were used for experimentation: U-87 MG (American Type Tissue Culture Collection [ATCC] no.HTB-14) derives from a male patient with suspected GBM, and is the prototypical GBM cell line used for investigations. The Mo59K line (ATCC no. CRL-2365) derives from a 33-year-old male patient with known GBM.
Cells were grown in Dulbecco’s Modified Eagles Medium – high glucose (DMEM; Aldrich) supplemented with 10% Fetal calf serum (FCS; Aldrich), 2 mM l-Glutamine (Aldrich), 100 U/ml Penicillin (Aldrich) and 0.1 mg ml−1 Streptomycin (Aldrich). Cells were incubated at 37 C in a 5% CO2 atmosphere and passaged when confluent (approximately every four days).
Growth rate determination
Cells were seeded at a density of 1 × 104 cells per well in a 96 well plate in 100 μl of growth medium (see Materials section). Cells were incubated at 37 °C in 5% CO2 atmosphere and allowed to adhere overnight. On sequential days, the plates were removed from the incubator and cells washed with PBS to remove nonadherent cells. Cells were then fixed with 100 μl of 1% glutaraldehyde for 30 min. The glutaraldehyde solution was removed and 100 μl of 0.5% (w/v) crystal violet solution added to each well for 1 h, to stain cells. The plate was then washed in copious amounts of water, and left to dry overnight. The dye was solubilized with 100 μl of solubiliser per well (1% Sodium dodecyl sulphate; 10% (w/v) acetic acid). After incubating for 1 h, the absorbance at 595 nm was measured. For each cell line the growth rate was determined by measuring cellular content at 2, 3, 4 and 5 days.
Cytological effects of rare earth oxide nanoparticles
Cells were seeded at a density of 1 × 104 cells per well in 96-well plate. Cells were incubated at 37 °C in 5% CO2 atmosphere overnight to allow adherence to the plate. Nanoparticles were then added to the media at concentrations of 10, 20, 30 and 40 μM. The cells were then returned to the incubator to allow for cellular uptake to occur overnight. To investigate whether the nanoparticles affected cell survival, U-87 MG and Mo59 cells were analysed 24 and 72 h after NP exposure respectively due to their differences in proliferation rate. To test for radiosensitization properties, the cells were exposed to 3 Gy radiation 24 h after NP exposure, and then incubated for a further 24 and 72 h after irradiation for U-87 MG and Mo59 respectively. To assess cell viability after treatment, the cells were washed with 100 μl of PBS to remove nonadherent dead cells. Adherent live cells were then detached using 50 μl Trypsin-EDTA (Sigma-Aldrich) and placed inside an incubator for 20 min. The enzyme activity was then neutralised with 50 μl of growth medium per well. The viable cells were then counted manually with a haemocytometer. These experiments were performed in triplicate on two separate occasions.
Irradiation schedule
Cells were exposed to 250 kV x-rays (0.25 mm copper filter, resulting in a half-value layer of 1.0 mm Cu). This results in a broad spectrum of x-ray energies up to 250 keV with an average energy of approximately 90 keV. The dose rate was 0.58 Gy min−1. Cells were exposed to a total of 3 Gy irradiation before being returned to the incubator for 24 h prior to determining cell proliferation.
Clonogenic assay
The clonogenic assay, or colony formation assay, is an in vitro cell survival assay based on the ability of a single cell to grow into a colony. A colony is defined as a cluster of at least 50 cells that can often only be determined microscopically. The assay essentially tests every cell in the population for its ability to undergo ‘unlimited’ division.
RE nanoparticles were added at 40 μM to the cell culture media, and cells incubated overnight. Cells were then irradiated at either 0 or 3 Gy. The media was then removed, and cells washed with PBS. Cells were then removed from the flasks using trypsin and cell number ascertained using a haemocytometer. Cells were added to the wells in triplicate for each treatment. (Results showed that 500 cells for U-87 MG, and 750 cells for Mo59K cells, were appropriate starting cultures). The cells were incubated for at least two weeks at 37 °C in a 5% CO2 atmosphere. Subsequently cells were stained for counting. Firstly, media was removed and cells washed with PBS. Cells were then fixed with glutaraldehyde for 30 min. Cells were then stained with crystal violet solution for at least 1 h. The cells were then washed with water and left to dry prior to counting.
Wound healing assay
A wound healing assay (scratch test) was performed to evaluate cell migration and invasion. In a typical test, U-87 MG or Mo59K cells were cultured into a 24-well sterile microplate (Corning). The cells were allowed to proliferate, spread and form a confluent monolayer. A 1000 µl pipette tip was used as a pin tool to scratch across the cell layer to form a cell-free zone in each well. Rare earth nanoparticles were then added to the cell culture media. Images were collected premigration and postmigration. The width was measured three times across both the vertical and the horizontal scratches to calculate the average.
Radical oxygen species assay
Fluorescence was used as a qualitative measure for ROS production. We used the ROS indicator CM-H2DCFDA which passively diffuses into cells, where it’s acetate groups are cleaved, allowing binding with intracellular components for longer retention within cells. Oxidation of the compound results in an increase in fluorescence which can be monitored using a fluorescence microscope.
Cells were seeded at a density of 1 × 104 cells per well in 200 μl of growth medium followed by incubation at 37 °C in 5% CO2 atmosphere overnight, to allow adherence. Cells were pre-stained with 1 μM 2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; Invitrogen) for 45 min, followed by washing in growth medium. Nanoparticles were then added at 40 μM concentration. The cells were then exposed to 0 Gy or 3 Gy radiation, and left for 1 h. Cells were then imaged using a Motic AE31 microscope with filters for Fluorescein (Ex. 465 nm; Em. 510 nm). This experiment was performed in triplicate on two occasions.
Labelling of autophagic vacuoles with monodansylcadaverine
Cells were allowed to adhere to plates overnight, followed by incubation with 40 μM nanoparticles for 16 h. Cells were washed with PBS and then incubated with 0.05 mM monodansylcadaverine (MDC) in PBS at 37 °C for 10 min. After incubation the cells were washed 4 times with PBS and collected in 10 mM Tris-HCL, pH 8 containing 0.1% Triton X-100. Intracellular MDC was measured by fluorescence (Ex. 380 nm/Em. 525 nm) in a Tecan microplate reader. To normalize to the number of cells present, a solution of ethidium bromide was added to a final concentration of 0.2 μM and the DNA fluorescence measured (Ex. 530 nm/Em. 590 nm)
Blood interaction studies
Defibrinated horse blood was used to assess the haemotoxicity of the melanin nanoparticles. Blood (10 ml) was transferred to a centrifuge tube, and centrifuged at 2000 g for 5 min to pellet the red blood cells. The pellet was resuspended in PBS and centrifuged for 2000 g for 5 min. The wash was repeated twice more. After the final wash the supernatant was removed and the pellet resuspended in 1:10 (v/v) PBS. The sample was then divided into equal volumes in separate tubes. A positive control comprised adding 400 μl water to the sample, and a negative control used the same volume of PBS. The MNPs were added at different concentrations in 400 μl PBS. All samples were incubated at 37 °C for an hour. The samples were then centrifuged at 10,000g for 5 min. Supernatant (100 μl) was taken from each tube and placed in a 96 well plate. The absorbance was measured at 595 nm. The experiment was repeated on three separate occasions.
Statistical analysis
Quantitative cell viability data were analysed using GraphPad Prism 7.0 software. Analysis involving comparison of grouped means was conducted with a one-tailed Student’s t-test if there was normal distribution; p values of <.001 (***), .001 to .01 (**), .01 to .05 (*) were considered to be significant.
Results
Physicochemical characterization
The rare earth elements are a group of 17 chemically similar metallic elements, including the 15 lanthanides, scandium and yttrium. The lanthanides have similarities in their electron configuration which explains most of the physical similarities. Generally speaking, the lanthanides follow the Aufbau rule, and the 4f sublevel is filled as atomic number increases (). The 4f electron configuration is very important and determines the magnetic and optical behaviours of the elements. In particular the unpaired electrons may affect the ways in which the elements interact with cells. Lanthanides also differ in their basicity which is the ease with which an atom will lose electrons. Basicity decreases in the following way:
Table 1. The zeta potential of NPs.
The series can be further divided into ‘heavy rare earth elements’ which includes Gadolinium, and ‘light rare earth elements’ which includes lanthanum, cerium and neodymium. All nanoparticles tested were purchased from Sigma-Aldrich, and were all less than 100 nm in diameter. The zeta potential of the nanoparticles was determined for all the nanoparticles, and shown to be positive for all the rare earth oxides (). Neodymium oxide was also coated with a thin layer of silica which then showed negative charge and could be used for comparison.
Cell doubling time
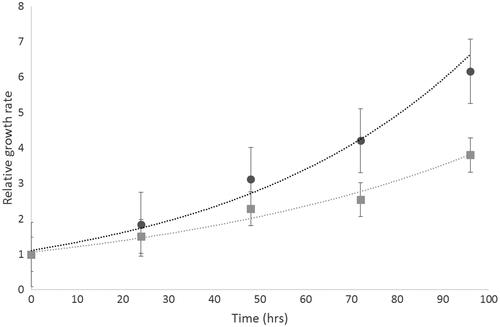
The growth rate of the GBM cell lines was determined over 96 h. It has previously been reported that the sensitivity of different cell lines to apoptotic agents is related to their doubling time [Citation15]. Both U87-MG and Mo59K cells showed an exponential increase in growth (R2 values = 0.987 and 0.9732, respectively). Faster growth rates were observed in U87-MG compared to Mo59K ().
Rare earth elements can cause cytotoxicity and radioenhancement
To assess the degree of cell death in the presence of the rare earth nanoparticles, the two GBM cell lines were tested with concentrations of the different nanoparticles, up to 40 μM. In the Mo59K cell line there was very little effect of the Rare earths in inducing cell death. The only statistically significant reductions were seen with Cerium oxide at 30 μM and gadolinium at 30 μM (p < .05 for both). In the U-87 MG cell line significant reductions in cell number were seen for neodymium oxide, and lanthanum oxide. The greater sensitivity to the chemical agents in U-87 MG rather than Mo59K cells is consistent with the results of [Citation15] whereby the faster growing cells were more susceptible to the compounds applied.
Cell numbers were then assessed in the presence of nanoparticles after being treated with either 0 Gy or 3 Gy radiation. U-87 MG cells were assessed after 24 post-irradiation, Mo59K cells after 72 h, due to the differences in their growth rates. Mo59K cells showed an average decrease in cell number of 41.5% compared to the more radioresistant U-87 MG cells (see ) which only showed a decrease of 30.8% (data not shown). After irradiation Mo59K cells showed decreases in cell number when incubated with neodymium oxide and lanthanum oxide (). However, the levels of reduction seen were not significantly different from the same concentrations of the specific rare earth when unirradiated, therefore, showing no radioenhancement with the elements in Mo59K cells. Conversely, U-87 MG cells () showed radioenhancement with neodymium oxide at 30 μM (61.3% without radiation versus 37.4% with radiation) and with the silica coating. Interestingly the latter showed biocompatibility at 40 μM in the absence of irradiation (94.3%), but reduced cell count to (54.3%) after 3 Gy irradiation thereby showing specific radioenhancement. A similar situation was seen for gadolinium oxide at 30 μM and 40 μM showing 91.4% versus 59.4% (p < .01) and 103.7% versus 62.6% (p < .05), respectively, for the presence and absence of irradiation. Lanthanum oxide, whilst not biocompatible, did show a degree of radioenhancement at 30 μM (30.3% vs. 15.0%, p < .05).
Figure 2 Viable cell count after incubation with nanoparticles, and subsequent irradiation with either 0 Gy or 3 Gy. Cells were counted using a hemocytometer 72 and 24 h after irradiation for (A) Mo59K, or (B) U-87 MG cells, respectively. Results are expressed relative to either the O Gy or 3 Gy cell only control, as appropriate. Data shows the average ± standard deviation of three independent experiments, each perfomed in triplicate. Statistical data was assessed by Student t-test, and significance is shown as * p < .05, ** p < .01, *** p < .005 when compared to the relevant cell only control.
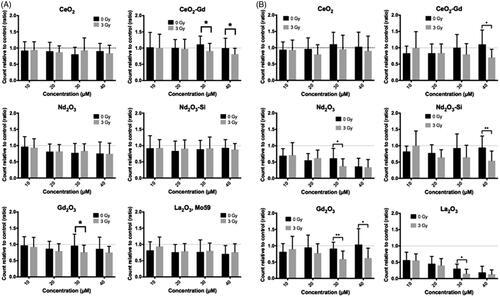
Table 2. The relative radiosensitivity of a small number of immortalized cancer cell lines for comparison against Mo59K and U-87 MG.
Rare earth elements can prevent cell division
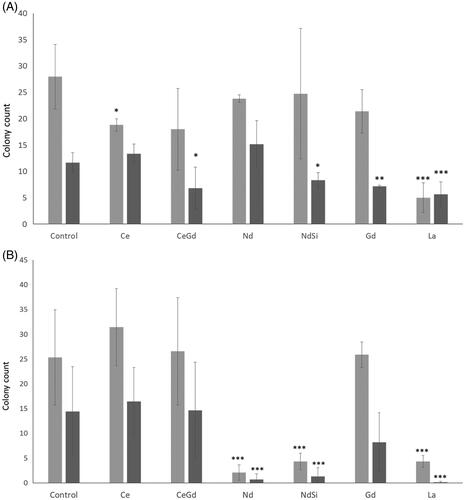
A clonogenic assay (or Colony Formation Assay; CFA) tests every cell in the population for its ability to undergo unlimited division. Therefore, the CFA is the gold standard method to show that a drug has irreversible/reversible effects on long term growth. In the absence of radiation Mo59K cells showed significant reductions in colony numbers () after incubation with cerium oxide nanoparticles (18.83 ± 1.18) and lanthanum oxide (5.00 ± 2.83) compared to control cells (28.00 ± 6.13) (student’s t-test, p < .05 and p < .005, respectively). After irradiation, significant reductions were seen in the presence of silica coated neodymium oxide (8.33 ± 1.41), gadolinium oxide (7.17 ± 0.24) and lanthanum oxide (5.67 ± 2.36), compared to the irradiated cell control (11.67 ± 1.89). However, there was no significant difference between colony numbers in the presence of lanthanum whether irradiated or not. U87 cells showed large reductions in colony numbers () in the presence of neodymium oxide (2.11 ± 1.54), silica coated neodymium oxide (4.33 ± 1.67) and lanthanum oxide (4.33 ± 1.20), compared the unirradiated control (25.33 ± 9.61). The same rare earths also showed reductions after irradiation, although only silica coated neodymium oxide and lanthanum oxide showed significant differences between unirradiated and irradiated samples (p < .01 and p < .005, respectively).
Figure 3 Clonogenic Assay. Rare earth elements were incubated with either (A) Mo59K, (B) U-87 MG cells. Cells were incubated with nanoparticles, and irradiated with either 0 Gy or 3 Gy. Cells were then plated and colonies counted. Technical replicates were performed in at least triplicates, experimental repeats were performed in triplicate. Data shows the average ± standard deviation. Statistical data was assessed by student t-test and is shown as * p < .05, ** p < .01, *** p < .005 when compared to the relevant cell only control.
U-87 MG cells generate additional ROS after irradiation in the presence of RE nanoparticles
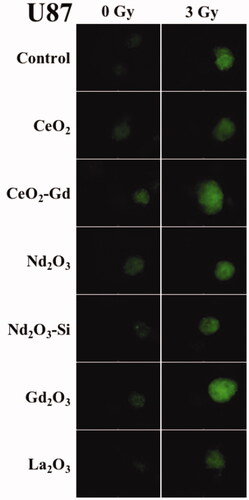
The production of local radical oxygen species (ROS) which are highly reactive compounds and can damage cellular DNA compromise cellular repair mechanisms and trigger apoptotic processes within cancer cells. It was, therefore, investigated whether the addition of the RE nanoparticles increased the amount of ROS generated after irradiation. A qualitative assay was used due to the interference of the nanoparticles with optical assays. The Mo59 cell line showed no fluorescence either before or after irradiation, nor in the presence or absence of the RE nanoparticles (data not shown). Conversely, fluorescence due to ROS production was observed in the U87 cell line radiated at both 0 Gy and 3 Gy (). Fluorescence was increased in the presence of NPs after irradiation, and most particularly in cells exposed to Gd2O3 and CeO2-Gd.
Figure 4 Cell fluorescence marker indicating ROS in U-87 MG cells. Adherent cells were incubated with a variety of Rare Earth nanoparticles at a concentration of 40 μM for 24 h. The cells were then exposed to either 0 Gy (no radiation) or 3 Gy irradiation. After a further 1 h fluorescence was assessed with an inverted microscope (Optimal Ex. 465; Em. 510 nm) using a 4 s exposure to record images. Brighter fluorescence indicates greater ROS production. Representative images are shown from n = 3 sample repeats, and n = 2 independent experiments.
Reduction in cell migration
A scratch test was performed to evaluate cell migration and invasion in the presence of the rare earth nanoparticles. In a typical test cells were cultured in 24-well sterile microplates until they formed a monolayer. A pipette tip was then used to scratch away the cell layer and the cells incubated with the different rare earth nanoparticles. Images were taken over a period of time to compare the closure of the gap in the control samples with those incubated with nanoparticles.
In the absence of radiation, Mo59K cells showed a decrease in metastatic potential (between 39.02% and 84.83% gap remaining) when incubated with any of the Rare Earth elements compared to the control (25.96% ± 11.59) (; blue bars). In particular gadolinium oxide nanoparticles, were shown to significantly reduce regrowth compared to the control (student t-test, p < .005).
Figure 5 Scratch test. Confluent adherent cell layers were scratched to produce a gap. Nanoparticles were added to the incubation media. Cells were then either exposed to 0 Gy or 3 Gy irradiation. Microscopic images were taken at time zero, and after regrowth, and the gap closure calculated. (A) Mo59K cells (B) U-87 MG cells. Statistical data is shown as * p < .05, ** p < .01, *** p < .005 when compared to the relevant cell only control. Data shows the average ± standard deviation of triplicate repeats, two independent experiments.
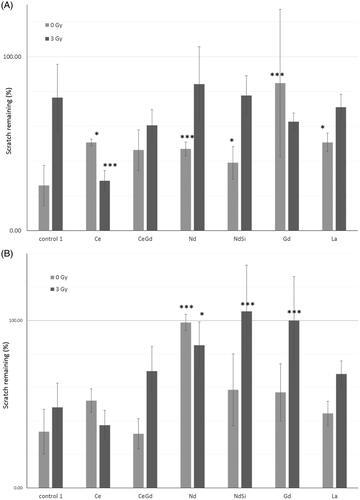
Irradiation reduced the gap closure and hence the metastatic potential in the control sample (76.5% ± 19.16 gap remaining). This indicates that Mo59K cells are fairly responsive to radiation treatment. This was also seen with the Rare Earth nanoparticles (between 60.52% and 84.23% gap remaining) with the exception of Ce where gap closure was actually increased after irradiation (28.68% ± 5.89). This could relate to the dual pro-oxidant and anti-oxidant functions seen with Ce.
In the absence of radiation U-87 MG cells are similarly affected by the presence of the rare earth nanoparticles as Mo59k cells. Neodymium oxide nanoparticles showed an almost complete arrest in the regrowth of the cells (98.82 ± 4.78%, student t-test p < .005). U87 cells were seen to be more radioresistant (data not shown) and this is reflected in the minimal change in re-growth in the control after irradiation (). Once again all of the rare earths except Ce decreased the amount of regrowth. Both neodymium oxide and gadolinium oxide nanopowders showed a highly significant reduction in regrowth (105.37 ± 27.56%, and 100 ± 26.11%, respectively; student t-test p < .005). This is particularly important given the radioresistant nature of U-87 MG cells.
Autophagy
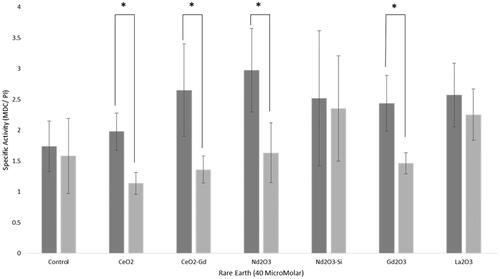
Since Neodymium oxide nanoparticles have been shown to induce vacuolization and autophagic cell death in non-small cell lung cancer NCI-H460 cells [Citation18], we further investigated whether autophagy occurred in the presence of the nanoparticles in glioma cells. It has been reported that the autofluorescent drug MDC is a selective marker for autophagic vacuoles [Citation19]. MDC was, therefore, used as a marker, and results normalized using a Propidium iodide signal as a proxy for cell number (). While there was a general trend for increasing amounts of autophagy in U-87 MG cells with the rare earth nanoparticles, the only statistically significant increase was seen with neodymium oxide nanoparticles (2.97 ± 0.68, compared to control value of 1.74 ± 0.41, student t-test p < .05). The Mo59K cells showed no significant change in autophagy compared to the control. Comparison between the two cell types showed that there was a statistically significant increase in autophagy in U87-MG compared to Mo59K cells for cerium oxide, cerium oxide doped with gadolinium, neodymium oxide and gadolinium oxide. This is consistent with the fact that cell death in the presence of nanoparticles was shown in U-87 MG and not Mo59K cells (). U87-MG cells were particularly affected by neodymium oxide and lanthanum oxide which could suggest that cell death in the former proceeds via autophagy but that a different mechanism may be involved in the latter.
Figure 6 Autophagy assay. Rare earth elements were incubated with either Mo59K cells (light grey bars) or U-87 MG cells (dark grey bars). Cells were incubated with 40 μM nanoparticles for 16 h, and irradiated with either 0 Gy or 3 Gy. Cells were then plated and colonies counted. Data shows the average ± standard deviation, of triplicate experiments. Statistical data is shown as * p < .05, ** p < .01, *** p < .005.
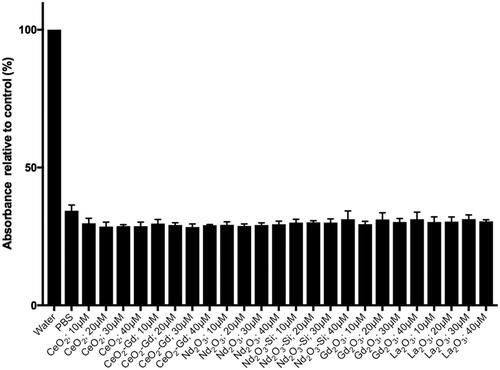
Haemotoxicity
Since there is the potential for RE nanoparticles to be administered intravenously, the degree of haemolysis induced by the nanoparticles was assessed (). Water is used as a control since the osmotic potential will cause the red blood cells to burst, releasing haemoglobin, which can be detected spectroscopically. None of the nanoparticles tested showed any significant degree of haemolysis, indicating the biocompatibility of the particles in blood.
Discussion
Cytotoxicity of REs
This study has investigated the potential of five RE oxide NPs to be cytotoxic and radiosensitizing in U-87 MG and Mo59K, two different GBM cell lines. There were significant findings found in both aspects, predominantly within the U-87 MG cell line. This includes a cytotoxic effect trend with Nd2O3 and La2O3 NPs and radiosensitizing effects associated with Gd2O3 and CeO2-Gd doped NPs in U-87 MG.
Significant cytotoxicity with RE oxide NPs was most evident in the U-87 MG cell line exposed to Nd2O3 and La2O3. It has long been postulated that the biological effects of rare earths may be due to their similar ionic radii to Ca++. This means that the RE elements could compete with Ca++ ions and interfere with the natural regulatory processes involving Ca++ ions [Citation20]. The propensity of certain RE elements to interfere in such processes may be connected to the ‘lanthanide contraction’. This relates to the fact that higher atomic number lanthanides have smaller ionic radii. This is due to the increase in nuclear charge which is not completely screened by the additional 4f electrons in the series of lanthanides. Consequently the increased effective charge draws in the electrons closer to the nucleus and, therefore, results in a smaller radius. This also accounts for the decreased basicity (see Results section).
Indeed, both Nd3+ and La3+ which had the most cytotoxic effect in both cell lines () have the closest ionic radii size to Ca2+ amongst the RE oxide NPs tested (). It is worth noting that toxic effects which have been commonly observed in other studies are for RE element concentrations between 100 nM to 100 μM [Citation20], a similar range to that used in this study. Previous studies investigating the use of rare earth chlorides found that lanthanum chloride, cerium chloride and mixed rare earth chlorides were able to inhibit the growth, morphology and microtubule structure of PAMC82 (human gastric cancer cell line) at concentrations between 0.5 and 1.5 mM [Citation8]. A similar study in melanoma cells showed that growth rates were significantly slowed by 1 mM concentrations of the rare earth elements La3+, Ce3+, Nd3+, Sm3+, Gd3+ and Yb3+ [2Citation1]. Lanthanum chloride and cerium chloride have also been shown to inhibit the leukaemic cells lines HL-60 and NB4. The anti-tumour activity of the rare earth elements appears to include changes to the cell membrane fluidity, permeability, ATP activity, intracellular and extracellular ion-exchange, cell mitosis and DNA synthesis; all of which affect the function of tumour tissues and cells [Citation22].
Radiosensitization using REs
Due to their unique electronic configuration there is the potential for rare earth elements to play a role in enhancing radiotherapy. Brain cancers have a particularly bleak prognosis and so we investigated the possibility that rare earth elements could act as radiosensitizers in our model cell lines. The lines that we chose were Mo59K and U-87 MG. The radiosensitivity of the lines, in comparison to other commonly used cell lines, is indicated in which shows the surviving fraction following a single 2 Gy dose of radiation (SF2) by clonogenic assay. The Mo59K is, therefore, defined as being ‘radioresistant’, whereas U-87 MG is ‘very radioresistant’. Our results also showed U-87 MG to be more radioresistant than Mo59K under the conditions tested.
Significant radiosensitization was observed in U-87 MG when incubated with Gd2O3, CeO2-Gd and Nd2O3:Si (). Importantly, these elements did not cause any intrinsic toxicity in the absence of irradiation and so could be considered biocompatible. A similar study by Mowat et al. showed a radiosensitizing effect with Gd2O3-based NP in U-87 MG by demonstrating a significant decrease in cell survival in the presence of NPs using the MTT assay following irradiation with 6 MV x-rays (similar to those used in routine X-ray therapy) in the presence of NPs [Citation23]. In addition, Le Duc et al. [Citation7] demonstrated significantly longer survival times in GBM 9LGS-inoculated mice after radiation with Gd2O3-based NPs, using a synchrotron produced white spectrum of photons ranging from 50 to 350 keV (mean energy of 90 keV).
The fact that CeO2-Gd doped NPs were radiosensitizing whilst CeO2 were not indicates that the effect is due to the gadolinium dopant, rather than the Ce++ core. In a similar fashion, it was shown that the Nd2O3 nanoparticles were cytotoxic, whereas the silica coating on the particles prevented toxicity, yet were still radiosensitizing. This may be connected to the findings of Li et al. [Citation24] who showed that rare earth oxide nanoparticles release the RE ions and are rapidly bound by free phosphates which results in the crystallization of REPO4 deposits on the particle surface. This leads to a morphological change and ‘urchin-shaped’ particles. Once all of the free phosphates have been depleted then the RE ions are capable of stripping phosphates from membranes leading to organelle damage. It is, therefore, possible that the silica coating is preventing such events in the case of the Nd2O3 nanoparticles.
In contrast to the effects seen in U-87 MG cells, no radiosensitization was seen in Mo59K from cell counts after 24 h. However, clonogenic assays which indicate the ability of a cell to divide over several generations, demonstrated that in a similar fashion to that seen in , the Mo59K cells were radiosensitized by incubation with Gd2O3, Ce2O3-Gd and Nd2O3:Si. The differences seen between the two cell lines may be a consequence of the different growth rates of the cell lines (), genotypic differences and potential differences in the ability of the different cells to internalize the nanoparticles.
Reduction of cell migration using RE elements
Rare earth elements may also have the potential to control cell migration. Our experiments showed that in U-87 MG cells, Nd2O3:Si and Gd2O3 prevented cell regrowth after a ‘scratch test’ and irradiation, and were biocompatible. In Mo59K cells Ce2O3, Nd2O3 and Gd2O3 stopped invasion into the cell free area in the absence of irradiation, but none of the elements tested in this cell line showed a further decrease in cell growth after irradiation. A previous study investigating nanoparticulate Nd2O3 showed S-phase arrest in NCI-H460 cells [Citation18]. Such cell cycle arrest could explain the prevention of regrowth in our experimental system. The restriction of regrowth may be important with respect to a cells ability to metastasise.
In normal cells, metastasis is prevented by the death of cells which become detached from the extracellular matrix; a process known as anoikis. However, metastatic cells are able to evade the process of anoikis which would result in programmed cell death, and are able to invade other tissues and form secondary tumours. Previously, rare earth elements have been shown to induce anoikis in cancer cells [Citation25]. The presence of low concentrations of lanthanum citrate (0.001 to 0.1 mmol/l) could induce anoikis in HeLa cells after 48 h of treatment.
Generation of additional ROS in the presence of RE nanoparticles
The use of radiation therapy to target cancer cells works primarily as a result of radiation induced DNA damage, as a result of both direct interaction with DNA and importantly indirectly through reactions with short-lived local radical oxygen species (ROS) generated by water radiolysis in the surrounding water. X-rays interact within the body resulting in the production of fast electrons and it is via these electrons that energy is deposited within the cells traversed. This is in the form of highly structured tracks of ionisation and excitation events as they lose energy and slow down, which result in an increase in the probability of producing correlated damage (e.g. DNA double-strand breaks) on DNA and other macromolecules. In addition to the generation of short-lived ROS (such as superoxide anion O2−, hydrogen peroxide H2O2 and hydroxyl radical HO●) resulting from these ionisation and excitation events, ionising radiation can also result in the modulation of the background level of ROS production which may continue for days and months following irradiation as a result of perturbation of intracellular and intercellular signalling [Citation26, for review see Ref. Citation27]. The main weakness of conventional radiation therapy is the lack of cancer specificity and control for sensitivity. If the amount of energy deposited at the tumour site could be enhanced by a radiation dose-enhancement mechanism, less damaging amounts of radiation would be needed to pass through healthy tissue. Our data shows that in terms of ROS generation the most effective nanoparticles were Gd2O3 and CeO2-Gd. This is particularly interesting since CeO2 has long been postulated as a radioprotectant. However, a study by Briggs et al. [Citation28] showed that whether the CeO2 acted as a radioprotectant or a radiosensitizer depends upon the energy of the incident irradiation. The generation of ROS as a consequence of incubation with RE nanoparticles may be due to the Auger effect, whereby there is a release of low-energy electrons from outer-shells to replenish inner-shells ionised by radiation leading to a series of additional energy emissions to that of the initial radiation dose and associated increase in biological efficiency for a given dose [Citation14,Citation29].
ROS can be an effective control mechanism for cancer treatment since noncancerous cells possess a greater antioxidant reserve that allows them to tolerate higher levels of ROS compared to cancerous cells [Citation30]. As can be seen in this study, the Gd2O3 and CeO2-Gd nanoparticles which were effective in generating ROS in U87 upon irradiation were also seen to cause cell death ().
These studies along with many other studies on nanoparticles have been performed using orthovoltage x-ray energies (max energy less than 500 keV). At these energies the photoelectric cross section scales approximately proportional to (Z/E)3, where Z is the atomic number and E is the energy of the incoming photon for high Z materials. These result in a substantially higher interaction cross section than that of soft tissue. However, clinical radiotherapy is usually delivered using MV X-rays; at these energies the photoelectric effect will no longer dominate and the interaction cross section of high Z material compared to soft tissue decreases at high energy and, therefore, the resulting dose enhancement is significantly reduced. However, despite the significantly reduced interaction cross section of these nanoparticles, experiments using clinical MV x-rays have still demonstrated a radiosensitization effect (e.g. Butterworth et al. [Citation31]) which is believed to be a result of increased oxidative stress
Autophagy and haemotoxicity
While both our study and others show that RE elements can inhibit proliferation and induce apoptosis in selected cancer cell lines (see review [Citation32]) more recently RE oxides have also been postulated to be autophagy inducers. We, therefore, tested our cell lines against the selected RE elements. The Mo59K cell line showed no significant autophagy with any of the RE elements tested. Nd2O3 nanoparticles, however, showed statistically significant increases in autophagy in the U-87 MG cell line. Chen et al. [Citation18] also found that Nd2O3 nanoparticles induced autophagic cell death, and massive vacuolization in NCI-H460 cells. Previously Man et al. [Citation12] had looked at Gd2O3 and Tb2O3 (heavy REE) and Sm2O3 and Eu2O3 (light REE) as autophagy inducers in HeLa cells. They observed autophagic structures and large transparent vacuoles by TEM. The use of autophagy inducers is particularly interesting for cancer therapies since normal cells, unlike the rapidly growing cancer cells, are expected to be less sensitive because of lower metabolic demands, and the normal activity of regulators such as PI3K and Akt.
While it is desirable for the nanoparticles to be cytotoxic or radiosensitizing, it would be important for intravenously applied drugs to be compatible with the components of blood. Our study indicated that none of the rare earth nanoparticles tested were haemolytic.
Differences in response based upon cell type
It was very obvious from our study that the Mo59K and U-87 MG cells responded very differently to exposure to the rare earth nanoparticles. The Mo59K glial cell line was isolated from a 33-year-old male patient with untreated GBM. The modal Chromosome Number is 75 (with a range of 65 to 79) and a polyploidy Rate of 22% (Number of cells examined = 59). The Mo59K is wild-type for p53 and proficient in DNA-PKCS which means that it is able to effect double strand break repairs (unlike its isogenic line Mo59J which is deficient in DNA-PKCS) [Citation33].
The U-87 MG cell line was derived from a stage four 44-year-old GBM patient. This is a hypodiploid cell line with a modal chromosome number of 44 occurring in 48% of cells, and is wild type for p53 expression [Citation16]. Both Mo59K and U-87 MG have been shown to express high levels of activated DNA-PKcs (i.e. p- DNA-PKcs) in contrast to both normal human astrocyte cells and also the glioma cell lines A172 and H4 [Citation17].
Mo59K cells were shown to be more radiosensitive than U-87 MG, but also slower growing. However, the Mo59k cell line showed no increased ROS after irradiation either in the presence or absence of RE nanoparticles, and very little effect on cell death rates (). This is contrast to that seen in U-87 MG which clearly shows that RE nanoparticles could be beneficial in either a cytotoxic or radiosensitizing manner. Thus, while RE nanoparticles have the potential to be a potent method of attack against GBM, it appears that the specific genotype may be important in the success of this treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Victor M. Lu
Victor M. Lu is a PhD candidate at the University of New South Wales. His focus is on neuro-oncology and paediatrics.
Felicity Crawshay-Williams
Felicity Crawshay-Williams is studying for an MChem at Bath University, with a placement year at Oxford University.
Benjamin White
Benjamin White studied for an MChem at Bath University and is a DPhil candidate at Oxford University.
Amy Elliot
Amy Elliot is a research assistant at the CRUK/MRC Oxford Institute for Radiation Oncology within the Department of Oncology.
Mark A. Hill
Mark A. Hill has been Head of the Radiation Biophysics Core at the CRUK/MRC Oxford Institute for Radiation Oncology within the Department of Oncology since 2008, following the move of his research group from the MRC Radiation and Genome Stability Unit.
Helen E. Townley
Helen E. Townley is a University Research Lecturer and Williams Fund Fellow in the Nuffield Department of Women’s and Reproductive Health, and a Senior visiting Fellow in the Department of Engineering, Oxford University.
References
- Stupp R, Mason WP, van den Bent MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996.
- Bleeker FE, Molenaar RJ, Leenstra S. Recent advances in the molecular understanding of glioblastoma. J Neurooncol. 2012;108:11–27.
- Young RM, Jamshidi A, Davis G, et al. Current trends in the surgical management and treatment of adult glioblastoma. Ann Transl Med. 2015;3:121.
- Brastianos PK, Nayyar N, Rosebrock D. Resolving the phylogenetic origin of glioblastoma via multifocal genomic analysis of pre-treatment and treatment-resistant autopsy specimens. Precis Oncol. 2017;1:33.
- Rao KS, Reddy MK, Horning JL, et al. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29:4429–4438.
- Shilo M, Motiei M, Hana P, et al. Transport of nanoparticles through the blood–brain barrier for imaging and therapeutic applications. Nanoscale. 2014;6:2146–2152.
- Le Duc G, Miladi I, Alric C. Toward an image-guided microbeam radiation therapy using gadolinium-based nanoparticles. ACS Nano. 2011;5:9566–9574.
- Ji Y, Xiao B, Wang Z, et al. The suppression effect of light rare earth elements on proliferation of two cancer cell lines. Biomed Environ Sci. 2000;13:287–292.
- Shi P, Huang Z. Proteomic detection of changes in protein synthesis induced by lanthanum in BGC-823 human gastric cancer cells. Biometals. 2005;18:89–95.
- An Y, Li R, Wang K. Effects of rare earth elements on proliferation of Human Hepatoma Cell SMMC-7721. J Chinese Rare Earth Soc. 2005;23:105–108.
- Liu H, Yang X, Wang K. Effects of lanthanide ions (La3+, Gd3+ and Yb3+) on growth of human normal liver line 7701 and human cervical carcinoma and the effect of cell apoptosis induced by lanthanide. J Chinese Rare Earth Soc. 2006;24:484–488.
- Man N, Yu L, Yu S-H, et al. Rare earth oxide nanocrystals as a new class of autophagy inducers. Autophagy. 2010;6:310–311.
- Carr DH, Brown J, Bydder GM, et al. Gadolinium-DTPA as a contrast agent in MRI: initial clinical experience in 20 patients. AJR Am J Roentgenol. 1984;143:215–224.
- Kobayashi K, Usami N, Porcel E, et al. Enhancement of radiation effect by heavy elements. Mutat Res. 2010;704:123–131.
- Assanga SB, Gil SAA, Lewis LL, et al. Cell growth curves for different cell lines and their relationship with biological activities. Int J Biotechnol Mol Biol Res. 2013;4:60–70.
- Badie B, Goh CS, Klaver J, et al. Combined radiation and p53 gene therapy of malignant glioma cells. Cancer Gene Ther. 1999;6:155–162.
- Lan T, Zhao Z, Qu Y, et al. Targeting hyperactivated DNA-PKcs by KU0060648 inhibits glioma progression and enhances temozolomide therapy via suppression of AKT signaling. Oncotarget. 2016;7:55555–55571.
- Chen Y, Yang L, Feng C, et al. Nano neodymium oxide induces massive vacuolization and autophagic cell death in non-small cell lung cancer NCI-H460 cells. Biochem Biophys Res Commun. 2005;337:52–60.
- Biederbick A, Kern HF, Elsässer HP. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol. 1995;66:3–14.
- Pałasz A, Czekaj P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim Pol. 2000;47:1107–1114.
- Sato T, Hashizume M, Hotta Y, et al. Morphology and proliferation of B16 melanoma cells in the presence of lanthanoid and Al3+ ions. Biometals. 1998;11:107–112.
- Dai Y, Li J, Li J, et al. Effects of rare earth compounds on growth and apoptosis of leukemic cell lines. In Vitro Cell Dev Biol Anim. 2002;38:373–375.
- Mowat P, Mignot A, Rima W, et al. In vitro radiosensitizing effects of ultrasmall gadolinium based particles on tumour cells 2011. J Nanosci Nanotechnol. 2011;11:7833. 9
- Li R, Ji Z, Chang CH, et al. Surface interactions with compartmentalized cellular phosphates explain rare earth oxide nanoparticle hazard and provide opportunities for safer design. ACS Nano. 2014;8:1771–1783.
- Su X, Zheng X, Ni J. Lanthanum citrate induces anoikis of Hela cells. Cancer Lett. 2009;285:200–209.
- Portess D, Bauer G, Hill MA, et al. Low dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Cancer Res. 2007;67:1246–1251.
- Azzam EI, Jay-Gerin J-P, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60.
- Briggs A, Corde S, Oktaria S, et al. Cerium oxide nanoparticles: influence of the high-Z component revealed on radioresistant 9L cell survival under X-ray irradiation. Nanomedicine. 2013;9:1098–1105.
- Hill MA. The variation in biological effectiveness of X-rays and gamma rays with energy. Radiat Prot Dosimetry. 2004;112:471–481.
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591.
- Butterworth KT, McMahon SJ, Taggart LE, et al. Radiosensitization by gold nanoparticles: effective at megavoltage energies and potential role of oxidative stress. Transl Cancer Res. 2013;2:269–279.
- Townley HE. Applications of the rare earth elements in cancer imaging and therapy. Cnano. 2013;9:686–691.
- Allalunis-Turner MJ, Zia PK, Barron GM, et al. Radiation-induced DNA damage and repair in cells of a radiosensitive human malignant glioma cell line. Radiat Res.1995;144:288–293.
- Prithivirajsingh S, Story M, Bergh S, et al. Accumulation of common mitochondrial DNA deletion induced by ionizing radiation. FEBS Lett. 2004;571:227–232.
- Hall J, Iype R, Senra J, et al. Investigation of radiosensitivity gene signatures in cancer cell lines. PLoS ONE. 2014;9(1):e86329.
- Ader I, Delmas C, Skuli N, et al. Preclinical evidence that SSR128129E–a novel small-molecule multi-fibroblast growth factor receptor blocker–radiosensitises human glioblastoma. Eur J Cancer. 2014;50:2351–2359.