?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
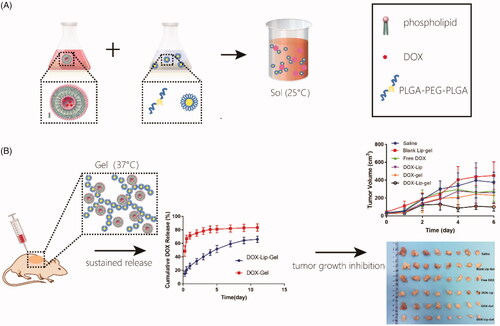
The aim of this research is to utilize a hybrid system of liposomal doxorubicin (DOX-Lip) loaded thermogel (DOX-Lip-Gel) to realize the steady sustained delivery of doxorubicin (DOX), a small hydrophilic drug, for the treatment of breast cancer locally. Herein, liposomal doxorubicin was prepared via the traditional film dispersion method with the particle size of 75 nm and drug entrapment efficiency of 86%. And, the triblock copolymer of poly (D, L-lactide-co-glycolide)-b-poly (ethylene glycol)-b-poly (D, L-lactide -co-glycolide) (PLGA-PEG-PLGA) was synthesized via ring-opening polymerization to prepare the thermosensitive hydrogel through dissolving the polymers in DOX-Lip solution. The liposome loaded hydrogel was in a sol state at room temperature and converted into the gel state at body temperature and would degrade gradually during the time in vivo. The drug release of DOX out of DOX-Lip-Gel could be in a steady sustained manner up to 11 days without significant burst release as compared to that of DOX-loaded hydrogel (DOX-Gel). An orthotopic breast cancer model was adopted to evaluate the in vivo antitumor efficacy. And, the results revealed DOX-Lip-Gel had better antitumor efficiency as well as lower side effects.
Introduction
Breast cancer is positioned as the first among all cancer diseases identified in females. And, a lot of research is conducted every year to discover proper medication method [Citation1,Citation2]. For now, in clinic, nearly all females who are diagnosed with breast tumour will adopt the lumpectomy surgery to get rid of the maximum amount of cancerous tissues. And then, chemotherapy or local radiotherapy is utilized to decrease the possibilities of tumour metastasis [Citation3].
Doxorubicin (DOX) is a class of anthracycline antibiotic and is regarded as the first-line antitumor drug for the treatment of breast cancer. However, its clinical application is restrained for its severe dose-dependent systematic toxicity, such as cardiotoxicity, which is usually caused by the poor targeting effect of free antitumor drugs in tumour tissues. Thus, the novel DOX delivery system of liposomal doxorubicin (Doxil®) was approved by FDA for its higher tumour accumulation via EPR effects and longer blood circulation time [Citation4,Citation5]. But, the fact is even with Doxil®, the drug accumulation in the tumour site is only less than 1% [Citation6,Citation7], which is far from satisfaction.
The local drug delivery system gives the chance to enhance the effectiveness and safety of cancer therapy due to its ability to enable the drug at the target site to the largest extent. A polymer-based hydrogel encapsulated with anticancer drugs can be placed beside an intended tumour and produce adequate drug concentration at a localized level [Citation8,Citation9]. Severe side effects due to the high concentration of drug will be reduced since the drug is administered topically instead of intravenous administration [Citation10]. And, the injectable and biodegradable hydrogels have pulled in large interests in practical use for its injectability and biodegradation [Citation11–13]. Meanwhile, both dose-dependent and time-dependent action could be realized by this kind of hydrogel [Citation14,Citation15]. Among these, the amphiphilic triblock copolymer of poly(D,L-lactide-co-glycolide)-b-poly(ethylene glycol)-b-poly(D,L-lactide-co-glycolide) (PLGA–PEG–PLGA) has been used to prepare the thermosensitive hydrogel formulations [Citation16–18]. For its good biocompatibility and biodegradability, the PLGA-PEG-PLGA based thermogel has attracted great consideration for their potentials in medical use [Citation19,Citation20]. It is a sol at room temperature and will convert to a gel at body temperature, which enabled its injectability and has been adopted as the sustained release matrix of a variety of drugs [Citation21,Citation22]. However, it is well known that for this kind of hydrogel, it is suitable for the sustained release of small hydrophobic drugs and macromolecular drugs [Citation23,Citation24]. And for the sustained release of DOX, which is a small hydrophilic molecule, the hydrogels are usually not appropriate because it contains a huge amount of water as well as large pores. And, the small molecular drug would be released quickly due to its diffusion release mechanism, which means huge burst release in the initial stage [Citation25–27]. Thus, how to achieve steady sustained release profile of small hydrophilic drugs out of the hydrogels is still worth further research.
Liposomes are familiar for its capability to entrap hydrophilic compound inside their aqueous core and also hydrophobic compound inside their bilayer membrane. The entrapped compound will tend to pass out from the bilayer into the outer liquid gradually, in this way, enabling liposomes to act as a suitable delivery dosage form for prolonged release by integrating with hydrogel. In the hybrid system of liposomes loaded hydrogel, the liposomes and hydrogel serve as the first and second barrier for the drug release, lowering the burst release and enabling steady sustained release.
In this work, we assumed that designing of hybrid drug delivery system based on encapsulation of DOX-loaded liposome into hydrogel matrix (DOX-Lip-Gel) can realize steady sustained drug release, improve the anticancer efficacy through localized therapy and manage to reduce the toxic side effects (Scheme 1).
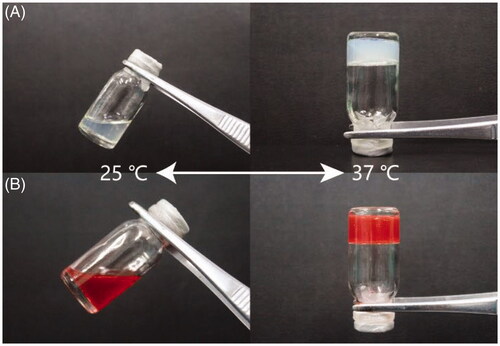
Scheme 1 (a) PLGA-PEG-PLGA copolymer was dissolved into DOX liposomes suspension to form DOX-Lip-Gel, which was in a sol state at 25 °C. (b) DOX-Lip-Gel solution was injected peritumorally and transformed into a solid gel in situ at body temperature (37 °C), enabling the sustained release of DOX and significant inhibition of tumour growth.
Materials and methods
Materials
Soybean phosphatidylcholine (SPC) was bought from Lipoid Corporation (Ludwigshafen am Rhein, Germany) and cholesterol (CHOL) was purchased from Aladdin Reagent Co., Ltd (Shanghai, China). Doxorubicin hydrochloride (DOX) was given by Zhejiang Hisun Pharmaceutical Co., Ltd. (Zhejiang, China). PEG (average MW 1500) was bought from Sigma Aldrich. D, L-lactide (LA) and glycolide (GA) were purchased from Purac. All the other reagents were analytical grade.
Cell lines and animals
Murine breast cancer cell line 4T1 was bought from KeyGen Biotech Co. Ltd (Nanjing, China). Cells were cultured in RPMI 1640 medium containing 1% antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) along with 10% fetal bovine serum (FBS, BI) at 37 °C in a 5% CO2 atmosphere.
The BALB/c mice (female, 20–22 g) of 6–8 weeks old were purchased from Comparative Medicine Center of Yangzhou University. All animals were held in the controlled condition. Standard laboratory water and food were provided as a feeding material. All animal experiments were performed in accordance with the guidance for the Care and Use of Laboratory Animals issued by China Pharmaceutical University.
Synthesis and characterization of the copolymer
PLGA-PEG-PLGA copolymer was synthesized via the open-ring polymerization. Briefly, 30 g PEG was firstly dehydrated via vacuum at 120 °C for 12 h. Then, 50.45 g LA and 13.55 g GA were added and dehydrated for another half an hour after cooling down to 80 °C. 0.1 g stannous octoate was added as a catalyst. After reaction at 150 °C for 12 h, the product was purified by washing in 80 °C hot water twice. The product was freeze-dried and stored at −20 °C before use.
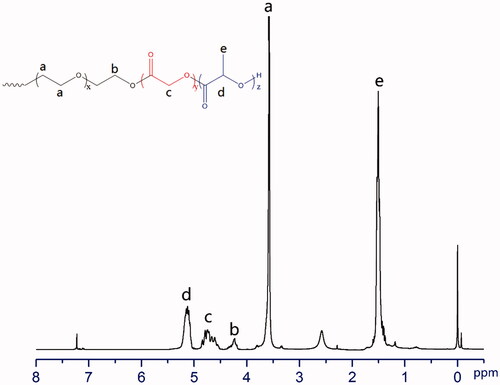
The PLGA-PEG-PLGA triblock copolymers were analyzed using 1H NMR to find out the structure and composition. All the data were noted at 300 MHz on a BRUKER AV-300 spectrometer at 25 °C using deuterated chloroform (CDCl3) as a solvent and tetramethyl silane (TMS) as an internal standard [Citation28]. The typical chemical shift: δ (ppm) 5.143 (1H, –C(=O)CH(CH3)O–), 4.743 (2H, –C(=O)CH2 O–), 3.577 (4H, –CH2CH2O–), 1.505 (3H, –COCH(CH3)O–). The molecular weight of LA and GA was calculated via the ratio between peak area of δ (ppm) 5.143 and δ (ppm) 4.743.
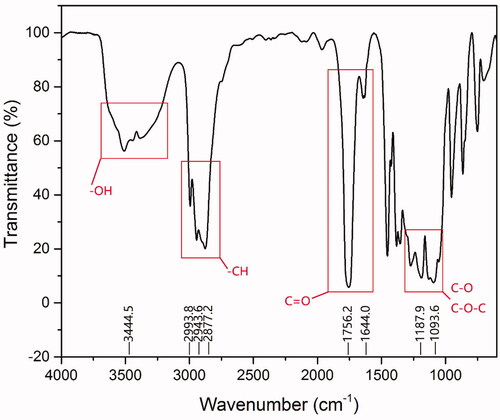
The characterization of the functional groups of PLGA-PEG-PLGA triblock copolymers was carried out by Fourier transform infrared (FT-IR) analysis using Perklin Elmer 2400 II (USA) FTIR spectroscopy. The FTIR spectrum was gained by spreading PLGA-PEG-PLGA on the potassium bromide tablet.
Preparation and characterization of liposomes
Liposomes were prepared by the thin film dispersion technique. The ammonium sulfate gradient technique was utilized for the preparing of doxorubicin-loaded liposomes (DOX-Lip) [Citation29,Citation30]. In brief, SPC and CHOL (10:1 ratio) were kept in round bottom flask and dissolved in dichloromethane followed by using rotary evaporator (RE52cs, Yali-wing Biochemical Instrument Factory, Shanghai China) to dry and make a thin lipid film. Afterwards, the dried film was hydrated with 4 ml ammonium sulfate (300 mM) at 37 °C for 1 h. The lipid dispersion was sonicated at 400 W for 16 min (4 s working and 2 s rest times) with an ultrasound probe (JY92 ultrasonic cell crusher, Xinzhi Institute of Scientific Instruments, Ningbo, China). The free ammonium sulfate was separated by the utilization of dialysis bag (MWCO 14,000 D) against 10% w/v sucrose solution for 12 h.
The liposomes preparation was kept in a refrigerator at 4 °C. Then, 2 mg/mL DOX solution was mixed with liposomal suspension as per drug-to-lipid ratio of 1/10 (w/w) and incubated at 50 °C for 1 h. Free DOX was removed from liposomes by the utilization dialysis bag (MWCO 2000 D) against purified water for 3 h.
Liposomes were dissolved in 10% Triton X-100 for measurement of DOX content by UV-Vis spectrophotometry at 480 nm. The entrapment efficiency (EE) was calculated as EquationEquation (1)(1)
(1) .
(1)
(1)
Preparation of blank hydrogel (Blank gel)
The hydrogel solution was made by dissolving triblock copolymer (PLGA-PEG-PLGA, 20 wt%) into the purified water following simple mixing technique with the help of magnetic stirrers under ice-water bath until a clear solution was formed [Citation31,Citation32].
Preparation of injectable liposomal hydrogel solution
Blank liposome in the blank hydrogel (Blank Lip-Gel) and DOX-Lip-Gel were obtained by using simple mixing technique. Briefly, the measured quantity of PLGA-PEG-PLGA copolymer (20 wt%) was dissolved into blank liposomes or DOX liposomes suspension with stirring at 4 °C till polymer was completely dissolved to become a clear solution [Citation33]. Subsequently, prepared hydrogel solution having drug encapsulated liposomes were kept at 4 °C [Citation34]. After that, hydrogel solution was taken into 1 ml of disposable syringe and pressed it through a 25-gauge needle to examine the injectability of gel solution at room temperature (25 °C) [Citation35].
Evaluation of particle size and zeta potential
The particle size and zeta potential of DOX-Lip were measured by dynamic laser-light scattering instrument (Brookhaven Instruments, USA). The hydrogel sample was diluted to 1 wt % with double distilled water before the measurement.
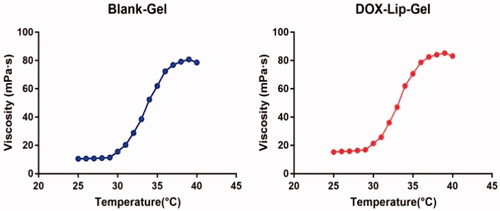
Rheological test
Rheological analysis of blank gel and DOX-Lip-Gel solution were completed by the utilization of Brookfield DV-III ultra-programmable rheometer (Brookfield Engineering Laboratories, USA) which was assembled with the CP-18 spindle. The temperature was gradually raised from 25 °C to 40 °C at 1 °C/min to determine the viscosity with respect to temperature.
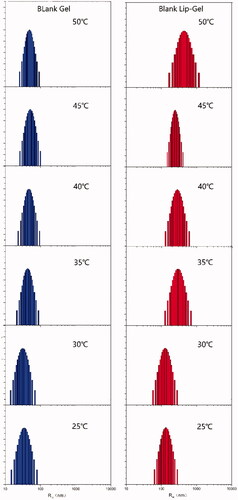
Particle size distribution
The particle size and size distribution of blank gel and blank Lip-Gel (20 wt%) were measured by dynamic light scattering instrument at a temperature from 25 °C to 50 °C. First, the particle size of the gel sample was diluted into 0.5 w/w% with purified water and filtered through the 0.45 μm filter membrane. Then kept the sample in a water bath for 10 min by setting required temperature before the measurements.
Thermosensitive evaluation of DOX-Lip-Gel
The gelling time and temperature of blank Lip-Gel and DOX-Lip-Gel were measured through the tube inverting technique [Citation36]. In brief, 3 ml sample from each group was kept in a little glass bottle having rubber caps. Then, placed these bottles in a water bath and increased the temperature gradually from 25 °C to 47 °C. At every temperature level, the gel sample was equilibrated for 10 min. After that, the flowability of each gel was monitored by inverting the tubes. The gelling point was measured by flowing or non-flowing principle over 30 s. The temperature point at which the gel solution became immovable was noted as gelling temperature.
Formation and degradation of gel in vivo
ICR female mice (23–25 g) were utilized for this experiment. Then, 0.8 ml of PLGA-PEG-PLGA copolymer solution (20 wt%) was subcutaneously injected into the back side of the neck. After predetermined time, the mice were sacrificed to examine the gel formation and its degradation.
Biocompatibility of gel in vivo
0.5 ml of PLGA-PEG-PLGA copolymer solution (20 wt%) was subcutaneously injected into the back side of BALB/c mice (female, 20–22 g). The rats were sacrificed at the predetermined time points (3 and 7 days). Then, Gel-located skin was collected and fixed in a 4.0% (W/V) paraformaldehyde solution. The skin slices were stained by hematoxylin and eosin (HE) for pathology analyses.
In vitro drug release
The in vitro drug release from DOX-Lip-Gel and DOX-loaded hydrogel (DOX-Gel) were determined through the tube technique. 0.5 ml of each hydrogel sample solution was precisely added into 30 ml tube and placed at 37 °C for 10 min to convert to solid gel. Afterwards, 30 ml of phosphate buffer saline (PBS, pH 7.4) as a release medium was added into each gel formulation, and these samples were kept in an air bath incubator at 37 °C by setting shake rate 100 rpm. At fixed time, 20 ml of released medium was taken out and same amount of fresh release medium was added. The concentration of DOX in the release medium was determined by fluorescence measurement (λex = 494 nm and λem = 554 nm). Each sample was repeated in triplicate (n = 3) to achieve the better reproducible results [Citation37]. The cumulative release of DOX was determined from the standard curve. All the experiment was performed by protecting from light.
In vivo antitumor activity
The in vivo antitumor efficacy was estimated by utilizing orthotopic 4T1 breast cancer models. In this model, a low dose (5 × 105 cells) of 4T1 cells was injected into female BALB/c (6–8 weeks old, 20–22 g) mouse mammary fat pad [Citation38]. Treatment was started once the tumour reached 60–120 mm3. Afterwards, tumour-bearing mice were weight and randomly distributed into six groups (n = 6) of normal saline as a negative control group, free DOX, DOX-Lip, DOX-Gel, blank Lip-Gel and DOX-Lip-Gel. The time was denoted as Day 0. Then, 0.2 ml of DOX solution (5 mg/kg), DOX-Lip (5 mg/kg) were administrated intravenously every second day for 6 times. And 0.2 ml of DOX-Lip-Gel (15 mg/kg), DOX-Gel (15 mg/kg) and blank Lip-Gel were administrated peritumorally on Day 0 and Day 7. Under the hygienic conditions, the length and width of the tumour and body weights of mice were measured every two days. All mice were sacrificed on Day 13. The body weight was observed to assess the toxicity of treatment.
The evaluation of antitumor efficacy based on the results of the tumour volume, relative tumour volume (RTV), relative tumour growth rate (T/C %), the tumour growth inhibition rate (TIR) and body weight respectively. Tumour Volume (V) was calculated as , where a is the longest diameter of the tumour, b is the shortest diameter of the tumour. Relative Tumor Volume (RTV) was calculated as
, where
is the measured tumour volume at the first administration (Day 0), and
is the tumour volume measured on Day n. Relative Tumor Growth Rate
, where
is RTV value in the treatment group;
is RTV value in the negative control group. Tumour growth inhibition rate (TIR %) was calculated on the basis of the weight of the solid tumour.
, where
for the control group tumour weight,
for the treatment group tumour weight.
Histological analysis
Animals were sacrificed after the cycle of therapy. The solid tumours were rapidly taken out from the mice body under sterile environment, cleaned with PBS and measured their weight. Afterwards, all these solid tumours were dipped in 10% formalin for 24 h at room temperature, regularly dried out and at last enclosed by paraffin. Tumour slices were stained by HE to investigate the histological features.
Statistical analysis
All the data were represented as the mean ± SD. Student’s t-test was used for the analysis of statistical significance. A p values <0.05 was considered to be significant.
Results
1H NMR analysis
PLGA-PEG-PLGA was synthesized via the ring-opening polymerization of lactide (LA) and glycolide (GA) initiated by the hydroxyl-terminated PEG. The chemical structure and composition of the synthesized copolymers were measured using 1H NMR spectrometry. The results were summarized in and . The characteristic peaks from 1H NMR at 1.505, 3.577, 4.743 and 5.143 ppm were used to determine the average MW of 1650(PLGA)–1500(PEG)–1650(PLGA) and LA/GA molar ratio of 2.9/1. A spectrum of PLGA-PEG-PLGA copolymer with its chemical structure was presented in .
Table 1. Chemical structure and chemical shift of PLGA-PEG-PLGA determined 1H NMR.
Table 2. Molecular parameters of the synthesized copolymer.
FT-IR analysis
PLGA-PEG-PLGA copolymer was successfully synthesized as evidenced by . The absorption peak at 3444.5 cm−1 was assigned to terminal hydroxyl groups of PLGA-PEG-PLGA. The band at 2993.8, 2943.6 and 2877.2 cm−1 attributed to the C-H stretches. The absorption peaks at 1756.2 and 1644.0 cm−1 were assigned to the characteristic of C = O group, indicated that polymerization of LA and GA initiated by the end hydroxy groups of PEG. Bands at 1187.9 and 1093.6 cm−1 due to C–O and C–O–C stretches.
Characterization of liposomes and thermogel
The entrapment efficiency of DOX-Lip was up to 86% with the particle size of 74.6 nm. The particle size and size distribution of blank gel and blank Lip-Gel were determined by dynamic light scattering. The particle size of blank gel was increased with the rising temperature, indicating the aggregation of micelles. Compared with blank gel, the particle size of blank Lip-Gel had a tendency of turning into a larger size along with the rise of the temperature. Hence, it presumed that there are some interactions between liposomes and polymeric micelles which made micelles easier to form aggregation, as shown in .
Thermosensitive characterization of thermogel
Viscosity
The sol-gel transition of blank Lip-Gel and DOX-Lip-Gel corresponding to the rise of temperature was shown in . Both blank Lip-Gel and DOX-Lip-Gel showed less viscosity below 30 °C which enabled the injectability of drug-loaded hydrogel formulation at room temperature. Though, once the temperature raised up to 30 °C, the viscosity of gel started to increase sharply, indicating the transition from sol to gel.
Gelation temperature of thermogel
The gel-forming nature of PLGA-PEG-PLGA based hydrogel was shown in . Both blank Lip-Gel and DOX-Lip-Gel were in liquid form at room temperature and gradually converted into solid gel when kept in an incubator at 37 °C. When the temperature was rising, it initiated the generation of hydrophobic bonds between PLGA-block of the triblock polymer, resulting in the formation of a gel [Citation39]. Temperature-activated gel formation of amphiphilic PLGA-PEG-PLGA triblock copolymer was due to the micellar aggregation and this micelle aggregation was initiated by increasing the hydrophobic interaction among the PLGA portions [Citation40]. And, the result suggested the gel-forming nature of hydrogel was not affected by the presence of DOX encapsulated liposomes inside the hydrogel formulation.
Formation and degradation of thermogel in vivo
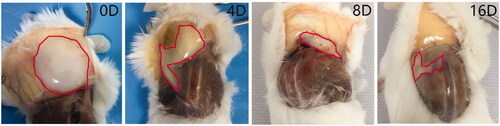
In vivo degradation of injected thermogel biomaterial is crucial for their biomedical utilization [Citation41]. Hence, the investigation of gel formation and degradation in vivo was performed by subcutaneous injection of the gel solution into the back of mice neck at room temperature. After the injection of PLGA-PEG-PLGA polymer solution into the mice, the gel was formed in situ at the subcutaneous layer due to the body temperature of mice and the formed gel could be felt by touching at the injection site. At predetermined time, the mice were sacrificed for further analysis of gel remaining. The amount of gel was reduced gradually with the past of time. After 16 days, most of the gel was degraded, as shown in .
Biocompatibility of gel in vivo
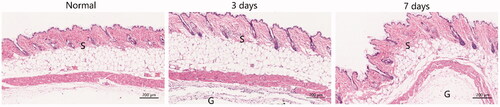
The local inflammatory response was evaluated by HE staining of 3 and 7 days after subcutaneous injection of the thermogel. As shown in , after 3 days of the gel injection, inflammatory cells and neutrophils were detected in the skin section when compared with the normal skin tissue, indicating the mild acute inflammatory response. After 7 days, as the gradual degradation of the hydrogel, the inflammation reduced markedly compared to the 3-day skin tissue. And, there was no necrosis in all the skin tissues. Above all, the results indicated that the good biocompatibility of gel in vivo.
In vitro DOX release
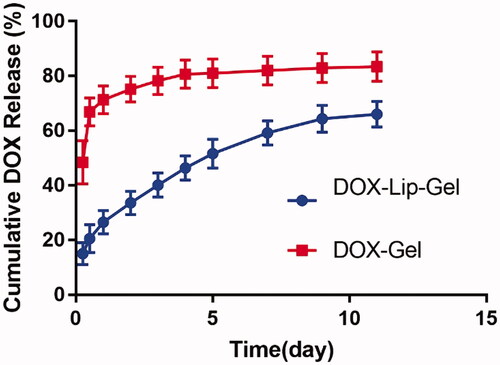
The drug release profile of DOX-Gel and DOX-Lip-Gel formulation was shown in . The drug release profile of DOX-Gel formulation demonstrated an initial high burst release. About 66% of DOX was released from hydrogel during 12 h. Whereas the DOX-Lip-Gel formulation demonstrated much lower initial drug release, only 20% of DOX was released during 12 h. Then, the drug could be gradually released during the releasing time. Prolonged release without a significant burst of the DOX was achieved up to 11 days and cumulative DOX release from DOX-Lip-Gel was 66%.
In vivo antitumor efficiency
The in vivo antitumor efficiency of DOX containing formulations was examined in orthotopic 4T1 breast tumour-bearing BALB/c female mice. The cycle of therapy was shown in , and the tumour volume was measured with the data presented in and the tumour inhibition effect of each group was presented in . The tumours of blank Lip-Gel groups increased quickly, which demonstrated that blank Lip-Gel group did not have tumour inhibition efficiency contrasted to normal saline. And each drug-containing group had a specific level of tumour inhibition efficiency as compared with negative control group. Tumour inhibition rate of free DOX, DOX-Lip, DOX-Gel and DOX-Lip-Gel groups were 56.2%, 59.7%, 75.9% and 86.5%, respectively. Hence, it was suggested that DOX is the main factor that delivered the antitumor activity. Although treatment with free DOX and DOX-Lip produced an inhibitory efficiency to some extent, tumour growth continued and increased to 493.4 ± 84 mm3 and 456.2 ± 84.3 mm3 at Day 13, respectively. Tumour volume of the sustained-release groups receiving DOX-Gel and DOX-Lip-Gel were much smaller than other groups, indicating that peritumoral administration had better anti-tumour efficacy. Among them, DOX-Lip-Gel group had the smallest tumour volume of 269.9 ± 61.7 mm3 (p < 0.01 vs other groups), indicating the superior tumour inhibition efficiency.
Figure 9 In vivo antitumor efficiency of different groups of saline, blank Lip-Gel, Free DOX, DOX-Lip, DOX-Gel and DOX-Lip-Gel on orthotopic tumor-bearing BABL/c mice model *p < 0.05, **p < 0.01, n = 6. (A) schematic of the time course of drug administration within the cycle of treatment; (B) tumor volume curves; (C) tumor weight of the different groups; (D) typical tumor images of all groups on Day 13; (E) body weight of different groups during the whole therapy.
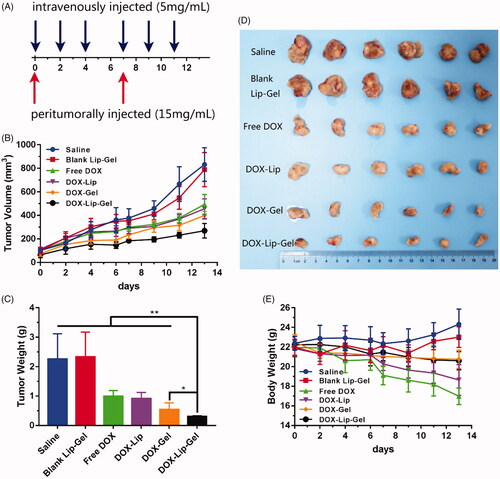
Table 3. Tumour inhibition parameters (n = 6).
Tumour-bearing mice of all groups were sacrificed on the Day 13. Then, the tumours were taken out and their weight was determined. As shown in , DOX-Lip-Gel group had much smaller tumour weight compared with DOX-Gel group (p < 0.05), suggesting that better anti-tumour efficiency could be achieved by optimizing drug release profile. The typical image of the tumour size was also shown in , which indicated that DOX-Lip-Gel group had better tumour growth inhibition efficiency, which is in consistence with the results of and .
Histological analysis
The tumour tissue was taken out after sacrificing the mice for histological investigation by utilizing HE staining. As shown in , the tumour stained of tumour-bearing mice treated with saline had adequate cytoplasm, a greater quantity of tumour cells nuclei and extremely constrict interstitial space. There was no obvious necrosis in the HE-stained slides of both saline and blank Lip-Gel groups. These histological examinations suggested that peritumoral injection of blank Lip-Gel had no tumour inhibition efficiency as compared to the saline group.
Figure 10 (a) HE-stained images of tumour cross-sections of breast tumour-bearing BALB/c female mice taken on the Day 13 after administration of saline, blank Lip-Gel, free DOX, DOX-Lip, DOX-Gel, and DOX-Lip-Gel. (b) HE-stained images of heart tissue obtained after treated with saline, blank Lip-Gel, free DOX, DOX-Lip, DOX-Gel and DOX-Lip-Gel. Some typical inflammatory cells were denoted by red arrows, disorganization of myofibrillar arrays was denoted by blank arrows. The scale bar is 50 μm.
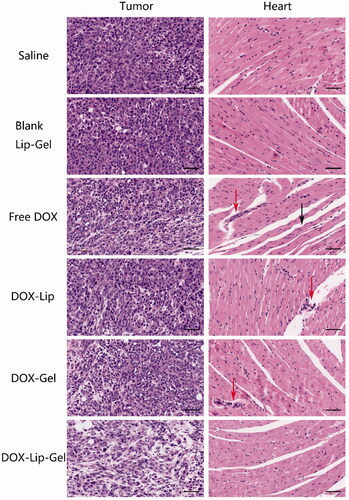
The HE-stained slides of free DOX and DOX-Lip groups exhibited more necrosis areas compared with the negative control group. After the peritumoral administration of DOX-Lip-Gel, the histological examination of tumour stained exhibited the least quantity of tumour cell nuclei, which was enclosed by an adequate interstitial space compared with other groups. This histological result indicated that thermosensitive hydrogel encapsulated with DOX-Lip (DOX-Lip-Gel) could cause a massive devastation of tumour tissue and improve the treatment efficacy.
Toxicology investigation
The potential systemic toxicity of antitumor drugs was another important criterion. Herein, the systemic toxicity of all formulations was assessed by evaluating the changes of body weight. During the treatment duration, the body weight of mice was noted, and the results were presented in . It was observed that there was no obvious body weight loss of tumour-bearing mice for the groups of saline, blank Lip-Gel, DOX-Gel and DOX-Lip-Gel. However, 22.6% and 14.6% body weight reduction were observed for mice treated with free DOX and DOX-Lip, respectively. The reason may attribute to the inadequate distribution of antitumor drugs to tumour regions [Citation42], which led to severe systematic toxicity.
DOX-Lip-Gel and DOX-Gel groups did not exhibit severe weight loss, implying that PLGA-PEG-PLGA based thermogel could alleviate the systemic toxicity of free DOX.
Cardiotoxicity investigation
Regarding the injury caused by free DOX to heart, such as swollen cardiac muscle fibres, interstitial oedema and inflammatory infiltration, a histological test was performed to examine the cardiotoxicity of DOX. As presented in , there was no any indication of tissue oedema and inflammation of cardiac muscles observed in the HE-stained slice of the blank Lip-Gel group when contrasted with the saline group. These results demonstrated that blank Lip-Gel group had no cardiotoxicity on heart muscles. Free DOX-induced more serious swollen of cardiac muscle, disorganization of myofibrillar arrays (denoted by blank arrows), myocardial constriction and a few inflammatory cells (denoted by red arrows), shown in , while fewer inflammation and swelling of cardiac muscles were detected in DOX-Lip treated slides compared with free DOX.
DOX-Gel treated heart tissue stained exhibited some inflammatory cells. Whereas DOX-Lip-Gel treated heart tissue stained demonstrated less inflammation and little cardiac muscles swollen as a contrasted with DOX-Gel. These results showed that DOX-Lip-Gel had minor cardiotoxicity effect.
As a result of the peritumoral administration of DOX-Lip-Gel, it enhanced the localized drug release straightly at the tumour regions and enabled the drug resided at tumour cells for a longer duration. That is, it reduced drug dissipation to the healthy organs compared with conventional intravenous delivery of free DOX and DOX-Lip. Such kind of beneficial results is achieved mainly because of more amount of DOX reside at the tumour site and fewer drug dissemination within the heart tissue. This was achieved by the quick transformation of hydrogel solution into the solid gel as drug reservoir instead of distributing throughout the administration place. In addition, the sustained release of DOX had no critical early burst release since drug should pass through the liposomal bilayer at the first time and then diffuse through the hydrogel barrier at the second time, which might impede the cardiotoxicity caused by DOX. While for DOX-Gel the gel matrix alone could not realize steady sustained release of DOX, that is, an obvious burst release happened, which means quick diffusion of DOX throughout the administration place and drug dissipating to the normal organ such as heart and induced the cardiac toxic effect. Consequently, the results suggested that liposomal doxorubicin loaded thermogel could lower the cardiac toxicity of DOX.
Discussion
Chemotherapy plays a vital role in the treatment of various cancers, but it is severely limited in the clinic due to the systemic toxicity and side effects. Therefore, increasing the drug efficiency and reducing the side effects are the main strategy for addressing the limitations of conventional chemotherapy. For example, the clinical practice DOX is limited to some extent by its undesired cardiac toxicity, such as arrhythmia, cardiomyopathy or congestive heart failure and destruction of cardiomyocytes. Free DOX solution exhibited excellent cellular uptake in vitro but it was hard to reach the tumour target regions because of the quick elimination by the reticuloendothelial system and poor targeting efficiency [Citation43]. Therefore, the liposomal preparations of DOX were approved by FDA in 1995 to reduce its side effects and prolong circulation lifetime is limited [Citation44]. However, the drug accumulation of nanoparticle formulations in tumour tissue would be less than 5% if only relied on passive targeting. Moreover, recent studies have shown that the accumulation of the active targeting particle is still not sufficient to effectively treat tumours [Citation45].
Local chemotherapy is capable of maximizing the concentration of anti-tumour drug in tumour tissues and decreasing systemic toxicity. Thermosensitive hydrogel such as PLGA-PEG-PLGA based thermogel has been widely employed as an anti-tumor local treatment for sustained release. As shown in , the polymer solution was a sol at 25 °C, then quickly transformed into a gel at 37 °C. Meanwhile, the rheology data presented that the gel in liquid form has very less viscosity which can simply move through the syringe and form the solid get at the tumour site (). The hydrophilic molecules released from PLGA-PEG-PLGA copolymers by the diffusion-controlled process whereas hydrophobic molecules released firstly by diffusion-controlled phase, then pursuing the sustained polymer degradation-controlled phase [Citation15]. Therefore, hydrogel could realize the sustained release of hydrophobic drugs and macromolecular drugs but is limited for small hydrophilic molecules. The rational design of this hybrid system of encapsulating drug-loaded liposomes into hydrogel could address the challenge for small molecular drug such as DOX and achieve steady sustained release. Liposomes were introduced to hydrogel could release the drug in a controlled way without quick and high initial burst release contrasted with gel alone and its prolonged release effect was excellent than liposomal drugs. The in vitro release studies showed that DOX-Lip-Gel achieved prolonged release without obvious burst of DOX up to 11 days and the cumulative DOX release was 66% (). Liposomes inside temperature-responsive gel could function as a matrix for the first barrier of hydrophilic drugs. That is, when the drug is encapsulated into the liposome incorporated gel complex, the drug will encounter two barriers to release, which could realize the sustained release and prevent the burst release of hydrophilic drug.
In addition, we investigated the anti-tumour efficiency and body toxicity of the hybrid system. DOX-Gel and DOX-Lip-Gel had lower tumour growth rate after twice peritumoral injections contrasted with intravenous injection groups (p < 0.05). Moreover, the results suggested that DOX-Lip-Gel had significantly higher tumour growth inhibition effect than DOX-Gel (p < 0.01). There were many factors which support the DOX-Lip-Gel group to exhibit the superior therapeutic effect when contrasted with other preparations. DOX-Lip-Gel was feasible to keep up DOX effectiveness over the span of treatment due to its prolonged sustained release, since the antitumor efficacy of DOX is dose and time-dependent [Citation46]. Moreover, the anticancer drugs themselves turned out to be more effective when given directly to the targeted tumour and also a greater concentration of drug at localized level can be accomplished contrasted with conventional method. Also, the result showed no significant weight change in the DOX-Lip-Gel, which was in sharp contrast to about a 22.6% body weight loss for mice treated with free DOX, indicating that gel matrix could alleviate the side effects of free DOX.
Conclusion
In this work, an injectable liposomal doxorubicin loaded PLGA-PEG-PLGA thermogel was developed. The thermogel was a sol at room temperature and could transform into a solid gel in vivo at body temperature. In situ formed gel was gradually degraded in vivo. DOX-Lip-Gel exhibited steady prolonged release of DOX without distinct initial burst compared with DOX-Gel, for the entrapped drug should pass through the liposomal bilayer and then diffuse out of the gel. In vivo antitumor examinations demonstrated that DOX-Lip-Gel could significantly inhibit the tumour growth compared with other groups (p < 0.01), no statistical difference in the body weight of treated mice with saline group (p < 0.05). HE-stained of myocardium showed lower cardiotoxicity for the group of DOX-Lip-Gel. This research suggests that hybrid drug delivery system consisting of liposome and hydrogel can sustain drug release and improve drug release profile, as well as enhancing the anticancer efficacy through localized therapy and manage to reduce cytotoxicity.
| Abbreviations | ||
| DOX | = | Doxorubicin |
| DOX-Lip | = | Liposomal doxorubicin |
| DOX-Gel | = | Doxorubicin loaded hydrogel |
| DOX-Lip-Gel | = | Liposomal doxorubicin loaded hydrogel |
| Blank Lip-Gel | = | Blank liposomes loaded hydrogel |
| LA | = | Lactic Acid |
| GA | = | Glycolic Acid |
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Swapana M, Padmavathy C. A critical review on breast cancer literature: screening, awareness and preventive measures. Mediterr J Social Sci. 2015;6:4.
- Varshosaz J, Hassanzadeh F, Aliabadi HS, et al. Targeted delivery of doxorubicin to breast cancer cells by magnetic LHRH chitosan bioconjugated nanoparticles. Int J Biol Macromol. 2016;93:1192–1205.
- Korfel A, Höcht S, Thiel E. Combined and sequential systemic chemotherapy and radiotherapy for treatment of brain metastases. Onkologe. 2008;14:255–259.
- Sabeti B, Noordin MI, Mohd S, et al. Development and characterization of liposomal doxorubicin hydrochloride with palm oil. Biomed Rese Int. 2014;2014:1.
- Jungsuwadee P. Doxorubicin-induced cardiomyopathy: an update beyond oxidative stress and myocardial cell death. Cardiovasc Regener Med. 2016;3:e1127.
- Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery. Chem Rev. 2015;115:10938.
- Mcneil SE. Evaluation of nanomedicines: stick to the basics. Nat Rev Mater. 2016;1:16073.
- Zheng Y, Cheng Y, Chen J, et al. Injectable hydrogel-microsphere construct with sequential degradation for locally synergistic chemotherapy. ACS Appl Mater Interfaces. 2017;9:3487–3496.
- Wei L, Chen J, Zhao S, et al. Thermo-sensitive polypeptide hydrogel for locally sequential delivery of two-pronged antitumor drugs. Acta Biomaterialia. 2017;58:44–53. doi:10.1016/j.actbio.2017.05.053.
- Shen W, Chen X, Luan J, et al. Sustained codelivery of cisplatin and paclitaxel via an injectable prodrug hydrogel for ovarian cancer treatment. ACS Applied Materials & Interfaces 2017;9:40031–40046.
- Liu H, Cheng Y, Chen J, et al. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018;73:103–111.
- Zhang Y, Zhang J, Chang F, et al. Repair of full-thickness articular cartilage defect using stem cell-encapsulated thermogel. Mater Sci Eng C Mater Biol Appl. 2018;88:79–87. doi:10.1016/j.msec.2018.02.028.
- Zhang W, Ning C, Xu W, et al. Precision-guided long-acting analgesia by gel-immobilized bupivacaine-loaded microsphere. Theranostics. 2018;8:3331–3347. doi:10.7150/thno.25276. PubMed PMID: 29930733; PubMed Central PMCID: PMC6010997.
- Kang GD, Cheon SH, Song SC. Controlled release of doxorubicin from thermosensitive poly(organophosphazene) hydrogels. Int J Pharm. 2006;319:29–36.
- Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49:1993–2007.
- Hui S, Chen X, Liu Y, et al. Cucurbit[7]-assisted sustained release of human calcitonin from thermosensitive block copolymer hydrogel. Int J Pharm. 2017;527:52.
- Hu C, Liu X, Ran W, et al. Regulating cancer associated fibroblasts with losartan-loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials 2017;144:60–72.
- Li X, Ding J, Zhang Z, et al. Kartogenin-incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl Mater Interfaces. 2016;8:5148–5159. doi:10.1021/acsami.5b12212.
- Gao Y, Sun Y, Ren F, et al. PLGA-PEG-PLGA hydrogel for ocular drug delivery of dexamethasone acetate. Drug Dev Ind Pharm. 2010;36:1131–1138.
- Liu Y, Chen X, Li S, et al. Calcitonin-loaded thermosensitive hydrogel for long-term antiosteopenia therapy. ACS Appl Mater Interfaces. 2017;9:23428–23440.
- Zhang W, Xu W, Ning C, et al. Long-acting hydrogel/microsphere composite sequentially releases dexmedetomidine and bupivacaine for prolonged synergistic analgesia. Biomaterials. 2018;181:378–391. doi:10.1016/j.biomaterials.2018.07.051.
- Liu L, Feng X, Pei Y, et al. Alpha-cyclodextrin concentration-controlled thermo-sensitive supramolecular hydrogels. Mater Sci Eng C Mater Biol Appl. 2018;82:25–28. doi:10.1016/j.msec.2017.08.045.
- Ci T, Chen L, Yu L, et al. Tumor regression achieved by encapsulating a moderately soluble drug into a polymeric thermogel. Sci Rep. 2014;4:5473.
- Zhang Y, Ding J, Sun D, et al. Thermogel-mediated sustained drug delivery for in situ malignancy chemotherapy. Mater Sci Eng C. 2015;49:262–268.
- Lee JH, Oh H, Baxa U, et al. Biopolymer-connected liposome networks as injectable biomaterials capable of sustained local drug delivery. Biomacromolecules. 2012;13:3388.
- Lalloo A, Chao P, Hu P, et al. Pharmacokinetic and pharmacodynamic evaluation of a novel in situ forming poly(ethylene glycol)-based hydrogel for the controlled delivery of the camptothecins. J Control Release. 2006;112:333–342.
- Yu L, Ci T, Zhou S, et al. The thermogelling PLGA–PEG–PLGA block copolymer as a sustained release matrix of doxorubicin. Biomater Sci. 2013;1:411–420.
- Chen S, Pieper R, Webster DC, et al. Triblock copolymers: synthesis, characterization, and delivery of a model protein. Int J Pharm. 2005;288:207–218.
- Bolotin EM, Cohen R, Bar LK, et al. Ammonium sulfate gradients for efficient and stable remote loading of amphipathic weak bases into liposomes and ligandoliposomes. J Liposome Res. 1994;4:455–479.
- Haran G, Cohen R, Bar LK, et al. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta. 1993;1151:201
- Mohajeri SA, Yaghoubi S, Abdollahi E, et al. In-vivo study of naltrexone hydrochloride release from an in-situ forming PLGA-PEG-PLGA system in the rabbit. J Drug Delivery Sci Technol. 2016;36:156–160.
- Ma H, He C, Cheng Y, et al. Localized co-delivery of doxorubicin, cisplatin, and methotrexate by thermosensitive hydrogels for enhanced osteosarcoma treatment. Biomaterials. 2014;35:8723–8734.
- Nie S, Hsiao WW, Pan W, et al. Thermoreversible Pluronic® F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: in vitro drug release, cell cytotoxicity, and uptake studies. Int J Nanomed. 2011;6:151.
- Mulik R, Kulkarni V, Murthy RS. Chitosan-based thermosensitive hydrogel containing liposomes for sustained delivery of cytarabine. Drug Dev Ind Pharm. 2009;35:49–56.
- Gao Y, Ren F, Ding B, et al. A thermo-sensitive PLGA-PEG-PLGA hydrogel for sustained release of docetaxel. J Drug Target. 2011;19:516
- Chung YM, Simmons KL, Gutowska A, et al. Sol-gel transition temperature of PLGA-g-PEG aqueous solutions. Biomacromolecules. 2002;3:511
- Xie B, Jin L, Luo Z, et al. An injectable thermosensitive polymeric hydrogel for sustained release of Avastin® to treat posterior segment disease. Int J Pharm. 2015;490:375–383.
- Sun H, Cao D, Liu Y, et al. Low molecular weight heparin-based reduction-sensitive nanoparticles for antitumor and anti-metastasis of orthotopic breast cancer. Biomater Sci. 2018;6:2172–2188. doi:10.1039/c8bm00486b.
- Chen L, Ci T, Li T, et al. Effects of molecular weight distribution of amphiphilic block copolymers on their solubility, micellization, and temperature-induced sol–gel transition in water. Macromolecules. 2014;47:5895–5903.
- Li T, Ci T, Chen L, et al. Salt-induced reentrant hydrogel of poly(ethylene glycol)–poly(lactide-co-glycolide) block copolymers. Polym Chem. 2014;5:979–991.
- Lei K, Chen Y, Wang J, et al. Non-invasive monitoring of in vivo degradation of a radiopaque thermoreversible hydrogel and its efficacy in preventing post-operative adhesions. Acta Biomater. 2017;55:396.
- Chun CJ, Sun ML, Chang WK, et al. Doxorubicin–polyphosphazene conjugate hydrogels for locally controlled delivery of cancer therapeutics. Biomaterials. 2009;30:4752–4762.
- Nie Y, Ji L, Ding H, et al. Cholesterol derivatives based charged liposomes for doxorubicin delivery: preparation, in vitro and in vivo characterization. Theranostics. 2012;2:1092.
- Abraham SA, Waterhouse DN, Mayer LD, et al. The liposomal formulation of doxorubicin. Meth Enzymol. 2005;391:71.
- Lee BK, Yun YH, Park K. Smart nanoparticles for drug delivery: boundaries and opportunities. Chem Eng Sci. 2015;125:158–164.
- Weiner AL, Carpenter-Green SS, Soehngen EC, et al. Liposome-collagen gel matrix: a novel sustained drug delivery system. J Pharm Sci. 1985;74:922–925.