Abstract
We herein describe a simple but efficient method for the determination of terminal transferase (TdT) activity, which relies on our finding that Fe(III)-quenched boron-dipyrromethene (BODIPY)-ATP is utilized as a switch-on monomer for polymerization and enables the facile synthesis of fluorescence oligonucleotides without additional, post-processing steps. As TdT carries out the synthesis of DNA by adding the monomers into growing chains, Fe(III) is displaced from BODIPY with the release of pyrophosphate group, which consequently leads to the generation of highly fluorescent long oligonucleotides. With this strategy, we selectively detected the TdT activity with high sensitivity. In addition, its practical applicability was successfully demonstrated by determining TdT activities in human serum.
Introduction
DNA molecules labelled with a fluorescent moiety, which is termed “fluorescence oligonucleotides”, have been utilized in various fields such as biotechnology, biomedicine, and molecular and cellular biology [Citation1–3]. In general, the synthesis of fluorescence oligonucleotides is broadly classified into two types: (i) chemical DNA synthesis based on phosphoramidite method [Citation4] and (ii) enzymatic DNA synthesis using modified mononucleotides [Citation5,Citation6]. In particular, the second one has gained momentum due to its simplicity, cost-effectiveness and especially, no limitation to form long DNA strands (up to thousands of bases) [Citation5,Citation6]. Many enzymes from DNA polymerase family that are capable of incorporating the modified dNTPs, have been exploited to synthesize the fluorescence oligonucleotides [Citation5–7]. However, previous methods require extra, post-processing steps such as separation of unreacted dNTPs or secondary labelling with fluorophore, which makes procedures tedious, time-consuming and complicated, consequently limiting the widespread application.
In this study, we devised a new, facile method for in situ formation of the fluorescence oligonucleotide based on the enzymatic DNA synthesis. As a key component, we employed ATP conjugated with fluorescent boron-dipyrromethene (BODIPY) analogues (BODIPY-ATP), which have been known to possess unique fluorescence properties including high quantum yield (almost 1.0 in water), outstanding photostability, high extinction coefficient (more than 80,000 cm−1M−1), and pH-insensitivity [Citation8–12]. In addition, on the basis of the effective fluorescence quenching effect of Fe(III) on BODIPY-ATP via its coordination with the triphosphate group and N7 nitrogen of BODIPY-ATP, the quenched form of BODIPY-ATP complexed with Fe(III) (BODIPY-ATP/Fe(III)) was prepared () [Citation13]. We expect that as DNA polymerase catalyses the synthesis of DNA by adding the specially designed monomers into the growing chains, Fe(III) would be liberated from BODIPY with the concomitant release of pyrophosphate group, which would lead to the high fluorescence signal from the produced, long oligonucleotides ().
Figure 1. (a) Fe(III)-induced fluorescence quenching of BODIPY-ATP. (b) Schematic illustration of the TdT activity assay utilizing BODIPY-ATP/Fe(III).
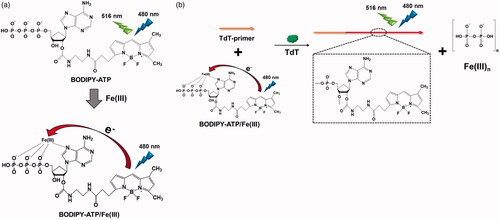
As a model DNA polymerase, we selected terminal transferase (TdT), a template-independent DNA polymerase that catalyses the addition of mononucleotides to the 3’ terminus of DNA [Citation14–17]. It is known that TdT serves as an indicator for acute leukaemia and thus there is high demand for a simple and novel strategy for the determination of TdT activity [Citation18,Citation19]. In the effort described below, we successfully demonstrated the proposed concept with TdT, which was applied for the simple determination of TdT activity with high sensitivity and selectivity.
Material and methods
Materials
TdT-primer (5′-AATACAACCTCTCA-3′) used in the study was synthesized by Genotech Co. (Daejeon, South Korea). TdT, phi29 DNA polymerase, exonuclease I (Exo I), Klenow fragment (exo-), Vent (exo-) DNA polymerase, T4 DNA ligase and T4 DNA polymerase were purchased from New England Biolabs Inc. (Beverly, MA), and i-pfu DNA polymerase was purchased from iNtRON Biotechnology (Seongnam, Korea). BODIPY-ATP and FeCl3·6H2O were purchased from Invitrogen (Carlsbad, CA) and Sigma-Aldrich (St. Louis, MO), respectively. All other chemicals were of analytical grade and used without further purification. Ultrapure DNase/RNase-free distilled water purchased from Bioneer® (Daejeon, Korea) was used in all experiments.
Procedure to determine TdT activity
TdT-primer (1 μM), BODIPY-ATP/Fe(III) (5 µM), and TdT at varying concentrations were incubated in 1X TdT reaction buffer (20 mM Tris-acetate, 50 mM potassium acetate, 10 mM magnesium acetate, 0.25 mM CoCl2, pH 7.9) at 37 °C for 1.5 h (Total volume: 20 μL). Then the mixture was heated to 75 °C for 20 min to inactivate the enzyme and the resulting fluorescence emission spectra were measured in the range of 510–600 nm at an excitation wavelength of 480 nm by using Tecan Infinite M200 pro-microplate reader (Mnnedorf, Switzerland).
Gel electrophoresis analysis for TdT-catalysed polymerization products
The reaction products were resolved on a 16% polyacrylamide gel using 1X TBE as a running buffer at a constant voltage of 100 V for 40 min. After staining with SYBR Green II (Invitrogen), a gel image was taken with Gel Doc EZ Imager (Bio-rad, Hercules, CA).
Determination of TdT activity in human serum (10%)
The known concentrations of TdT were first spiked into the human serum to prepare a set of standards and then a calibration curve was created according to the procedures explained above. Based on this calibration curve, the concentrations of TdT of unknown samples were determined from the resulting fluorescence intensities.
Results and discussion
Detection feasibility
First, we investigated the quenching effect of Fe(III) on BODIPY-ATP by measuring the fluorescence signals at 516 nm, an emission maximum of BODIPY, in the presence of varying concentrations of Fe(III). As shown in Figure S1(a), Fe(III) quenches the fluorescence signals of BODIPY-ATP in a dose-dependent manner through a process of the photo-induced electron transfer [Citation13]. It was also confirmed that Fe(III)-induced fluorescence quenching of BODIPY-ATP is stable at temperatures up to 90 °C (Figure S1(b)).
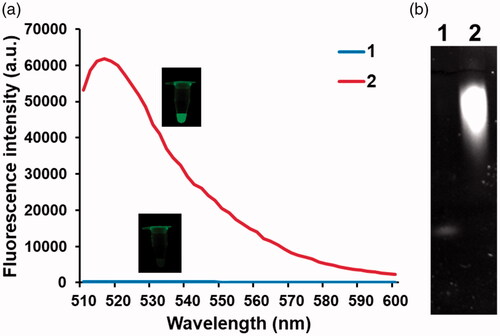
Next, the detection feasibility of this strategy was verified by employing BODIPY-ATP/Fe(III) as the monomer for TdT-catalysed polymerization. As envisioned, the presence of TdT produced the significantly increased fluorescence signal compared to that in the absence of TdT, which was supported by the formation of long oligonucleotides (. In addition, TdT-catalysed polymerization was monitored at different time points. The results in Figure S2 show that fluorescence signal increases by TdT as the reaction time increases until 90 min, over which it reaches a plateau. Overall, these observations confirm that TdT transforms BODIPY-ATP/Fe(III) into BODIPY-adenosine of the long oligonucleotides, resulting in the significantly increased fluorescence signal, which can be used for the simple, fluorescence turn-on determination of enzyme activities.
Figure 2. Feasibility of the TdT activity assay. (a) Fluorescence spectra from BODIPY and (b) polyacrylamide gel electrophoresis images under different conditions (1: TdT-primer + BODIPY-ATP/Fe(III); 2: TdT-primer + BODIPY-ATP/Fe(III) + TdT). The insets in (a) show the corresponding photographs under UV light. The final concentrations of Fe(III) and TdT are 1 mM and 200 U/mL, respectively.
Detection sensitivity and selectivity
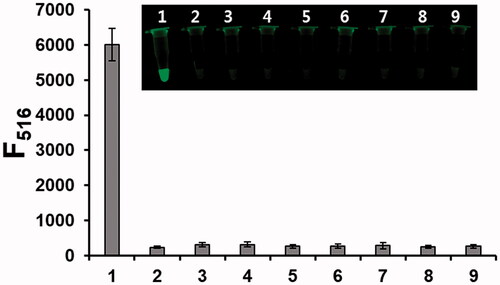
To assess the sensitivity of the proposed strategy, the fluorescence intensities at 516 nm were measured as a function of TdT concentration. As shown in , the linear relationship is observed in the range from 0 to 50 U/mL (R2=0.9942, F516 = 1141.4 × CTdT/U mL−1 + 3416.9, where CTdT is the concentration of TdT) with the detection limit of 0.64 U/mL (3σ/slope), a value that is comparable or superior to those associated with other fluorescence-based TdT assays [Citation20–24]. The selectivity was also determined by employing other enzymes such as exonucleases, ligases and template-dependent DNA polymerases. The results in show that the fluorescence of BODIPY is efficiently switched on by the action of TdT, while other enzymes induce the negligible fluorescence enhancement even though they are present at 10 times higher concentration than that of TdT, confirming that it has high selectivity toward TdT.
Figure 3. Sensitivity of the TdT activity assay. (a) Fluorescence spectra from BODIPY and (b) fluorescence intensities at 516 nm from BODIPY in the presence of TdT at varying concentrations. Insets in (a) and (b) indicate the corresponding photographs under UV light and linear relationship between fluorescence intensity at 516 nm and TdT concentration, respectively.
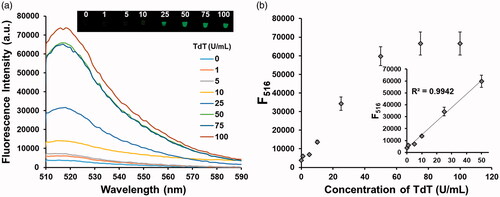
Figure 4. Selectivity of the TdT activity assay. Fluorescence intensities at 516 nm from BODIPY in the presence of TdT (1 U/mL) and other enzymes (10 U/mL) (1: TdT; 2: Blank; 3: phi29 DNA polymerase; 4: Exo I; 5: Klenow fragment (exo-); 6: Vent (exo-) DNA polymerase; 7: T4 DNA ligase; 8: T4 DNA polymerase; 9: i-pfu DNA polymerase). The inset shows the corresponding photographs under UV light.
Practical applicability
Finally, the ability of this strategy to determine TdT activity in human serum was evaluated to check its practical applicability. Following that the known amounts of TdT were spiked into diluted human serum (10%), the recovery experiments were carried out. As evidenced by good recovery ratio between 98% and 102% in , it worked well even in the serum containing various interfering agents, which indicates that it can be applied for the determination of TdT in real samples.
Table 1. Determination of the TdT activity in diluted human serum.
Conclusion
In summary, we found that BODIPY-ATP/Fe(III) serves as the effective monomer for the in situ synthesis of fluorescence oligonucleotides, which was utilized for the sensitive and selective determination of TdT activity. Importantly, the use of BODIPY-ATP/Fe(III) enables the cheap and convenient synthesis of fluorescence oligonucleotides while overcoming the drawbacks of previous methods. To the best of our knowledge, this is the first report that exploits BODIPY-ATP/Fe(III) in DNA polymerization to develop biosensing platforms. We believe this strategy has a great potential to be applied for the simple and sensitive detection of other biological targets such as small molecules, nucleic acids, and proteins, and even for the formation of versatile nanostructures.
181009_ACNB_TdT_activity_assay_Supporting.doc
Download ()Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Li Z, Bai X, Ruparel H, et al. A photocleavable fluorescent nucleotide for DNA sequencing and analysis. Proc Natl Acad Sci U S A. 2003;100:414–419.
- Seo TS, Bai X, Ruparel H, et al. Photocleavable fluorescent nucleotides for DNA sequencing on a chip constructed by site-specific coupling chemistry. Proc Natl Acad Sci U S A. 2004;101:5488–5493.
- Tan L, Liu Y, Yang Q, et al. Design and synthesis of fluorescence-labeled nucleotide with a cleavable azo linker for DNA sequencing. Chem Commun (Camb). 2016;52:954–957.
- Kosuri S, Church GM. Large-scale de novo DNA synthesis: technologies and applications. Nat Methods. 2014;11:499–507.
- Conrad F, Hanne A, Gaur RK, et al. Enzymatic synthesis of 2′-modified nucleic acids: identification of important phosphate and ribose moieties in RNase P substrates. Nucl Acids Res. 1995;23:1845–1853.
- Hocek M. Synthesis of base-modified 2′-deoxyribonucleoside triphosphates and their use in enzymatic synthesis of modified DNA for applications in bioanalysis and chemical biology. J Org Chem. 2014;79:9914–9921.
- Verga D, Welter M, Steck A-L, et al. DNA polymerase-catalyzed incorporation of nucleotides modified with a G-quadruplex-derived DNAzyme. Chem Commun. 2015;51:7379–7381.
- Guliyev R, Ozturk S, Sahin E, et al. Expanded bodipy dyes: anion sensing using a bodipy analog with an additional difluoroboron bridge. Org Lett. 2012;14:1528–1531.
- Zhu S, Dorh N, Zhang J, et al. Highly water-soluble neutral near-infrared emissive BODIPY polymeric dyes. J Mater Chem. 2012;22:2781–2790.
- Fan G, Yang L, Chen Z. Water-soluble BODIPY and aza-BODIPY dyes: synthetic progress and applications. Front Chem Sci Eng. 2014;8:405–417.
- Xu J, Li Q, Yue Y, et al. A water-soluble BODIPY derivative as a highly selective “Turn-On” fluorescent sensor for H2O2 sensing in vivo. Biosens Bioelectron. 2014;56:58–63.
- Zhang J, Peng F, Dong X, et al. Water-soluble BODIPY derivative as a highly selective “Turn-On” fluorescent probe for hydrogen sulfide in living cells. Chem Lett. 2015;44:1524–1526.
- Lin J-H, Yang Y-C, Shih Y-C, et al. Photoinduced electron transfer between Fe(III) and adenosine triphosphate-BODIPY conjugates: application to alkaline-phosphatase-linked immunoassay. Biosens Bioelectron. 2016;77:242–248.
- Zhao B, Gong Z, Ma Z, et al. Simple and sensitive microRNA labeling by terminal deoxynucleotidyl transferase. Acta Biochim Biophys Sin. 2012;44:129–135.
- Boulé J-B, Rougeon F, Papanicolaou C. Terminal deoxynucleotidyl transferase indiscriminately incorporates ribonucleotides and deoxyribonucleotides. J Biol Chem. 2001;276:31388–31393.
- Wang L-J, Ren M, Liang L, et al. Controllable fabrication of bio-bar codes for dendritically amplified sensing of human T-lymphotropic viruses. Chem Sci. 2018;9:4942–4949.
- Zhang Y, Wang X-Y, Zhang Q, et al. Label-free sensitive detection of DNA methyltransferase by target-induced hyperbranched amplification with zero background signal. Anal Chem. 2017;89:12408–12415.
- McCaffrey R, Lillquist A, Sallan S, et al. Clinical utility of leukemia cell terminal transferase measurements. Cancer Res. 1981;41:4814–4820.
- Wang L-J, Luo M-L, Zhang Q, et al. Single quantum dot-based nanosensor for rapid and sensitive detection of terminal deoxynucleotidyl transferase. Chem Commun. 2017;53:11016–11019.
- Lu L, Wang M, Liu L-J, et al. A luminescence switch-on probe for terminal deoxynucleotidyl transferase (TdT) activity detection by using an iridium(III)-based i-motif probe. Chem Commun. 2015;51:9953–9956.
- Yuan Y, Li W, Liu Z, et al. A versatile biosensing system for DNA-related enzyme activity assay via the synthesis of silver nanoclusters using enzymatically-generated DNA as template. Biosens Bioelectron. 2014;61:321–327.
- Ye T, Li C, Su C, et al. Enzymatic polymerization of poly (thymine) for the synthesis of copper nanoparticles with tunable size and their application in enzyme sensing. Chem Commun. 2015;51:8644–8647.
- Peng F, Liu Z, Li W, et al. Enzymatically generated long polyT-templated copper nanoparticles for versatile biosensing assay of DNA-related enzyme activity. Anal Methods. 2015;7:4355–4361.
- Liu Z, Li W, Nie Z, et al. Randomly arrayed G-quadruplexes for label-free and real-time assay of enzyme activity. Chem Commun (Camb). 2014;50:6875–6878.
