Abstract
A novel multifunctional nanoplatform constructed from methoxy-PEGylated poly(amidoamine) (PAMAM) generation 3 dendrimers with superparamagnetic iron oxide nanoparticles (SPIONs) entrapped in their core, containing curcumin as the payload drug and folic acid (folate) as the targeting ligand (abbreviated as FA-mPEG-PAMAM G3-CUR@SPIONs), is presented in this study. SPIONs entrapped in the core of the nanocomplex may act as a hyperthermia agent and generate localized heat upon excitation with an alternating magnetic field (AMF), thus enabling a thermo-chemotherapy strategy for cancer treatment. Accordingly, the cytotoxic effect and the mode of cell death triggered by the nanocomplex in combination with AMF were evaluated on two different cancer cell lines with various folate receptor (FR) expression levels, including KB nasopharyngeal cancer cells overexpressing FRs as the model and MCF-7 breast cancer cells with low level of FRs as the blank sample. The obtained results showed that KB cell death was greater than the cell death observed in MCF-7 cells. Moreover, a majority of cell death in both cell lines were related to apoptosis when the folate-modified nanocomplex was used instated of the non-folate-modified nanocomplex. Therefore, functionalizing the nanocomplex with folate modulated the response to thermo-chemotherapy by shifting the cell death pathways toward apoptosis.
Graphical Abstract
Introduction
Chemotherapy, as one of the primary cancer treatment modality in its current form, encounters serious drawbacks that cannot provide a satisfactory therapeutic outcome. Multidrug resistance (MDR), as a result of repeated drug administration over a chemotherapy treatment course, is known as the major factor responsible for chemotherapy failure [Citation1,Citation2]. Following the administration of anticancer drugs, they are distributed throughout the body without preferential accumulation within the tumour that may cause normal tissue toxicity and then impart additional complexity to the patient, thereby reducing the quality of life [Citation3,Citation4]. The incorporation of anticancer drugs into nanocarriers has recently emerged as a promising strategy to break the limitations of traditional chemotherapy. Site-specific tumour targeting and thus a reduction of off-target side effects, the ability to control the rate and the site of payload release, and improved solubility and stability of drugs are some of the favourable consequences of drug delivery using nanocarriers [Citation5,Citation6].
Curcumin, a natural polyphenol found in herbal remedies and the dietary spice turmeric, has been recently introduced as a new anticancer agent capable of inhibiting proliferation and inducing apoptosis [Citation7,Citation8]. The recent trend of using curcumin in cancer chemotherapy not only arises from its anticancer activity but also from the fact that it may be highly tolerated by patients without the development of side effects, even at high doses in comparison to other chemotherapeutic agents. Curcumin has been utilized in many clinical trials for the treatment of various cancers including colorectal, prostate, head and neck, pancreatic, and breast cancer thus far [Citation9,Citation10]. However, due to its very poor bioavailability, low water solubility, rapid metabolism, and systemic elimination, curcumin has not yet been considered in routine clinical practice [Citation7]. The encapsulation of curcumin into nanocarriers appears to be a good strategy in addressing these problems, thus expanding the clinical utility of curcumin.
Dendrimers are a class of highly branched polymers with well-defined interior void spaces that are suitable for accommodating guest molecules and have been proposed as an ideal drug delivery system [Citation11]. Recent studies have demonstrated the enhanced solubility and the bioavailability of curcumin when incorporated with dendrimers [Citation12]. Overcoming the drawbacks of curcumin by using dendrimers can provide an ideal opportunity for curcumin to further progress in clinical applications. On the other hand, the unique chemical features of dendrimers can be exploited for the attachment of additional substrates (e.g. nanoparticles) in order to expand their biomedical applications. In this regard, the functionalization of dendrimers with magnetic nanoparticles (MNPs) will create a versatile multifunctional nanoplatforms capable of the diagnosis and treatment of cancer [Citation13,Citation14]. Upon excitation of alternating magnetic fields (AMFs), MNPs generate localized heat through hysteresis loss and relaxation loss, which are used to mediate cancer hyperthermia [Citation15]. Consequently, curcumin-encapsulated dendrimers, when functionalized with MNPs, may enable a combinatorial cancer treatment strategy through the concurrent delivery of hyperthermia and chemotherapy, termed as thermo-chemotherapy; this results in synergistic therapeutic effects. Furthermore, MNPs have been widely utilized as contrast agents to enhance the sensitivity and the specificity of magnetic resonance imaging (MRI). Therefore, an image-guided strategy to monitor the drug delivery process and evaluate therapeutic responses can be obtained when MNPs are incorporated with drug delivery systems.
Aside from the functionalization of dendrimers with therapeutic and diagnostic agents, their unique surface chemistry allows the attachment of active targeting ligands for the specific recognition of cancer cells. Among various targeting ligands, folic acid (folate) has been shown to be well-suited for targeted therapy and the imaging of cancer [Citation16–19]. Therefore, surface modification of dendrimers with folate allows the dendrimers to be specifically targeted toward folate receptors (FRs), which are overexpressed on the surface of several types of cancer cells.
In this study, we synthesized methoxy-PEGylated poly(amidoamine) (PAMAM) generation 3 dendrimers co-loaded with curcumin and superparamagnetic iron oxide nanoparticles (SPIONs), and decorated their surface with folic acid to form a novel multifunctional nanoplatforms (abbreviated as FA-mPEG-PAMAM G3-CUR@SPIONs) for the targeted thermo-chemotherapy of cancer cells. To this end, the cytotoxic effect of the as-prepared nanocomplex in combination with AMF excitation was evaluated on two different cancer cell lines with various FR expression levels, including KB nasopharyngeal cancer cells overexpressing FRs as the model and MCF-7 breast cancer cells with low level of FRs as the blank sample. Moreover, the extent of apoptosis triggered by this thermo-chemotherapy modality was investigated by using Annexin V-Fluorescein Isothiocyanate (FITC) propidium iodide staining.
Material and methods
Materials
Ferric chloride hexahydrate (FeCl3·6H2O, 99%, Merck), ferrous sulphate heptahydrate (FeSO4·7H2O, 99%, Merck), and dimethyl sulphoxide (DMSO) were purchased from Merck (Darmstadt, Germany). Amino-propyl triethoxysilane trimethoxysilane (APTMS, 99%, Sigma-Aldrich), methyl acrylate (MA, 99%, Aldrich), ethylene diamine (EDA, 99%, Sigma-Aldrich), Roswell Park Memorial Institute (RPMI) 1640 cell culture medium, penicillin–streptomycin, trypsin–ethylene diamine tetraacetic acid (EDTA), 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and propidium iodide were purchased from the Sigma-Aldrich Company (St Louis, MO, USA ). Foetal bovine serum (FBS) was purchased from Gibco® (USA).
Synthesis of SPIONs
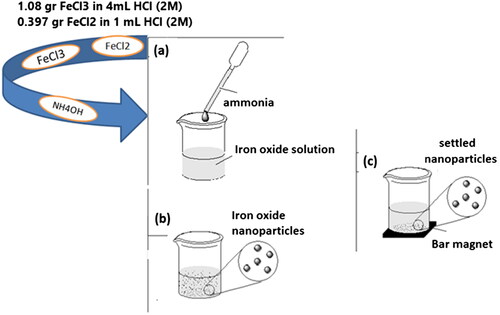
SPIONs were synthesized by co-precipitation method. For this purpose, the SPIONs were precipitated from iron (II) sulphate and iron (III) chloride solutions with the regulation of pH by the use of an aqueous ammonia solution. Ammonia solution (70 ml, 1 M) was added dropwise to the stirred solution of FeSO4 (0.397 g in HCl (1 ml, 2 M)) and FeCl3 (1.08 g in HCl (4 ml, 2 M)) at room temperature. The final product was purified by using the magnetic separation method three times and it was dispersed in 50 ml methanol (Scheme 1).
Aminosilane coating of SPIONs
Iron oxide suspension containing methanol was sonicated for 30 min. Then, 10 ml of the solution was poured into a container and toluene (20 ml) was added to it and the mixture was sonicated for 15 min. An amount equaling 50 µL of amino-propyl triethoxysilane trimethoxysilane (APTMS) was added to the mixture and sonication was continued for 15 min more. The container was closed and it was kept in an oven at 60 °C for 6 h. The final product was washed with methanol three times using magnetic separation and it was dispersed in 25 ml methanol.
Synthesis of PAMAM dendrimer on SPIONs
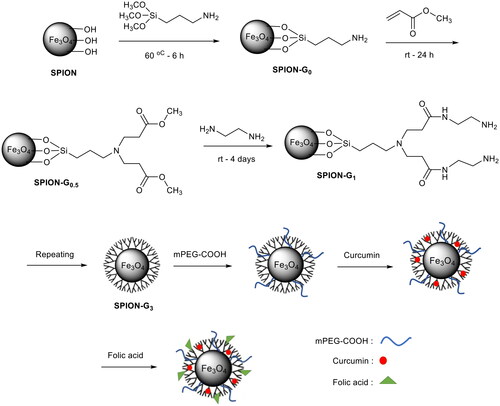
PAMAM G0.5@SPIONs
Dendritic polymer synthesis was carried out according to the reported procedure [Citation20]. First, 200 µl of methyl acrylate was added to 25 ml of methanol and the solution was vigorously stirred. Then APTMS coated SPIONs were added dropwise to this solution under nitrogen gas. Thereafter, the solution was placed on a shaker for 24 h at room temperature. The final product was washed with methanol three times using magnetic separation and then it was dispersed in 25 ml methanol.
PAMAM G1@SPIONs
Next, 500 µl of ethylenediamine was added to 25 ml of methanol and the solution was vigorously stirred. This step was carried out in an ice-water bath for temperature control. Then, G0.5 product synthesized in the previous step was added dropwise to the prepared solution under nitrogen gas. Thereafter, the solution was placed on a shaker for 4 days at room temperature. The resulting product was washed with methanol three times using magnetic separation, and then, it was dispersed in 25 ml methanol.
PAMAM G1.5@SPIONs
The solution of methyl acrylate (350 µl) was added to 25 ml of methanol and the resulting mixture was vigorously stirred. Then, G1 product obtained from the previous step was added dropwise to the prepared solution under nitrogen gas. Thereafter, the solution was placed on a shaker for 24 h at room temperature. The final product was washed with methanol three times using magnetic separation, and then, it was dispersed in 25 ml methanol.
PAMAM G2@SPIONs
The solution of ethylene diamine (1000 µl) was added to 25 ml of methanol and the mixture was vigorously stirred. This step was carried out in an ice-water bath for temperature control. Then, G1.5 product synthesized in the previous step was added dropwise to the prepared solution under nitrogen gas. Thereafter, the solution was placed on a shaker for 4 days at room temperature. The resulting product was washed with methanol three times using magnetic separation and then it was dispersed in 25 ml methanol.
PAMAM G2.5@SPIONs
The solution of methyl acrylate (700 µl) was added to 25 ml of methanol and the mixture was vigorously stirred. Then, G2 product resulted from the previous step was added dropwise to the prepared solution under nitrogen gas. Thereafter, the solution was placed on a shaker for 24 h at room temperature. The final product was washed with methanol three times using magnetic separation and then it was dispersed in 25 ml methanol.
PAMAM G3@SPIONs
The solution of ethylenediamine (1500 µl) was added to 25 ml of methanol and the mixture was vigorously stirred. This step was carried out in an ice-water bath for temperature control. Then, G2.5 product synthesized in the previous step was added dropwise to the prepared solution under nitrogen gas. Thereafter, the solution was placed on a shaker for 4 days at room temperature. The resulting product was washed with methanol three times using magnetic separation, and then, it was dispersed in 25 ml methanol.
Synthesize of mPEG-PAMAM G3@SPIONs
Methoxy polyethylene glycol (mPEG) carboxylic acid (10000, 0.5 g) was dissolved in 10 ml of distilled water and then mixed with the synthesized PAMAM G3 and the solution pH was adjusted about 6. The solution was sonicated for 15 min and then EDC (1 mg) and NHS (1 mg) were added to it. The sonication was continued for 15 min more and thereafter the solution was placed on a shaker for 1 h at room temperature. The final product was separated by a permanent magnet and washed once with methanol and water mixture (50:50). Finally, the resulting product was dispersed in 10 ml methanol.
Synthesize of mPEG-PAMAM G3-CUR@SPIONs
Curcumin (20 mg) was dissolved in the dispersion product prepared in the previous step and then methanol was evaporated from the solution by a rotary evaporator (40 °C) to obtain the dried powder sample. The resulting powder was dispersed in 10 ml of distilled water. Since the curcumin dissolves in methanol but does not dissolve in water, so it penetrates into the dendrimers' cavities.
Synthesize of FA-mPEG-PAMAM G3-CUR@SPIONs
For conjugation of folic acid to the primary amine end groups of dendrimers; first, 1 ml solutions of EDC (100 mg) and NHS (55 mg) were dissolved in the obtained dispersion in the previous step and the solution pH was adjusted between 6 and 7. The resultant mixture was stirred for 10 min in room temperature. Finally, 200 mg folic acid which was dissolved in DMSO (0.5 ml) was added to the mixture while vigorously stirred, and the reaction was continued for 2 h. The final product was washed by permanent magnet several times and it was dispersed in distilled water (Scheme 2).
Characterization
The UV-Visible (UV-Vis) absorption spectra of the nanocomplex dispersed in water were recorded using the Rayleigh UV-1601 instrument (Beijing, China). Fourier transform infrared (FTIR) spectra were recorded using the Shimadzu FT-IR 4300 instrument (Kyoto, Japan), which was equipped with pressed potassium bromide pellets at room temperature. FTIR studies were considered in order to confirm the surface functionalization of the nanoconjugate. The morphology and the size distribution of the synthesized nanocomplex were analysed by high-resolution transmission electron microscopy (TEM, LEO906,Zeiss, Germany). The hydrodynamic diameter of the nanocomplex was measured by dynamic light scattering (DLS) by using the Malvern Zetasizer Nano ZS-90 instrument (Worcestershire, UK). The magnetic properties of the nanocomplex were determined using a vibrating sample magnetometer (VSM; MDK6, Kavir, Iran). The powder X-ray diffraction pattern (XRD, X’Pert Pro MPD-PANalytical (Cambridge, UK), Cu Kλ, 40 kV, 30 mA) was derived from the sample prepared by dropping 200 µl of the products dispersed in water on a silicon substrate and allowing the solvent to evaporate spontaneously at ambient temperature.
In vitro drug release
The in vitro release of curcumin from the nanocomplex was studied at pH 7.4 and pH 5.5 using the dialysis method. The dialysis bag (molecular weight cut-off = 12,400 Da) was soaked in preheated double-distilled water before use. Then, 5 ml solutions of the final product in water and pure curcumin in ethanol were prepared and transferred into the dialysis bag. The bags were separately placed into 45 ml of buffer, which acted as a release medium. The release study was performed in an incubator shaker at 37 °C. At selected time intervals, 3 ml of the solution outside the dialysis bag was sampled and replaced with the same volume of fresh buffer solution. The curcumin content of the samples was measured by high-performance liquid chromatography (HPLC) at 425 nm. Finally, the cumulative release profile of curcumin from the nanocomplex was plotted versus time.
Cell culture
Two different cell lines were used in this study, KB human nasopharyngeal carcinoma cell line and MCF-7 human breast carcinoma cell line. The cells were cultured in RPMI-1640 medium with L-glutamine and NaHCO3 plus 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. The cells were maintained in an incubator with 5% CO2 and 95% air at 37 °C. To harvest the cells, they were trypsinized with 1 mM EDTA/0.25% Trypsin (w/v) diluted in PBS.
Cytotoxicity effects of FA-mPEG-PAMAM G3-CUR@SPIONs
After three passages, the cells were prepared to investigate the cytotoxicity of the nanocomplex. To this end, the cells were counted using a regular hemocytometer and 1 × 104 cells were seeded in each well of 96-well plates. The plates were maintained in an incubator overnight to allow for cell attachment. Then, the cells were incubated with various concentrations of the nanocomplex ranging from 0–150 µg/ml for 12 h. Thereafter, MTT assay was performed according to the manufacturer's manual to assess the cell viability. The detailed information about MTT assay method can be found in our recent paper [Citation21].
Cytotoxicity effects of FA-mPEG-PAMAM G3-CUR@SPIONs in combination with AMF
After incubation of KB and MCF-7 cells with the nanocomplex (30 µg/ml) with and without folate modification for 12 h, the cells were washed three times with PBS to remove excess free nanocomplexes from the culture medium, and then, a fresh medium was added to the cells. The cell culture plate was placed at the centre of the coil of a magnetic field generator operating at 100 kHz. The cells were exposed to AMF at a magnetic field strength of 10 kOe for 20 minutes and then incubated at 37 °C in a humidified 5% CO2 atmosphere. MTT assay was performed to evaluate cell viability.
Flow cytometry
Flow cytometry study was performed to assess the level of apoptosis induction in both cancer cell lines due to various treatments. For this purpose, the untreated cells and cells treated with nanocomplex with and without folic acid modification followed by AMF excitation were considered. The cells were prepared by following the manufacturer’s protocol for Annexin V–FITC apoptosis detection kit (eBioscience, USA), and then underwent flow cytometry (BD FACSCanto II, San Jose, CA, USA ). The detailed information about how to prepare the cells for this experiment was reported in our recent paper [Citation21].
Statistical analysis
The experiments were performed at least three times. Statistical analysis was done using the SPSS software (version 16). All measurement data were expressed as mean values ± SD (standard deviation). The one-way ANOVA test was used to determine if the differences between the means of various groups are statistically significant. Moreover, the Tukey’s test was performed as the post hoc analysis for pairwise comparison of the means of various groups. A p values <.05 was considered statistically significant.
Results
Nanocomplex characterization
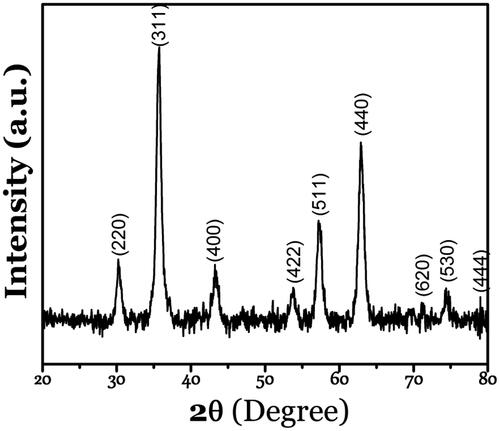
The results of XRD crystalline structure analysis confirmed the formation of the highly purified magnetite phase of iron oxide with diffraction peaks at (220), (311), (400), (422), (511), (440), (530), and (444), which are the characteristic peaks of the Fe3O4 inverse spinel structure (JCPDS file No. 19–0629), without any interference with other phases of FexOy (). Among Fe2+and Fe3+, ferric is the most stable form of iron; thus ferrous ions (Fe2+) could easily change into ferric ones. Broadening of the dominant intense peak in the XRD pattern (311) confirms the small size of the resultant particles. The crystalline size of the magnetite nanoparticles was calculated as 42 nm using the Debye–Scherrer Equation [Citation22].
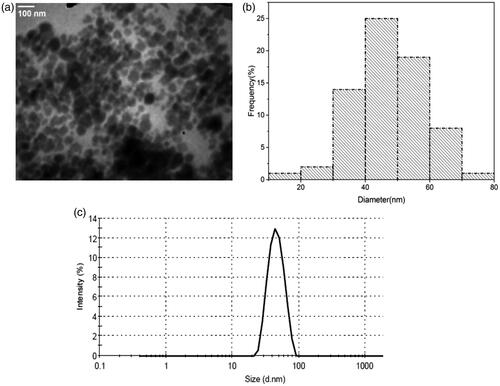
The morphology and the size distribution of the nanocomplex were studied by TEM (). The TEM micrograph shows that the nanocomplex is spherical in shape with a size distribution ranging from 30 to 70 nm (most frequently between 30 nm to 40 nm). shows the result of DLS analysis, in which the hydrodynamic diameter of the nanocomplex ranged from 20 to 90 nm, with the highest frequency at 46 nm.
Figure 2. (a, b) TEM micrograph and corresponding size distribution histogram of the synthesized nanocomplex. (c) Hydrodynamic size distribution of the nanocomplex obtained by DLS analysis.
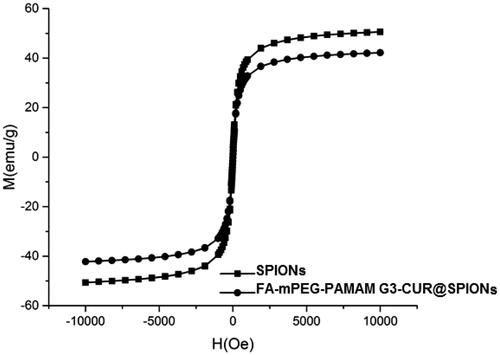
The magnetic properties of MNPs and the nanocomplex were determined using VSM. As seen in , for the synthesized nanocomplex, no hysteresis was detected. This indicates that the SPIONs act as the magnetic core of the synthesized nanocomplex.
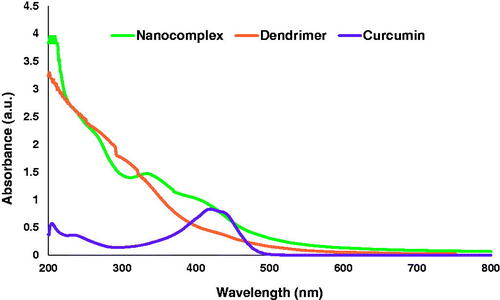
The UV-Vis spectrum of the synthesized nanocomplex has a peak at about 420 nm, which coincides with the absorbance peak of curcumin, indicating the presence of curcumin in the dendrimer. In addition, the broad peak at 320 nm is related to the interaction of the dendrimer with curcumin and curcumin with SPIONs ().
Figure 4. UV-vis spectra show the surface plasmon resonance of curcumin (purple line), dendrimer nanoparticles without drug (orange line) and dendrimer nanoparticles with drug (green line).
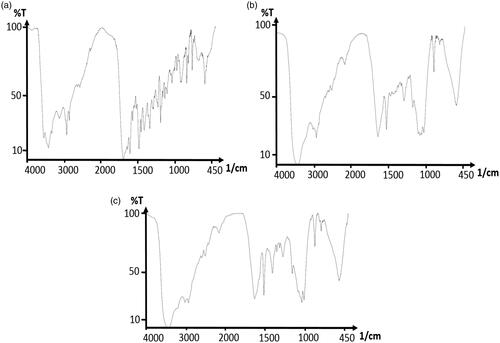
FTIR spectroscopy technically confirms the formation of FA-mPEG-PAMAM G3-CUR@SPIONs. The FTIR spectra of folic acid () indicates the band at 1315 cm−1 and 1338 cm−1, which corresponds to the asymmetric stretching vibration of -NH2 and C-O in folate, respectively. On the other hand, the bands at 1606 cm−1 and 1693 cm−1 relate to C = O stretching in carboxyl acid and the -CONH group, respectively. The absorption bands at 1512 cm−1 and 1629 cm−1 confirm the existence of the nanodendrimer dendrimer with the magnetite core and curcumin (). The peak at 1512 cm−1 belongs to the C-NH bond stretching vibration and the peak at 1629 cm−1 corresponds to the C = O bond of the amide group of the dendrimer. indicates the band formation between the carboxyl group of folic acid and the terminal amine of the dendrimer. The stretching vibration of the carbonyl group at 1606 cm−1 and the C-O band at 1388 cm−1 disappears, while the amid band is formed (the peak at 1631 cm−1 indicates the presence of the C = O bond in the newly formed amide group and may overlap with the band related to the amide group of the dendrimer).
In vitro drug release
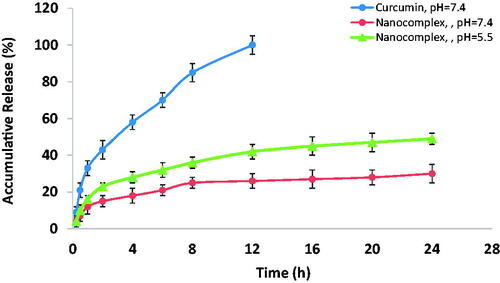
shows the in vitro release profile of the encapsulated curcumin from the nanocomplex at various pH values. While free-form curcumin was completely released into PBS out of the dialysis bag within 12 h, the percentage of curcumin released from the nanocomplex was 30% after 24 h. Therefore, the synthesized nanocomplex displayed a sustained release behavior and caused a significant prolongation of curcumin release. It was also determined that the release profile is pH dependent. The release rate of curcumin from the nanocomplex at pH 5.5 was higher than that observed for pH 7.4. This means that curcumin may be released from the nanocomplex in the tumour region (where pH is lower than 7) with a higher rate.
MTT assay results
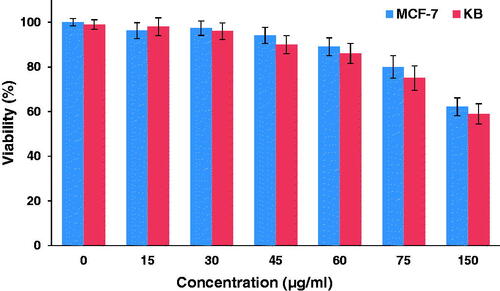
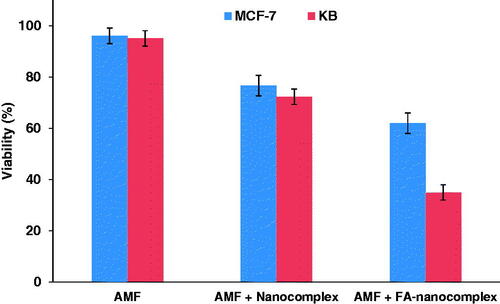
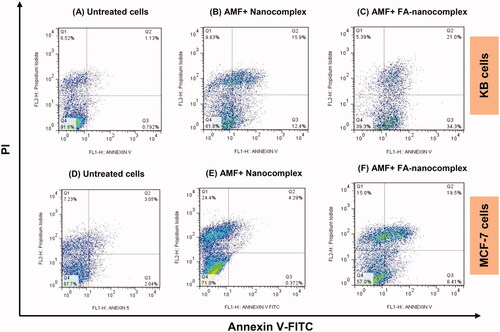
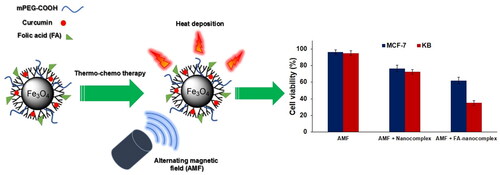
The cytotoxicity of the nanocomplex was investigated against the KB and the MCF-7 cell lines at different concentrations for a 12-h incubation period using MTT assay. As shown in , no significant reduction in cell viability was found for both cell lines when treated with the nanocomplex at concentrations below 60 µg/ml in comparison to the control. To evaluate the combined effect of magnetic hyperthermia and chemotherapy in the presence of the nanocomplex, KB and MCF-7 cells were pre-treated with the nanocomplex (30 µg/ml; 12 h) and were subjected to AMF excitation (100 kHz, 10 kOe; 20 min). The separate administration of the nanocomplex and AMF alone had no significant cytotoxic effect, whereas the use of the nanocomplex coupled with AMF induced significant cell lethality in both cell lines (). Moreover, the folate-modified nanocomplex can further induce cell death in both cell lines following thermo-chemotherapy in comparison to the non-folate-modified nanocomplex at the same concentration. However, there was a significant difference between the cell lethality of the KB and the MCF-7 cells, where 65% of the KB cells and 38% of the MCF-7 cells were killed following thermo-chemotherapy using FA-PEG-PAMAM G3-CUR@SPIONs.
Flow cytometry results
The kinetics of cell death triggered by thermo-chemotherapy and the ability of the nanocomplex to induce apoptosis was monitored by Annexin V-FITC-propidium iodide staining. As shown in , the KB and the MCF-7 cells show a differential cell death mode when exposed to non-folate-modified nanocomplex plus AMF. The MCF-7 cells exhibited negligible apoptosis induction (∼4%) and the majority of the cell death was caused due to necrosis (∼24%). Conversely, the KB cells treated with non-folate-modified nanocomplex followed by AMF were majorly killed via apoptosis (∼28% apoptosis versus ∼10% necrosis). For both cancer cell lines, the percentage of the cells undergoing apoptosis significantly increased when the folate-modified nanocomplex was used as a thermo-chemotherapy agent instead of the non-folate-modified nanocomplex. In this condition, the major cell death pathway for both cancer cell lines was found to be apoptosis rather than necrosis.
Discussion
Owing to rapid metabolism and clearance, curcumin has very poor bioavailability, which hinders its clinical translation. In a report by Ireson et al., the oral administration of curcumin to rats at a dose of 500 mg/kg resulted in a peak concentration of only 1.8 ng/ml in plasma and intravenously administrated curcumin (40 mg/kg) was rapidly eliminated from blood with no detectable concentration in plasma within 1 h of administration [Citation23]. In a clinical report by Sharma et al., the oral administration of curcumin at a dose of 3.6 g daily resulted in a nanomolar concentration in plasma one-hour post-injection [Citation24]. However, a 10−5 to 10−4 M curcumin concentration is required to have a significant therapeutic effect [Citation23]. Accordingly, one primary objective in order to expand the biomedical application of curcumin is to enhance its bioavailability, which was pursued in the present study. shows that 30% of curcumin is released into PBS after 24 h, when it is encapsulated in the PAMAM dendrimers. Consequently, a remarkable prolongation of curcumin release from the dendrimers would provide the potential to enhance the blood circulation half-life of curcumin, and, therefore, it is in vivo bioavailability.
It is generally demonstrated that the tumour’s cell sensitivity against anticancer drugs can be enhanced at an elevated temperature [Citation25]. There are many physiology-related mechanisms that make the combination of heat and drug synergistic. Mild hyperthermia can increase the vascular permeability of the tumour, which, in turn, increases the tumour uptake of drugs [Citation26]. Hyperthermia can inhibit the activity of MDR transporter proteins, which actively pump the chemotherapeutic agents out of the cells, thus affording the potential of overcoming MDR [Citation27]. Hyperthermia can also stabilize DNA damage caused by anticancer drugs by inhibiting DNA-repair pathways [Citation28]. Additionally, hyperthermia can be utilized as an external stimulus to trigger drug payload release from the nanocarriers in a controlled manner [Citation29]. Based on these beneficiary outcomes, many efforts have been devoted to integrate hyperthermia and chemotherapy potencies into a single platform. Accordingly, we utilized the hyperthermic effect of MNPs by incorporating them into the dendrimer core in order to realize concurrent thermo-chemotherapy. Compared to other conventional hyperthermia methods, magnetic hyperthermia presents a completely non-invasive and safe strategy for selective heat deposition into the tumor based on magnetic energy losses of MNPs in the presence of AMF. As shown in , the chemotherapy effect of the nanocomplex was remarkably enhanced by the addition of magnetic hyperthermia, proving the synergistic effect of heat and the drug when applied simultaneously.
The decoration of dendrimers with folate-targeting ligands can further enhance their therapeutic and diagnostic performance [Citation17,Citation30,Citation31]. Given the high affinity of folates to FRs, which are overexpressed on the membrane of cancer cells, the surface functionalization of dendrimers with folates can specifically direct them toward cancer cells and accelerate the cellular internalization of dendrimers, eventually increasing the intracellular content of the dendrimer payload. As observable in , FA-mPEG-PAMAM G3-CUR@SPIONs in combination with AMF can induce greater cell death in both cancer cell lines than in non-folate-modified nanocomplex at the same concentration. This may be attributable to the increased cellular uptake of the nanocomplex due to folate-modification, which, in turn, increases the intracellular concentration of curcumin and SPIONs. The increased curcumin concentration is associated with an enhanced chemotherapy effect, while the increased SPIONs concentration may lead to higher heat-generation efficiency upon AMF excitation, ultimately resulting in a stronger thermo-chemotherapy outcome. Moreover, the higher lethality rate of the KB cell line versus the MCF-7 cell line can be explained by the higher expression level of FRs on the membrane of the KB cell line, which is expected to have higher uptake from the nanocomplex.
Recent studies have described apoptosis as the proposed pathway by which curcumin exhibits an anticancer effect [Citation32]. Apoptosis is the preferred cell death pathway during cancer therapy as it prevents the detrimental and the inflammatory effects of necrosis on the neighbouring healthy cells. Therefore, one of the primary objectives of cancer treatment approaches is to skew the response toward apoptotic cell death rather than necrotic death. Curcumin can initiate the intrinsic mitochondrial pathway of apoptosis through the activation of the p53 tumour-suppressor gene. Upon activation of the p53 gene, the pro-apoptotic members of the Bcl-2 family (Bak and Bax) are activated, which promotes apoptosis through mitochondrial outer membrane permeabilization, thereby releasing the apoptotic mediators into the cytoplasm. This ultimately results in cell death and phagocytosis [Citation33]. Further mechanistic insights into the anti-proliferative effect of curcumin on cancer cells have been elucidated by Guo et al. [Citation32]. The loss of the mitochondrial membrane potential, the activation of caspase-3 and caspase-9, the upregulation of cytochrome c, p53, and Bax in association with the downregulation of Bcl-2 and survivin in the LoVo colorectal carcinoma cells treated with curcumin, all confirmed the mitochondrial pathway of apoptosis as the major route for curcumin-triggered cell death. In the present study, we evaluated the extent of apoptosis induced by the combination of the nanocomplex and AMF through Annexin V-FITC-propidium iodide staining. As shown in , KB and MCF-7 cells treated with non-folate-modified nanocomplex plus AMF differentially show apoptosis and necrosis as the major routes for cell death, respectively. However, functionalizing the nanocomplex with folate shifted the cell death pattern triggered by thermo-chemotherapy from necrosis towards apoptosis. The apoptosis to necrosis ratios for MCF-7 and KB cancer cells treated with non-folate-modified nanocomplex plus AMF were 1: 6 and 2.8: 1, respectively. When a folate-modified nanocomplex was used instead of a non-folate modified nanocomplex, these values altered in the favor of apoptosis to 1.9: 1 and 11: 1, respectively. Therefore, cell death occurred mainly via apoptosis in both cancer cell lines.
Conclusion
A novel multifunctional folate-modified and curcumin-loaded dendritic magnetite nanocarrier was constructed as a promising platform for combined magnetic hyperthermia and targeted drug delivery. The enhanced prolongation of curcumin release from this nanocomplex provides the potential to address the poor bioavailability issue of curcumin that hinders its clinical translation. The nanocomplex could selectively bind to FRs overexpressing cancer cells and greatly enhance the targeted thermo-chemotherapy against these cancer cells upon AMF excitation. Furthermore, the decoration of the nanocomplex with folate-targeting ligands modulated the response to thermo-chemotherapy by shifting the cell death pathways toward the apoptotic route. Owing to the existence of SPIONs in the core of the nanocomplex, a future study investigating MRI contrast enhancement performance of the nanocomplex would be interesting.
Acknowledgement
All supports received from ZaUMS are acknowledged.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer. 2002;2:48–58.
- Peer D, Margalit R. Fluoxetine and reversal of multidrug resistance. Cancer Lett. 2006;237:180–187.
- Shakeri-Zadeh A, Shiran M-B, Khoee S, et al. A new magnetic nanocapsule containing 5-fluorouracil: in vivo drug release, anti-tumor, and pro-apoptotic effects on CT26 cells allograft model. J Biomater Appl. 2014;29:548–556.
- Abed Z, Khoei S, Ghalandari B, et al. The measurement and mathematical analysis of 5-Fu release from magnetic polymeric nanocapsules, following the application of ultrasound. Anticancer Agents Med Chem. 2018;18:438–449.
- Raju GSR, Benton L, Pavitra E, et al. Multifunctional nanoparticles: Recent progress in cancer therapeutics. Chem Commun. 2015;51:13248–13259.
- Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotech. 2007;2:751–760.
- Salem M, Rohani S, Gillies ER. Curcumin, a promising anti-cancer therapeutic: a review of its chemical properties, bioactivity and approaches to cancer cell delivery. Rsc Adv. 2014;4:10815–10829.
- Ghalandarlaki N, Alizadeh AM, Ashkani-Esfahani S. Nanotechnology-applied curcumin for different diseases therapy. BioMed Res Int. 2014;2014:1.
- Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors. 2013;39:2–13.
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. Aaps J. 2013;15:195–218.
- Esfand R, Tomalia DA. Poly (amidoamine)(PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Disc Today. 2001;6:427–436.
- Shi W, Dolai S, Rizk S, et al. Synthesis of monofunctional curcumin derivatives, clicked curcumin dimer, and a PAMAM dendrimer curcumin conjugate for therapeutic applications. Org Lett. 2007;9:5461–5464.
- Chandra S, Mehta S, Nigam S, et al. Dendritic magnetite nanocarriers for drug delivery applications. New J Chem. 2010;34:648–655.
- He X, Wu X, Cai X, et al. Functionalization of magnetic nanoparticles with dendritic–linear–brush-like triblock copolymers and their drug release properties. Langmuir. 2012;28:11929–11938.
- Beik J, Abed Z, Ghoreishi FS, et al. Nanotechnology in hyperthermia cancer therapy: from fundamental principles to advanced applications. J Control Release. 2016;235:205–221.
- Ghaznavi H, Hosseini-Nami S, Kamrava SK, et al. Folic acid conjugated PEG coated gold–iron oxide core–shell nanocomplex as a potential agent for targeted photothermal therapy of cancer. Artif Cells Nanomed Biotechnol. 2018;46(8):1594-1604.
- Beik J, Jafariyan M, Montazerabadi A, et al. The benefits of folic acid-modified gold nanoparticles in CT-based molecular imaging: radiation dose reduction and image contrast enhancement. Artif Cells Nanomed Biotechnol. 2017;1–9.
- Beik J, Khademi S, Attaran N, et al. A nanotechnology based strategy to increase the efficiency of cancer diagnosis and therapy: folate conjugated gold nanoparticles. Curr Med Chem. 2017;24(39):4399-4416.
- Mirrahimi M, Hosseini V, Kamrava SK, et al. Selective heat generation in cancer cells using a combination of 808 nm laser irradiation and the folate-conjugated Fe2O3@ Au nanocomplex. Artif Cells Nanomed Biotechnol. 2017;1–13.
- Madani M, Sharifi‐Sanjani N, Iraji‐Rad R. Aureole nanofibers by electrospinning of PAMAM‐PEO solution. J Appl Polym Sci. 2009;113:3005–3011.
- Zeinizade E, Tabei M, Shakeri-Zadeh A, et al. Selective apoptosis induction in cancer cells using folate-conjugated gold nanoparticles and controlling the laser irradiation conditions. Artif Cells Nanomed Biotechnol. 2018;1–13.
- Faiyas A, Vinod E, Joseph J, et al. Dependence of pH and surfactant effect in the synthesis of magnetite (Fe 3 O 4) nanoparticles and its properties. J Magn Magn Mater. 2010;322:400–404.
- Ireson C, Orr S, Jones DJ, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–1064.
- Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–6854.
- Dahl O. Interaction of heat and drugs in vitro and in vivo. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Thermoradiotherapy and thermochemotherapy. Springer-Verlag Berlin Heidelberg: Springer; 1995. p. 103–121.
- Masunaga SI. Tumor microenvironment and hyperthermia. In: Kokura S, Yoshikawa T, Ohnishi T, editors. Hyperthermic oncology from bench to bedside. Singapore: Springer; 2016. p. 151–169.
- May JP, Li S-D. Hyperthermia-induced drug targeting. Expert Opin Drug Deliv. 2013;10:511–527.
- Schaaf L, Schwab M, Ulmer C, et al. Hyperthermia synergizes with chemotherapy by inhibiting PARP1-dependent DNA replication arrest. Cancer Res. 2016;76:2868–2875.
- Yavuz MS, Cheng Y, Chen J, et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nature Mater. 2009;8:935.
- Shakeri-Zadeh A, Eshghi H, Mansoori G, et al. Gold nanoparticles conjugated with folic acid using mercaptohexanol as the linker. J Nanotech Prog Intl. 2009;1:13–23.
- Hashemian AR, Eshghi H, Mansoori GA, Shakeri-Zadeh A, Mehdizadeh AR. Folate-conjugated gold nanoparticles (synthesis, characterization and design for cancer cells nanotechnology-based targeting). Int J Nanosci Nanotechnol. 2009;5(1):25-34.
- Guo L, Chen X, Hu Y, et al. Curcumin inhibits proliferation and induces apoptosis of human colorectal cancer cells by activating the mitochondria apoptotic pathway. Phytother Res. 2013;27:422–430.
- Shehzad A, Lee YS. Molecular mechanisms of curcumin action: signal transduction. Biofactors 2013;39:27–36.