?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Blood compatibility is a key requirement to fulfil for intravenous administration of drug and oxygen carrier system. Recently, we published the fabrication of oxidised-dextran (Odex)-crosslinked protein particles by one-pot formulation. In the current study we investigate the haemocompatibility of these Odex - particles including albumin particles (Odex-APs) and haemoglobin particles (Odex-HbMPs). Odex-APs and Odex-HbMPs have a submicron size ranged 800–1000 nm with peanut-like shape and a negative surface charge. In vitro haemocompatibility assays included haemolysis test, indirect phagocytosis test and platelet activation test in human blood. Odex-APs and Odex-HbMPs did not provoke any undesirable effects on the blood cells. Firstly, the ratio of haemolysis after contacted with Odex-crosslinked protein particles were less than 5% and therefore the particles may be considered non-haemolytic. Secondly, the incubation of leukocyte with Odex-APs/HbMPs did not influence the phagocytosis of leukocyte. We conclude that our particles are not recognized by monocytes or granulocytes. Finally, exposure of Odex-APs/HbMPs to platelets did not cause an activation of platelets. Additionally, Odex-HbMP/AP did not enhance or attenuate agonist-induced platelet activation. We conclude that Odex-crosslinked protein particles exhibit a very good haemocompatibility and represent highly promising carriers for drugs or oxygen.
Introduction
A major part of the micro- and nanometer size particles in clinical and preclinical investigations are biomolecule-based particles fabricated from polysaccharides, polyaminoacids, polypeptides, proteins or lipids. Among them, protein-based micro- and nanoparticles have been extensively investigated [Citation1] as a site-specific drug carrier for cancer [Citation2–7] as well as haemoglobin-based oxygen carriers (HBOCs) [Citation8–16].
However, questions concerning the safety of prolonged use of nano- and micro-particles have been raised since most biomedical nanoparticles, for therapeutic and/or diagnostic purposes, are typically intravenously administrated and directly interact with the blood. Hence, the haemocompatibility of the nano- and micro- particles becomes important and critical. Materials are considered to be haemocompatible, if they are able to remain effectual after being exposed to blood but do not bring about any form of toxicity to the blood cells and do not cause any changes in composition and viscosity of the blood plasma [Citation17]. For instance, the rupture of red blood cells (RBCs) and subsequent haemoglobin releasing can cause the symptoms haemoglobinuria or anuria followed by renal failure [Citation18]. Additionally, platelets are critical to haemostasis by virtue of their ability to adhere, aggregate and release the contents of their granules as well as their capacity to alter their surface characteristics to support blood coagulation. Therefore, thrombotic and thromboembolic complications, as well as bleeding risks associated with the disseminated intravascular coagulopathy (DIC) remain of serious concern. Besides, the phagocytic cells (e.g. granulocytes and monocytes) can proficiently engulf particles in the blood which results in serious limitation of their blood circulation time and extravasation into target tissues [Citation19]. Therefore, to avoid these events, the use of biocompatible and biodegradable materials are crucial factors for the fabrication of particles suitable for clinical applications.
The haemocompatibility of particles is mainly affected by their physicochemical characteristics such as chemical composition, size, shape, surface charge, hydrophobicity or hydrophilicity [Citation20–22]. The fabrication techniques and selection of biomaterials are the fundamental steps, which can drastically impact the haemocompatibility of the particles as well as the efficacy of the proteins in the particles. Recently, we described a new promising formulation of protein submicron particles called “One-Pot formulation” [Citation23]. The new procedure is based on the coprecipitation-crosslinking-dissolution (CCD) method where the biopolymer particles are obtained in three main steps exploiting the ability of insoluble inorganic salts to incorporate macromolecules during precipitation in aqueous solutions. These macromolecules are subsequently crosslinked, and the inorganic precipitate is dissolved usually by complexation of the metal ions or pH change. With the one-pot procedure, coprecipitation and crosslinking were combined in one step due to the use of a biopolymer crosslinker, oxidised-dextran (Odex), which is coprecipitated together with a protein into a manganese carbonate (MnCO3) template. Odex-HbMPs fabricated by one-pot demonstrated an improved oxygen storage capability. We hypothesized that dextran is able to improve not only the protein function and stability but also the haemocompatibility of the particles because of its protein-rejecting and cell repelling abilities [Citation36,Citation37]. In addition, the multivalent nature of dextran is advantageous for surface immobilization of biologically active molecules.
Therefore, the aim of this study is to investigate the haemocompatibility of Odex-APs and Odex-HbMPs using several methods including RBCs haemolysis, phagocytic activity of leukocytes and activation/aggregation of thrombocytes, in vitro.
Materials and methods
Materials
Dextran (M.W. 40,000 and 70,000 Da named as 40 T and 70 T, respectively) were purchased from AppliChem GmbH, Germany. Human serum albumin (HSA) solution for injection (200 g/L HSA, contains 16 mM sodium caprylate, 16 mM sodium N-acetyltryptophanate, 100–130 mM sodium chloride) was purchased from Baxalta Deutschland GmbH, Germany. Bovine haemoglobin solution (50 g/L in 0.9% NaCl) was obtained from Biophyll GmbH, Germany. Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA-Na2), glycine, manganese chloride, sodium carbonate, sodium chloride, sodium hydroxide, and sodium (meta) periodate were purchased from Sigma-Aldrich, Germany. Phosphate buffered saline pH 7.4 (PBS, solutions contains 10 mM sodium phosphate dibasic, 1.9 mM potassium phosphate monobasic, 137 mM sodium chloride and 2.7 mM potassium chloride) was purchased from Fisher Scientific, USA. PHAGOTEST™ and PHAGOBURST™ kits were purchased from Glycotope Biotechnology GmbH, Germany.
Odex-crosslinked protein particles
50 mL of 10% dextran solutions (40 T and 70 T, 30.9 mmoL glucose subunits) were oxidised by sodium periodate (6.6 g, 30.9 mmoL) for 1 h at room temperature [Citation24]. Then, the resulting oxidised dextran (Odex) was transferred to a cellulose dialysis tube (MWCO of 12,000: Carl Roth GmbH, Germany) and dialysed against water. The amount of aldehyde in Odex was quantified by hydroxylamine hydrochloride titration method with unmodified dextran as a reference [Citation25].
Albumin particles (APs) and haemoglobin (HbMPs) were prepared by the one-pot procedure using 40T- and 70T-Odex as crosslinking as described earlier [Citation23]. Briefly, equal volumes of solution 1 consisting of 0.25 M of MnCl2 and 50 mg/mL of HSA or Hb were rapidly mixed with solution 2 containing 0.25 M of Na2CO3 and 40 mg/mL of Odex (40 T or 70 T, individually) in the beaker under vigorous stirring at room temperature. After 30 s, 5 mg/mL of HSA was added to the suspension and incubated for 5 min under stirring to allow the HSA to absorb into particles surface. The resulting particle suspensions were then proceeded to the dissolution of the MnCO3 template by 0.25 M EDTA/0.05 M Glycine for 30 min and the reduction by 0.4 mg/mL of NaBH4. Finally, the obtained Odex cross-linked albumin particles (Odex-APs) or haemoglobin particles (Odex-HbMPs) were washed three times with PBS (6000 g for 5 min) and finally suspended in sterile PBS (final particle concentration 2⋅× 1011/mL). APs and HbMPs crosslinked with 40T-Odex and 70T-Odex are named 40T-APs, 70T-APs, 40T-HbMPs and 70T-HbMPs, correspondingly.
For SEM imaging, samples were prepared by applying a drop of particles suspension onto glass slide followed by drying overnight. After sputtering with gold, measurements were conducted at an operation voltage of 3 keV using Gemini Leo 1550 instrument (Oberkochen, Germany) and ImageJ 1.44p software (Wayne Rasband, National Institute of Mental Health, Bethesda, MD, USA).
The hydrodynamic diameter and the zeta-potential of the Odex-APs and Odex-HbMPs were measured by dynamic light scattering using a Zetasizer Nano ZS instrument (Malvern Instruments Ltd., Malvern, UK) in PBS solution. Additionally, Odex-crosslinked particles were dispersed in PBS and analysed for autofluorescence using flow cytometry (BD FACSCanto II), FITC and PE channel were gated. BD FACSDIVA software (BD Biosciences, USA) was employed for data analysis.
Blood collection
Venous blood anticoagulated by lithium heparin or sodium citrate was collected from healthy volunteers. Informed consent was obtained from all donors in written form. The blood samples were withdrawn in accordance with the transfusion law of Germany. The use of donor blood samples for scientific purposes was approved by the ethics committee of the Charité – Universitätsmedizin Berlin (# EA1/137/14). Immediately after blood collection, tubes were slowly agitated to ensure an appropriate mixing of anticoagulant and blood and discarded if there was any evidence of clotting. The assays were performed within 2 h after blood collection.
Haemolysis test
The haemolytic test is based on the release of haemoglobin from damaged erythrocytes in vitro. Human heparinized erythrocytes were washed (3000 g, 5 min) in PBS until the supernatant was clear and colourless. RBCs were then to obtain a cell suspension with a volume concentration of 2% in PBS. 0.5 mL of 2% Odex-crosslinked particles suspension was mixed with 0.5 mL of the 2% washed human erythrocyte suspension. This ratio of particles to RBC corresponds to an exchange of 50% blood with particle suspension. 0.5 mL of double distilled water and PBS were employed as the positive (PC) and negative (NC) control, respectively. After incubation at 37 °C for 3 h, the erythrocyte suspensions were centrifuged at 3000 g for 5 min, the supernatants were collected and pipetted into a 96-well plate. The haemolytic ratio was determined by measuring the absorbance of the supernatants at 545 nm using a microplate reader (Cytation™ 3 Cell Imaging Multi-Mode Reader, BioTek) and calculated according to the following equation:
(1)
(1)
All results were estimated from the data of three individual experiments, and all data were expressed as the mean ± SD [Citation26]. The value of the positive control (water) was set to 100% haemolysis.
Phagocytosis test
The activation of phagocytosis performance of granulocytes and monocytes in whole blood was investigated using an indirect method based on a modified PHAGOTEST. The method is suitable for non- fluorescing particles. The different particles (10 µL of 2 × 1011 per mL) were added to 50 µL of heparinized-whole blood and incubated at 37 °C for 0, 10, 30, 60, 120 min to allow uptake by the leucocytes. Samples with non-fluorescent Escherichia coli (2 × 109 bacteria per mL from PHAGOBURST kit) and PBS were used as a positive and negative control, respectively. After reaching the corresponding incubation time, the standard test for phagocytosis activity (PHAGOTEST) was performed with all samples. Briefly, 10 µL of fluorescein isothiocyanate labelled E. coli (FITC-E. coli, 2 × 109 bacteria per mL from Phagotest kit) were added to each sample and incubated at 37 °C for further 10 min. Afterwards, the fluorescence of non-phagocytosed E. coli was quenched, the RBCs were lysed and the leucocytes were fixed and DNA-stained by propidium iodide. Washing steps with PBS were performed between each preparation step. Finally, the leukocyte populations were analysed by flow cytometry (BD FACSCanto II) to obtain the percentage of granulocytes and monocytes which have phagocytosed FITC-E. coli. Leukocytes previously saturated with non-fluorescent bacteria or particles are not able to uptake the FITC-E. coli, and therefore decreased phagocytic activity to FITC-E. coli indicates phagocytic activity to the non-fluorescent bacteria or particles.
Platelet activation test
The influence of Odex-AP and Odex-HbMP on human platelets was investigated in platelet-rich plasma (PRP) samples. Citrated human whole blood was centrifuged at 150 g for 15 min at 20–25 °C, and the PRP fraction was collected. The platelet amount was detected before the test using a haematology analyser ABX Micros 60 (HORIBA Europe GmbH, Germany). 45 µL of PRP was gently mixed with 5 µL of particle suspensions (to a ratio of 10 = particles per 1 platelet). Platelets incubated with PBS were used as a control. The samples were incubated at 37 °C for 30 min with gently shaking. Then, the pre-incubated human platelets were activated with platelet agonists (0.5 mg/mL arachidonic acid (AA), 0.2 mg/mL collagen and 0.01 mM epinephrine (Epi): möLab GmbH, Germany) to induce platelet activation and aggregation. The mixtures were incubated at 37 °C for further 30 min with shaking and the samples were then fixed by 0.5% formaldehyde in PBS. Finally, the platelets were stained by APC anti-human CD42b (GPIbα) antibody and Alexa Fluor® 488 anti-human CD62P (P-Selectin) antibody (BioLegend, San Diego, USA) and analysed by flow cytometry (BD FACSCanto II). Double-stained events were counted as activated platelets [Citation27,Citation28].
Statistical analysis
Data were presented as means ± standard deviation (SD), and statistical differences between groups were compared using ANOVA-like test. GraphPad Prism 6 software (GraphPad, La Jolla, CA, USA) was employed for graphs and statistical analyses. p Values <.05 was considered statistically significant.
Results
Particle characterisation
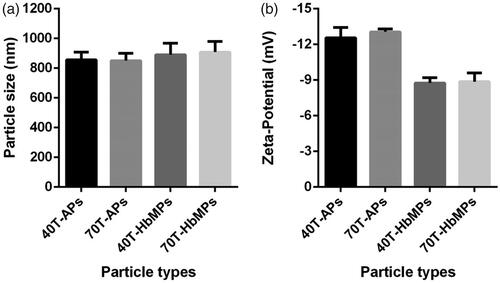
Odex-APs and Odex-HbMPs were successfully fabricated by the One-pot procedure. Like the CCD-technique using MnCO3 as inorganic template, the morphology and the size of the obtained haemoglobin and albumin particles by the One-pot procedure were not significantly different as observed by SEM (). The dynamic light scattering analysis of the particles is represented in . The size of Odex-APs and Odex-HbMPs was about 800–1000 nm and the zeta-potential was approximately −13 mV and −9 mV as measured in PBS (conductivity 18–20 mS/cm) for Odex-APs and Odex-HbMPs, respectively. No significant differences in size and zeta-potential could be detected for the particles prepared with 40T- and 70T-Odex.
Figure 1 SEM images of Odex-AP (a) and Odex-HbMP (b) fabricated by One-pot formulation. The insets show peanut-like single particles.
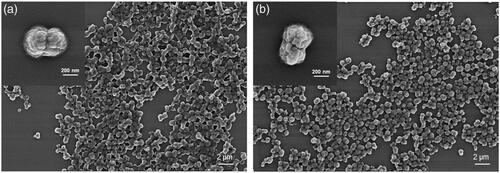
Figure 2 Characteristics of Odex-crosslinked protein particles. (a) Size and (b) Zeta-potential measured in 10 mM PBS (conductivity 18–20 mS/cm) at room temperature by Dynamic Light Scattering analysis. Data are presented as mean ± SD (n = 5).
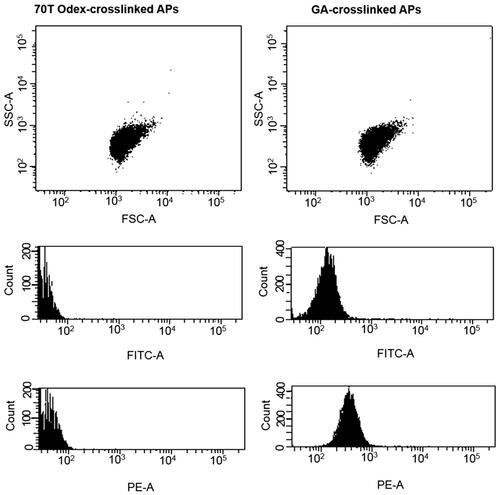
The results of flow cytometry analysis in also demonstrated that Odex-particles had no auto-fluorescence in contrast to the protein particles prepared using the CCD technique and crosslinked by glutaraldehyde [Citation8–10,Citation29,Citation30].
Haemolysis test
Haemolysis refers to the release of haemoglobin from RBCs due to damage of RBCs membrane, which is extensively applied to evaluate the biosafety of particles. A haemolytic ratio higher than 5% is considered as a significant damage of RBCs. The haemolytic ratio of the RBCs incubated with different Odex-crosslinked particles for 3 h at 37 °C is demonstrated in . It can be seen clearly that the haemolysis ratio for the samples with Odex-APs and Odex-HbMPs was between 1% and 2% and so far, they obviously did not deteriorate significantly the RBCs.
Table 1. Haemolytic ratio of odex-crosslinked particles.
Phagocytosis test
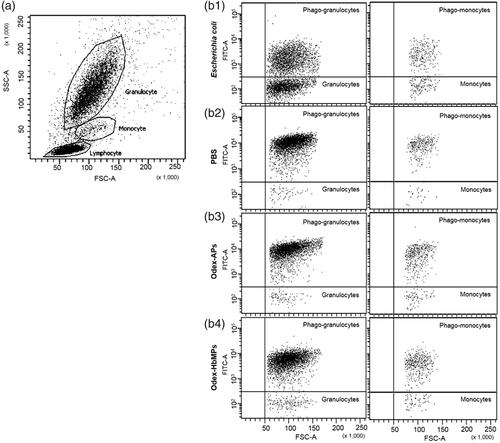
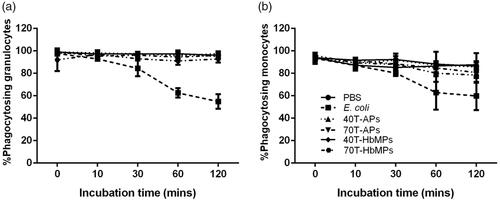
The commercial phagotest kit allows determinations of the percentage of phagocytes (in whole blood samples) which ingest FITC-labelled opsonized E. coli bacteria and is used as a diagnostic tool for functional testing of leukocytes. The test can be directly used to determine the phagocytosis of fluorescent particles by the phagocytes in the blood (mainly granulocytes and monocytes). However, in the case of the non-fluorescent particles, an additional fluorescent staining is needed which may alter their surface properties and influence the phagocytosis. Therefore, we employed an indirect method as described in the part Materials and Methods. Non-fluorescent E. coli, Odex-APs and Odex-HbMPs were added to the whole blood sample and incubated at 37 °C for 10–120 min in order to reach a saturated phagocytosis by the granulocytes and monocytes. Then, the standard test with FITC– labelled E. coli at 37 °C was performed with all samples. Results of the flow cytometry analysis of samples pre-incubated with non-stained E. coli, PBS, Odex-APs and Odex-HbMPs for 120 min is presented in as dot plots of granulocyte and monocyte populations detectable in the FITC channel. It can be seen that the distribution of the FITC-labelled cells is similar for the samples pre-incubated with PBS, 40T-APs and 40T-HbMPs. Only the positive control (pre-incubated with non-fluorescent E.coli) shows different behavior with an increased number of non-fluorescent cells.
Figure 4 Flow cytometric dot plot of human peripheral blood leukocytes. (a) Sub-populations of leukocytes can be distinguished based on forward scatter (relative size) and side scatter (relative complexity). Granulocyte are large and complex; monocytes are slightly larger than lymphocytes and less complex than granulocyte, while lymphocytes are smaller and less complex. (b) Dot plots FITC/FSC demonstrate the phagocytic activity of granulocytes (left panel) and monocytes (right panel) detected with FITC-labelled E. coli. Preincubation with different particles was performed for 120 min. (b1) Preincubation of leukocytes with non-stained E. coli (positive control), (b2) Preincubation of leukocytes with PBS (negative control), (b3) Preincubation of leukocytes with Odex-APs, (b4) Preincubation of leukocytes with Odex-HbMPs.
The summarized results in dependency on the pre-incubation time are shown in . The number of granulocytes and monocytes phagocytosing FITC-E. coli was not significantly different for Odex-crosslinked particles and PBS (negative control) over all pre-incubation times. In contrast, the positive control, E. coli, demonstrated a decrease in the percentage of the cells engulfing FITC-E. coli in a time-dependent manner, due to the phagocytosis of non-fluorescing E. coli.
Figure 5 Percentage of phagocytic activity of (a) granulocyte and (b) monocyte on Odex-APs and Odex-HbMPs compared with PBS (negative control) and E. coli (positive control). On one hand, the fluorescence signal of detective FITC-E. coli decreased grammatically across incubation periods in positive control. On the other hand, leukocyte preincubated with PBS as well as Odex-APs and Odex-HbMPs showed a strong detective FITC-E. coli and there was no change over the incubation times. Data are presented as mean ± SD (n = 3).
Platelet activation test
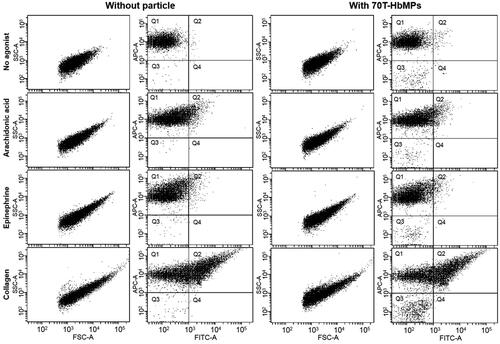
To evaluate whether our Odex-APs and Odex-HbMPs alter the haemostasis system, their influence on the activation of platelets was investigated. shows results of the flow cytometry analysis of platelets using the platelet-specific membrane receptor CD42b (GPIbα), and then distinguished those platelets that were activated using the activation marker CD62P. The results showed that the platelet activation assay was able to distinguish between platelets that are resting with high fluorescence level for CD42b and lower fluorescence levels for activation markers, CD62P, and those that have been activated by agonists with higher fluorescence levels for the two activation markers, CD42b and CD62P. Simultaneously, upon the stimulation of platelets by agonists, the aggregation of platelets was also observed as the shift of forward scatter/side scatter positioning compared with the control.
Figure 6 Flow cytometric analysis of the activation of platelets. Example of 70T-HbMPs compared to negative control. The events of the forward and side scatter as well as APC and FITC fluorescence channel are shown as dot plot. Platelets stained with APC-Antihuman CD42b (GPIbα) are enclosed in quadrant Q1. Double-stained with APC Anti-CD42ba and AlexaFlour 488 Antihuman-CD62P (P-selectin) events in quadrant Q2 represent activated-platelets. Non-stained particles were presented in Q3.
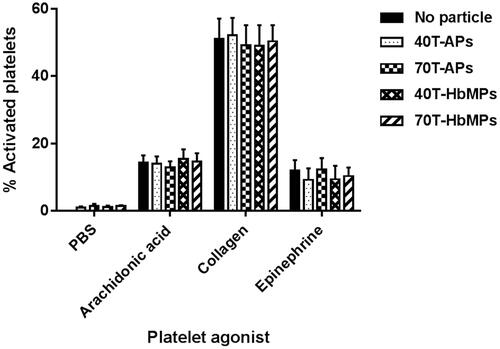
summarizes the results of the flow cytometry analysis. Obviously, the Odex-APs or Odex-HbMPs did not cause activation of the platelets as compared to the control sample which was incubated with PBS. Additionally, platelet activation induced by agonists including arachidonic acid, collagen and epinephrine of pre-incubated PRP with particles was comparable to the control samples.
Figure 7 Platelet activation (as determined by %CD62P expression) is not influenced by Odex-APs and Odex-HbMPs. Fresh citrated PRP was treated with the different particles or PBS as a control. The presence of particles did not influence on arachidonic acid-, collagen- and epinephrine-induced platelet activation. Non-stimulated labelled platelets are shown as a baseline for spontaneous activation. Data are presented as mean ± SD (n = 3).
It can be concluded that Odex-APs or Odex-HbMPs do not influence the function of platelets and therefore no negative side effects on the haemostasis are expected.
Discussion
Prior to all intravenous administration applications, the influence of particles on the blood cells needs to be evaluated. The haemocompatibility of particles is affected by their physical attributes, including size, shape, and flexibility, as well as their chemical composition, for instance, the incorporation of toxic compounds or active ligands for recognition and triggering of biological receptors. It has been well described that small particle size and positive charge caused thrombocyte and granulocyte activation, and haemolysis. Negatively charged particles larger than 60 nm hydrodynamic diameter appear to be considerably less haemotoxic than smaller ones [Citation21]. Our new particles fit to these criteria. Additionally, a successful perfusion of isolated mouse glomeruli with concentrated HbMP suspensions of the same size in vitro without vasoconstriction of the afferent arterioles could be shown [Citation10]. As the size of a particle decreases, its surface area per unit volume (or mass) increases and also allows a greater proportion of its atoms or molecules to be displayed on the surface rather than the interior of the material [Citation31,Citation32]. Therefore, it is very important to utilize procedures that allow preparation of particles with high degrees of uniformity, and with control over their physical and chemical characterisations.
Odex-APs and Odex-HbMPs fabricated by One-pot formulation have a size in the submicron range, uniform morphology and negative surface charge. The coating of the particles with human serum albumin improves significantly their blood compatibility by reducing the adsorption of other proteins as well the interaction with platelets and leukocytes [Citation33]. Due to the covalent binding of albumin to haemoglobin the stability of the coating is sufficient to protect the particles during their circulation in the blood stream against non-specific adsorption of other plasma proteins.
Currently described in vitro assays for haemocompatibility testing of particulate materials are not standardized. We evaluated the haemocompatibility of the Odex-crosslinked particles by testing haemolysis (destruction of RBCs), phagocytosis by leucocytes and platelet activation in order to explore possible adverse effects of Odex-crosslinked protein particles to blood cells
RBCs are the most abundant compartment in the bloodstream and are an important component determining the haemocompatibility. Once they are lysed, they release not only haemoglobin but also procoagulant factors which can cause serious adverse effects [Citation34]. According to the ISO 10993–4:2017, the haemolysis assays of Odex-AP and Odex-HbMP were considered to be non-haemolytic because these particles induced less than 5% haemolysis. Particle-induced haemolysis can be caused by the release of toxic substances from a biomaterial surface or from the interaction between particles and RBCs which result in the disruption and integrity of the RBC membrane and release of haemoglobin into the plasma. Additionally, it is generally agreed that surface properties (especially surface charge) are important, and there are several studies which have demonstrated this. For example, among a set of similar-sized fullerenes (C60-derivatives) bearing different numbers of anionic and cationic surface moieties, those with negative surface charge were not haemolytic, and haemolytic tendency increased in proportion to the number of attached cationic surface groups (positive surface charge) [Citation18].
The charge of particles stemming from distinct surface chemistries influences opsonisation, circulation times and interaction with resident macrophages of organs comprising the phagocytic system. On the one hand, positively charged particles more prone to sequestration by macrophages in the lungs, liver and spleen. On the other hand, neutral and slightly negatively charged nanoparticles have longer circulation lifetimes and less accumulation in the aforementioned organs [Citation35]. Our indirect phagotest assay showed that Odex-APs and Odex-HbMPs particles were not recognized by phagocytic cells including granulocytes and monocytes. As the adsorption of plasma proteins on the nanoparticle surface can have an important influence on the interactions between cells and the particles. Therefore, diminishing the susceptibility of particles to recognition by the phagocytes through coverage of their surface with hydrophilic polymers such as polyethylene glycol, dextrans or mimic the surface using human serum albumin is another strategy to prolong the residence time of particles in the circular system [Citation22].
Activation of platelet plays a crucial role in the coagulation cascade. They are very sensitive to the presence of foreign materials that can enhance or attenuate the activity of platelets and further affect the blood coagulation.
It should also be considered that on the one dextran used as plasma expander influences the function of platelets due to its adsorption on platelets surface [Citation36]. On the other hand, dextran forms not only a depletion layer around the blood cells and reduces the adsorption of proteins but can also be adsorbed to the cell surface. The dextran in the Odex-HbMP is covalently bound to haemoglobin and not free available for adsorption. But the moieties of dextran partially presented on the particle surface contribute to a repulsive force against other macromolecules [Citation37].
Therefore, the interaction between platelets and particle is an important evaluation of biomaterials for blood compatibility. In this study, the presence of Odex-APs and Odex-HbMPs influenced neither the platelet activation/aggregation nor the agonist induced-platelet activation. The mechanisms through which particles induce platelet aggregation are largely unknown. Nevertheless, trends observed in studies of polymer-based nanoparticles are similar in their charge-dependence to those described above for haemolysis [Citation18]. We assume that the negative surface charge as well as the albumin coating of Odex-APs and Odex-HbMPs prevent interaction between the particles and the platelets and therefore we do not expect negative effects on haemostasis.
Conclusions
In the current study, we demonstrated the in vitro evaluation of the compatibility of Odex-APs and Odex-HbMPs fabricated by “One-pot formulation” with human blood. The results of the haemolysis test, the direct phagocytosis test and of the platelet activation tests reflected good haemocompatibility. No biologically relevant alterations during blood contact were observed and therefore Odex-APs and Odex-HbMPs fabricated by “One-pot formulation” fulfill the requirements according to ISO 10993–4 for blood contacting materials. Therefore, their application potential is worthwhile to be further developed and explored.
Acknowledgements
C.K. and N.S. hold an academic development scholarship from the University of Phayao, A.P. from Naresuan University and S.C. from Payap University.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jao D, Xue Y, Medina J, et al. Protein-based drug-delivery materials. Materials (Basel) 2017;10:1–24.
- Nitta SK, Numata K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci. 2013;14:1629–1654.
- Elzoghby AO, Samy WM, Elgindy NA. Protein-based nanocarriers as promising drug and gene delivery systems. J Control Release. 2012;161:38–49.
- Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157:168–182.
- Lohcharoenkal W, Wang L, Chen YC, et al. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res Int. 2014;2014:1.
- Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine [Internet]. Adv Drug Deliv Rev. 2008;60:876–885.
- Kudarha RR, Sawant KK. Albumin based versatile multifunctional nanocarriers for cancer therapy: fabrication, surface modification, multimodal therapeutics and imaging approaches. Mater Sci Eng C. 2017;81:607–626.
- Xiong Y, Steffen A, Andreas K, et al. Hemoglobin-based oxygen carrier microparticles: synthesis, properties, and in vitro and in vivo investigations. Biomacromolecules. 2012;13:3292–3300.
- Bäumler H, Xiong Y, Liu ZZ, et al. Novel hemoglobin particles-promising new-generation hemoglobin-based oxygen carriers. Artif Organs. 2014;38:708–714.
- Xiong Y, Liu ZZ, Georgieva R, et al. Nonvasoconstrictive hemoglobin particles as oxygen carriers. ACS Nano. 2013;7:7454–7461.
- Jia Y, Duan L, Li J. Hemoglobin-based nanoarchitectonic assemblies as oxygen carriers. Adv Mater. 2016;28:1312–1318.
- Tao Z, Peter Ghoroghchian P. Microparticle, nanoparticle, and stem cell-based oxygen carriers as advanced blood substitutes. Trends Biotechnol. 2014;32:466–473.
- De Frates K, Markiewicz T, Gallo P, et al. Protein polymer-based nanoparticles: fabrication and medical applications. Int J Mol Sci. 2018;19:1–20.
- Duan L, Yan X, Wang A, et al. Highly loaded hemoglobin spheres as promising artificial oxygen carriers. ACS Nano. 2012;6:6897–6904.
- Tsuchida E, Sou K, Nakagawa A, et al. Artificial oxygen carriers, hemoglobin vesicles and albumin-hemes, based on bioconjugate chemistry. Bioconjugate Chem. 2009;20:1419–1440.
- Piras AM, Dessy A, Chiellini F, et al. Polymeric nanoparticles for hemoglobin-based oxygen carriers. Biochim Biophys Acta - Proteins Proteomics. 2008;1784:1454–1461.
- Naeye B, Deschout H, Röding M, et al. Hemocompatibility of siRNA loaded dextran nanogels. Biomaterials. 2011;32:9120–9127.
- Dobrovolskaia MA, Aggarwal P, Hall JB, et al. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5:487–495.
- Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles [Internet]. Int J Pharm. 2006;307:93–102.
- Albanese A, Tang PS, Chan WCW. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16.
- Mayer A, Vadon M, Rinner B, et al. The role of nanoparticle size in hemocompatibility. Toxicology. 2009;258:139–147.
- Lundqvist M, Stigler J, Elia G, et al. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Appl Phys Sci. 2008;105:14265–14270.
- Kloypan C, Prapan A, Suwannasom N, et al. Improved oxygen storage capacity of haemoglobin submicron particles by one-pot formulation. Artif Cells, Blood Substit Biotechnol. 2018; doi:10.1080/21691401.2018.1521819
- Berillo D, Elowsson L, Kirsebom H. Oxidized dextran as crosslinker for chitosan cryogel scaffolds and formation of polyelectrolyte complexes between chitosan and gelatin. Macromol Biosci. 2012;12:1090–1099.
- Lisman A, Butruk B, Wasiak I, et al. Dextran/albumin hydrogel sealant for Dacron(R) vascular prosthesis. J Biomater Appl. 2014;28:1386–1396.
- Lu M, Zhao C, Wang Q, et al. Preparation, characterization and in vivo investigation of blood-compatible hemoglobin-loaded nanoparticles as oxygen carriers. Colloids Surf B Biointerfaces. 2016 ;1:171–179.
- Braune S, Walter M, Schulze F, et al. Changes in platelet morphology and function during 24 hours of storage. Clin Hemorheol Microcirc. 2014;58:159–170.
- Cuyper IMD, Meinders M, Vijver EVD, et al. A novel fl ow cytometry – based platelet aggregation assay. Blood. 2013;121:70–80.
- Ba¨umler H, Georgieva R. Coupled enzyme reactions in multicompartment microparticles. Biomacromolecules. 2010;11:1480–1487.
- Xiong Y, Georgieva R, Steffen A, et al. Structure and properties of hybrid biopolymer particles fabricated by co-precipitation cross-linking dissolution procedure. J Colloid Interface Sci. 2018;15:156–164.
- Nel A, Xia T, Mädler L, et al. Toxic potential of materials at the nanolevel [Internet]. Science. 2006;311:622–627.
- Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951.
- Tanzi MC. Bioactive technologies for hemocompatibility. Expert Rev Med Devices. 2005;2:473–492.
- Helms CC, Marvel M, Zhao W, et al. Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost. 2013;11:2148–2154.
- Elci SG, Jiang Y, Yan B, et al. Surface charge controls the suborgan biodistributions of gold nanoparticles. ACS Nano. 2016;10:5536–5542.
- Bäumler H, Donath E, Krabi A, et al. Electrophoresis of human red blood cells and platelets. Evidence for depletion of dextran. Biorheology. 1996; 33:333–351.
- Bäumler H, Neu B, Donath E, et al. Basic phenomena of red blood cell rouleaux formation. Biorheology. 1999; 36:439–442.