Abstract
Nanoparticle albumin-bound paclitaxel (Nab-paclitaxel) offer more efficient paclitaxel delivery into tumor cells with fewer side effects than conventional chemotherapies. However, whether the efficacy of Nab-paclitaxel for non-small cell lung cancer (NSCLC) patients is age-related remains unknown. We performed this meta-analysis to evaluate the anticancer efficacy and safety of Nab-paclitaxel in NSCLC. Hazard ratios (HRs) or rate ratios (RRs) with corresponding 95% confidence intervals (CIs) for outcomes were retrieved. Thirteen high-quality studies with 4613 patients were included, of which five were comparative trials comparing nab-paclitaxel plus carboplatin (nab-P/C) with solvent-based paclitaxel plus carboplatin (sb-P/C), and the others were non-comparative trials investigating the nab-paclitaxel efficacy. Pooled comparative trial estimates showed that nab-P/C significantly improved overall response rates ([RR]: 1.31) and prolonged progression-free survival (PFS) (HR: 0.89) and overall survival (OS) (HR: 0.93) compared with the control. However, meta-analysis of the younger subgroup indicated that PFS (HR: 93) and OS (HR: 96) were similar between the two arms. Regarding safety, nab-paclitaxel significantly increased risk for grade ≥3 anaemia. For non-hematological adverse events, grade ≥3 sensory neuropathy and arthralgia occurred more frequently in the control arm than in the experimental arm. In conclusion: Nab-paclitaxel is effective and safe for NSCLC patients, especially the elderly.
Introduction
Lung cancer is the most common malignancy and the leading cause of cancer-associated morbidity and mortality worldwide [Citation1]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers, and more than 50% of patients with NSCLC have metastatic disease or/and have reached advanced stages at the time of their first diagnosis. The 5-year survival rate for these patients is only 3.9% [Citation2].
Paclitaxel, extracted from the bark of the Pacific yew, plays an essential role in the treatment of advanced NSCLC [Citation3,Citation4]. Because of its poor aqueous solubility, the standard commercial formulation of paclitaxel consists of the cremophor EL (CrEL) solvent system and ethanol, which can provoke serious toxicities, such as nephrotoxicity, endothelial toxicity, neurotoxicity, hypersensitivity and even irreversible sensory neuropathy [Citation5,Citation6]. To minimize the risk of hypersensitivity reactions caused by cremophor, the administration of CrEL requires a long infusion period (typically 3–24 h), in-line filters, and premedication with steroids and antihistamines [Citation4]. Regardless of these precautions, severe and even fatal hypersensitivity reactions still occur. Additionally, the sb-paclitaxel cremophor excipient traps paclitaxel in micelles, which results in nonlinear pharmacokinetics [Citation7]. Therefore, we urgently need to develop additional treatment strategies with high efficiencies and less toxicities for patients with NSCLC.
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is a cremophor-free, 130-nanometer particle form of paclitaxel that has been approved by the Food and Drug Administration, in January 2005, as a treatment for breast cancer. Compared with conventional sb-paclitaxel, this new formulation avoids CrEL-related toxicities and allows a shorter infusion period, because there is no need for steroid and antihistamine premedication. In addition, a recent pharmacokinetic/pharmacodynamic study demonstrated that nab-paclitaxel, in preclinical xenograft models, achieves a higher concentration of paclitaxel delivery and a higher mean free paclitaxel maximum serum concentration, compared with sb-paclitaxel, which contribute to its antitumor activity [Citation8]. The safety benefits and efficacy of nab-paclitaxel were investigated in many other clinical trials. However, single studies often exhibit large heterogeneities and biases owing to variable study designs and limited sample sizes, which may negatively affect study accuracy and conclusions. Based on published evidence, we herein performed a systematic meta-analysis according to standard methods, to evaluate the efficacy and toxicity of nab-paclitaxel in patients with advanced NSCLC.
Methods
Literature search
A systematic literature search was independently conducted by two authors. The PubMed, Clinical Trials.gov, Embase, Cochrane Library, and Web of Science English databases were searched for relevant studies that were published before 1 December, 2017. The major search terms that were used were (“Non-small Cell Lung Cancer” or “Non-small Cell Lung Neoplasm” or “Lung Neoplasm” or “Lung Cancer”) and (“nab-paclitaxel” or “nab-PTX” or “albumin-PTX” or “Abraxane”) AND “therapy” and “efficacy.” Any disagreements between the two investigators who performed the literature search were resolved through a consensus.
Inclusion and exclusion criteria
Studies that met the following eligibility criteria were included in this meta-analysis: (1) the articles were based on randomized controlled trials (RCTs) or non-comparative studies with more than 15 eligible patients; (2) the disease was histologically or cytologically confirmed as NSCLC; (3) the full paper or conference abstract was available; (4) the primary endpoint was overall response rate (ORR); (5) the outcomes involved hazard ratios (HRs) or rate ratios (RRs) and 95% CIs for RCTs; and (6) the adverse events were graded according to the National Cancer Institute Common Terminology Criteria, version 3.0. Exclusion criteria were as follows: (1) the studies included patients with other cancers; (2) the data was incomplete; (3) the study design was unclear; and (4) articles were duplicate.
Quality assessment of the studies
The quality assessment of the included RCTs was conducted according to the Cochrane Handbook, using the following criteria: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and study personnel; (4) blinding of outcome assessments; (5) incomplete outcome data; (6) selective reporting. Each term was definitively graded as a low, unclear, or high bias risk. Furthermore, the methodological qualities of the included non-comparative studies were assessed using the Methodological Index for Non-Randomized Studies form [Citation9].
Data extraction
Two investigators (Liu S, Hu J) independently extracted data from each eligible full-text and reached a consensus after discussion. The following information was obtained from each study: first author, year of publication, stage of NSCLC, study design, treatment regimen, median age and number of patients in each arm, the RRs with corresponding 95% CIs for primary end-point ORR and the HRs with 95% CIs for second end-point data (progression-free survival [PFS] and overall survival [OS]). In order to measure the safety, data of the major adverse events rates in each study were retrieved.
Statistical Analysis
RRs and HRs were derived from each study as primary and secondary endpoints, respectively, and the corresponding 95% CIs were also extracted. Statistical heterogeneity among these studies was determined by Cochran's Q test and I2 index, in which I2<50% or p values of <.10 indicated that significant heterogeneity did not exist. The fixed-effects model was applied if heterogeneity was not observed among the studies; otherwise, the random-effects model was adopted for pooled estimates. For adverse events analysis, the fixed-effect model with the Mantel-Haenszel method was applied to pool the odds ratios by using event counts and total sample sizes that were extracted from each arm. Statistically significant differences were represented by p values of <.05 and 95% CIs that did not overlap. Publication bias was assessed using funnel plots. All of the meta-analyses were performed with the Cochrane Review Manager (RevMan, software version 5.2).
Results
Study identification and selection
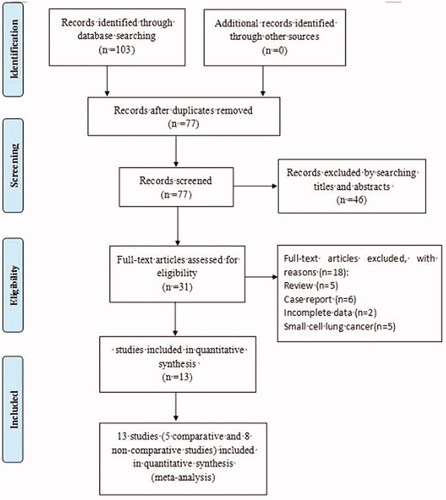
As shown in the flowchart (), 103 potentially related papers were initially retrieved from PubMed, ClinicalTrials.gov, Embase, Cochrane Library, and Web of Science. After excluding duplicates, 77 records were reserved. Following title and abstract screening, 46 irrelevant publications were excluded. From the 31 remaining articles, 18 were removed due to review, case report, small cell lung cancer and incomplete data. Finally, a total of 13 studies, including five comparative [Citation10–14] and eight non-comparative [Citation15–22] studies, were considered eligible for this meta-analysis, according to the selection criteria.
Characteristics of the comparative studies
The characteristics of the included comparative articles are presented in , including authors, publication year, stage of NSCLC, treatment arms, median age and number of patients, and outcome data. As shown, all of the studies were published between 2006 and 2017. This meta-analysis evaluated 4342 patients with stage IIIB or IV NSCLC, of whom 2192 were included in the control arm and 2150 in the experimental arm. Nab-paclitaxel was administered weekly, at a dose of 100 mg/m2, followed by carboplatin at an under the curve (AUC) of six, every 3 weeks, in the experiment arm. A dose of 200 mg/m2 solvent-based paclitaxel was given every 3 weeks followed by carboplatin (AUC =6) in the control arm.
Table 1. Characteristics of the included comparative studies.
Quality assessment
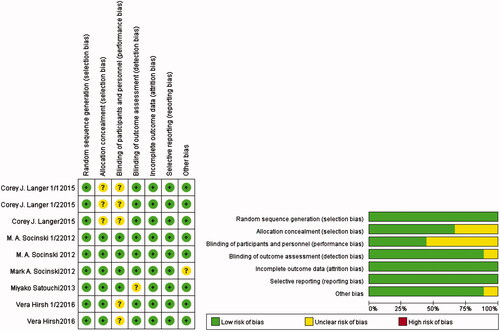
The quality assessment details for the comparative studies are shown in . Overall, two trials were defined as having lows risk of bias, and seven had unclear risks of biases. The major reasons why the seven trials had unclear risks of biases were due to unclear blinding of outcome assessments, blinding of participants and study personnel, and allocation concealment. Adequate randomized sequences were reported in all included trials, which had minor incomplete outcome data, selective reporting, or other biases. In conclusion, no significant biases were present in this meta-analysis.
Efficacy
ORR
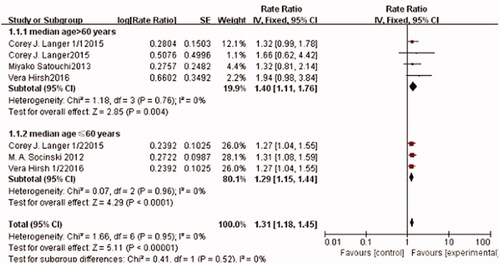
Seven trials, including 3280 patients, reported ORR. The pooled estimates showed that there was no significant heterogeneity among these trials (I2 =0%, p = .95). Therefore, the fixed-effects model was used for the meta-analysis. The meta-analysis results showed that Nab-paclitaxel treatment followed by carboplatin significantly improved the ORR compared with solvent-based paclitaxel followed by carboplatin chemotherapy (RR: 1.31; 95% CI: [1.18, 1.45], p < .0001) (). A subgroup analysis was also conducted to estimate the impact of different treatment arms, according to median patient. Compared with that of the sb-paclitaxel arm, ORR of the nab-paclitaxel arm was better in both younger (RR: 1.29; 95% CI: [1.15, 1.44], p < .0001) and older (RR: 1.40; 95% CI: [1.11, 1.76], p = .004) patient subgroups.
PFS
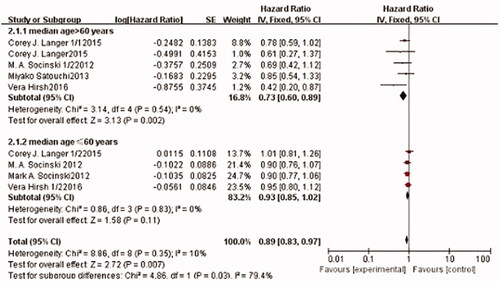
Nine trials, which included 4342 patients, reported PFS. The pooled estimates showed that there was no significant heterogeneity among these trials (I2=0%, p = .35). Therefore, the fixed-effects model was used for the meta-analysis. The pooled overall PFS results showed that there was a significant difference between the experimental and control arms (HR: 0.89; 95% CI: [0.83, 0.97], p = .007) (), which indicates that nab-paclitaxel-based chemotherapy significantly prolongs PFS. In the subgroup analysis, the pooled HR for PFS was evaluated according to patient age. In the elderly subgroup, a significant PFS benefit was observed in the nab-P/C arm, compared to sb-P/C arm (HR: 0.73; 95% CI: [0.60, 0.89], p = .002). However, no significant difference was found in the PFS of patients aged <60 years (p > .05 with overlapping 95% CI; HR: 93; 95% CI: [0.85, 1.02], p = .11). Therefore, Nab-P/C more effectively prolongs PFS in patients aged >60 years.
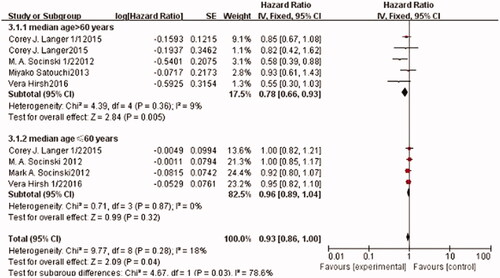
OS
Nine trials, with a total of 4342 patients, reported OS. The pooled estimates showed that there was no significant heterogeneity among these trials (I2=18%, p = .28). Therefore, the fixed-effects model was used for the meta-analysis. Compared to the control arm, patients in the corresponding experimental arm gained benefits from no hypertension (HR: 0.93; 95% CI: [0.86, 1.00], p = .04) (). Considering that age is a potential factor for the efficacy of nab-P/C based chemotherapy, studies that were included in this group were further stratified by patient age (median age >60 years or median age ≤60 years). The results showed that the effect of nab-P/C on OS was dramatically different between these two subgroups. The OS of the nab-P/C arm significantly improved in the patients of the >60 years of age subgroup compared with the sb-P/C arm (HR: 0.78; 95% CI: [0.66, 0.93], p = .005), but not in the other subgroup (HR: 96; 95% CI: [0.89, 1.04], p = .32).
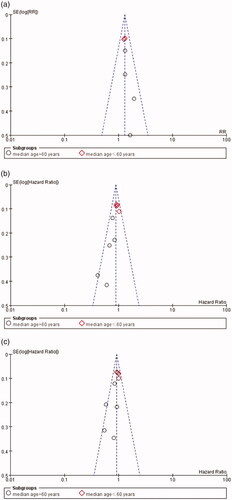
Analysis of publication bias
Funnel plots were used to assess publication bias. As reflected in , there was no obvious evidence for publication bias, as the funnel-shape was basically symmetrical.
Toxicity
Treatment-related adverse effects (AEs) (grades ≥3) for patients with advanced NSCLC were investigated in the five collected comparative studies, using event counts and total sample sizes that were extracted from each arm. The details of the toxicity analysis are summarized in . The patients in the nab-paclitaxel group showed more obvious side effects than those in the sb-paclitaxel group, such as nausea (OR: 1.90; CI: [0.86, 4.21], p = .11), anemia (OR: 5.04; CI: [4.16, 6.10], p < .00001), thrombocytopenia (OR: 2.37; CI: [1.97, 2.86], p < .00001), alopecia (OR: 7.18; CI:[1.29, 39.86], p = .02), and anorexia (OR: 2.06; CI: [1.22, 3.50], p = .007). Patients who received nab-paclitaxel therapy experienced three other AEs, which were generally equivalent to those of the control group: fatigue (OR: 0.80; CI: [0.63, 1.01], p = .06) and febrile neutropenia (OR: 0.82; CI: [0.48, 1.40], p = .46). The patients in the nab-paclitaxel treatment group showed fewer side effects than those in the control group, which included neutropenia (OR: 0.65; CI: [0.57, 0.73], p < .00001), sensory neuropathy (OR: 0.22; CI: [0.16, 0.31], p < .00001), arthralgia (OR: 0.08; CI: [0.03, 0.23], p < .00001), myalgia (OR: 0.39; CI: [0.22, 0.67], p < .00001), and peripheral neuropathy (OR: 0.26; CI: [0.15, 0.46], p < .00001). Heterogeneity was calculated, and the fixed-effects models were used for AE analysis.
Table 2. The meta-analysis result of the toxic effects in comparative studies.
Non-comparative studies analysis
The characteristics of the eight included non-comparative studies are summarized in . The sample size of these studies ranged from 21 to 48 patients. All patients in these studies received nab-paclitaxel or nab-paclitaxel followed by carboplatin, and the ORRs ranged from 16.30% to 50.00%. With respect to safety, the most major serious AEs are listed in . The most frequently observed AE was neutropenia (22.1%), and the other treatment-related AEs were leukopenia (14.8%), anaemia (8.0%), fatigue (12.1%), anorexia (5.0%), and thrombocytopenia (8%). However, no known treatment-related death has been reported after nab-paclitaxel treatment. The result of the quality assessment of included non-comparative studies is shown in .
Table 3. Characteristics of the non-comparative studies.
Table 4. The Summaries of the major serious adverse events in non-comparative studies.
Table 5. The Study designs and MINORS appraisal scores for the non-comparative studies.
Discussion
Nanoparticle albumin-bound paclitaxel, a novel formulation of paclitaxel, can reach higher tumor accumulation than sb-paclitaxel, due to a receptor-mediated transport process [Citation23–25] and an enhanced permeability and retention (EPR) effect [Citation26]. Additionally, since this formulation is free of cremophor, it exhibits promising tolerability with less side effects than sb-paclitaxel. Therefore, Nab-paclitaxel has great advantages in the treatment of cancer and has attracted great attention.
In this systematic review and meta-analysis, we demonstrated that Nab-paclitaxel is associated with significantly improved antitumor efficacy and generally reasonable toxicities. To our knowledge, this is the first meta-analysis to systematically investigate the efficacy and safety of nab-paclitaxel for patients with NSCLC. In this study, 13 clinical trials, including five comparative and eight non-comparative studies, were included. There were 4342 patients with stage IIIB or IV NSCLC aged between 24 and 84 years in the comparative studies, which insured a sufficient sample scale for meta-analysis. We evaluated the ORR, PFS and OS of Nab-paclitaxel followed by carboplatin, to treat locally advanced NSCLC, and our results showed that there was a significant difference between the experimental and control arms.
Nab-paclitaxel followed by carboplatin achieved a higher ORR, longer PFS and longer OS compared to the control arm. Furthermore, we performed a subgroup analysis to evaluate the ORR, PFS and OS of nab-paclitaxel treatment in the elderly and younger subgroups. The PFS and OS meta-analysis result showed that there was significant difference between the two arms of the elderly patient subgroup. Regarding ORRs, significant benefits were observed for both younger and elderly patients, when administrated nab-paclitaxel chemotherapy. It is necessary to mention here that pooled estimates from our study were similar to those from the large clinical study by Socinski et al. [Citation11], which included 1052 patients with stage IIIB/IV NSCLC. Their results showed that in elderly patients with advanced NSCLC, nab-P/C improved ORR and was associated with significantly longer PFS and OS than to sb-P/C. Furthermore, from the non-comparative studies analysis, we also found that nab-paclitaxel demonstrates promising antitumour efficacy for patients.
In terms of treatment-related toxicities, those of nab-paclitaxel chemotherapy were relatively controllable, which was consistent with a previous review conducted by Socinski et al. [Citation27]. The comparative meta-analysis showed that hematological toxicities, such as anaemia and thrombocytopenia, significantly increased in patients who received nab-paclitaxel. However, lower incidences of neutropenia were observed in the nab-P/C arm. With respect to non-hematological toxic effects, grade ≥3 alopecia, nausea and anorexia occurred more frequently in the nab-P/C group than in the sb-P/C group. Nevertheless, significantly less grade ≥3 sensory neuropathy, myalgia, arthralgia and peripheral neuropathy were observed in the nab-P/C cohort than in the sb-P/C cohort. In the non-comparative studies, the most frequent AEs were neutropenia, leukopenia, anemia, fatigue, anorexia and thrombocytopenia. However, no known treatment-related deaths were reported after nab-paclitaxel treatment. Thus, it is conceivable that nab-P/C has improved tolerability, which may allow higher dose delivery and contributed to the survival benefits in patients with NSCLC. In the present analysis, a large number of high-quality studies were included, and no significant heterogeneity or potential publication bias was detected among these studies. However, several limitations of the present meta-analysis should be considered. First, ORR outcome data were not available from one study [Citation11]. Second, most of the included patients were men, which might lead to a sex bias. Third, one of the included trials failed to provide detailed treatment information, which could lead to a potential risk of bias [Citation13]. In conclusion, this systematic review and meta-analysis demonstrated that albumin-bound paclitaxel significantly increases ORR and prolongs PFS and OS compared to sb-paclitaxel based chemotherapy for patients with NSCLC, especially those who are elderly. Therefore, nab-paclitaxel is an appropriate clinical drug that may achieve greater anticancer efficacy with generally tolerable toxicities than traditional chemotherapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
- Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw. 2013;11:645–653.
- Wani MC, Taylor HL, Wall ME, et al. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327.
- Socinski MA. Single-agent paclitaxel in the treatment of advanced non-small cell lung cancer. Oncologist. 1999;4:408–416.
- ten Tije AJ, Verweij J, Loos WJ, et al. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–685.
- Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. JCO. 1990;8:1263–1268.
- Sparreboom A, van Zuylen L, Brouwer E, et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999;59:1454–1457.
- Gardner ER, Dahut WL, Scripture CD, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14:4200–4205.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716.
- Satouchi M, Okamoto I, Sakai H, et al. Efficacy and safety of weekly nab-paclitaxel plus carboplatin in patients with advanced non-small cell lung cancer. Lung Cancer. 2013;81:97–101.
- Socinski MA, Langer CJ, Okamoto I, et al. Safety and efficacy of weekly nab(R)-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:314–321.
- Hirsh V, Ko A, Pilot R, et al. Weekly nab-paclitaxel in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: analysis of safety and efficacy in patients with diabetes. Clin Lung Cancer. 2016;17:367–374.
- Langer CJ, Hirsh V, Ko A, et al. Weekly nab-paclitaxel in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: analysis of safety and efficacy in patients with renal impairment. Clin Lung Cancer. 2015;16:112–120.
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. JCO. 2012;30:2055–2062.
- Green MR, Manikhas GM, Orlov S, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17:1263–1268.
- Reynolds C, Barrera D, Jotte R, et al. Phase II trial of nanoparticle albumin-bound paclitaxel, carboplatin, and bevacizumab in first-line patients with advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2009;4:1537–1543.
- Xing PY, Li JL, Wang Y, et al. Efficacy and safety of albumin-bound paclitaxel in treating recurrent advanced non-small-cell lung cancer. Chin J Cancer Res. 2013;25:200–205.
- Fang Y, Wang L, Xia GH, et al. Clinical investigation of efficacy of albumin bound paclitaxel plus platinum compounds as first-line chemotherapy for stage III/IV squamous non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15:7453–7457.
- Jin F, Zhu H, Shi F, et al. A retrospective analysis of safety and efficacy of weekly nab-paclitaxel as second-line chemotherapy in elderly patients with advanced squamous non-small-cell lung carcinoma. Clin Interv Aging. 2016;11:167–173.
- Zheng Q, Yao Y, Nan K. Weekly intravenous nanoparticle albumin-bound paclitaxel for elderly patients with stage IV non-small-cell lung cancer: a series of 20 cases. J Biomed Res. 2012;26:159–164.
- Higuchi M, Takagi H, Owada Y, et al. Efficacy and tolerability of nanoparticle albumin-bound paclitaxel in combination with carboplatin as a late-phase chemotherapy for recurrent and advanced non-small-cell lung cancer: a multi-center study of the Fukushima lung cancer association group of surgeons. Oncol Lett. 2017;13:4315–4321.
- Miyauchi E, Inoue A, Usui K, et al. Phase II study of modified carboplatin plus weekly nab-paclitaxel in elderly patients with non-small cell lung cancer: North Japan lung cancer study group trial 1301. Oncologist. 2017;22:640–e59.
- Li HH, Li J, Wasserloos KJ, et al. Caveolae-dependent and -independent uptake of albumin in cultured rodent pulmonary endothelial cells. PloS One. 2013;8:e81903.
- Schnitzer JE, Oh P. Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J Biol Chem. 1994;269:6072–6082.
- Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324.
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392.
- Socinski MA. Update on taxanes in the first-line treatment of advanced non-small-cell lung cancer. Curr Oncol. 2014;21:e691–e703.