Abstract
A single outcome in a biological procedure at the time of cancer therapy is due to multiple changes happening simultaneously. Hence to mimic such complex biological processes, an understanding of stimuli responsiveness is needed to sense specific changes and respond in a predictable manner. Such responses due to polymers may take place either simultaneously at the site or in a sequential manner from preparation to transporting pathways to cellular compartments. The present review comprehends the stimuli-responsive polymers and multi-responsiveness with respect to cancer therapy. It focuses on the exploitation of different stimuli like temperature, pH and enzymes responsiveness in a multi-stimuli setting. Nanogels and micelles being two of the most commonly used responsive polymeric carriers have also been discussed. The role of multiple stimuli delivery system is significant due to multiple changes happening in the near surroundings of cancer cells. These responsive materials are able to mimic some biological processes and recognize at the molecular level itself to manipulate development of custom-designed molecules for targeting cancer cells.
Introduction
Cancer is a set of heterogeneous diseases. Despite the availability of a great number of chemotherapeutic and molecularly-targeted drugs and other treatment modalities such as radiotherapy and surgery, cancer is considered as one of the top five leading causes of mortality globally. Millions of patients are suffering from various cancers; most losing the battle along the way during treatment. The situation is grim as seen from the fact that the overall survival rate has improved by only 3.4 months in 2013 compared to the treatments available in 2003 [Citation1]. Successfully treating and curing cancer still seems to be a distant dream.
Every single cell type in the human body has the latent ability to undergo malignant transformation leading to cancer of that cell type; some cancers are more prevalent than the others [Citation2]. Chemotherapeutic drugs, being used in therapy for several decades, suffer from several disadvantages such as severe toxicity, non-selectivity (normal versus cancer cells) and drug resistance. One way of mitigating their extreme toxicity is to deliver these drugs selectively at the tumour site. Innumerable number of approaches have been explored and reported for this purpose over the last decade-and-half or so [Citation3–5]. The advantage is taken of the fact that the tumour cells differ substantially from normal cells in their properties such as temperature, redox state, pH, elevated vascular permeability (enhanced permeability and retention, EPR) and overexpression of certain enzymes, for example, matrix metalloproteinases (MMPs) among many others.
The approaches for selective direction of drugs to the tumour tissue include passive as well as active targeting. Passive delivery approaches mainly exploit the EPR effect (higher vascular permeability and impaired lymphatic drainage) whereby the drugs encapsulated within nanoparticles (particle size <100 nm) have shown to accumulate to an extent of 10- to 100-fold in tumours compared to their free forms [Citation6]. DOXIL®, PEGylated liposomal doxorubicin, is used to treat >300,000 patients/year for ovarian cancer and Kaposi’s sarcoma [Citation7]. This formulation has substantially low or no cardiotoxicity associated with doxorubicin. In contrast, the active delivery uses the concept of ‘magic bullet’ wherein key cell macromolecules/receptors especially expressed on cancer cell surface or in cytoplasm (e.g. CD40, CD80, integrins, etc.) are utilized by attaching a peptide/small molecule ligand to the drug. The ligand attaches to the cancer cell and the drug is released at its site of action [Citation8–10]. Both these approaches have shown promise in cancer treatment with substantial reduction in the side effects. But the heterogenous nature of the tumour tissue, complex tumour microenvironment, little or no vasculature in certain parts of the tumour tissue has put breaks on the pan-usefulness of these methods in treating cancers [Citation4].
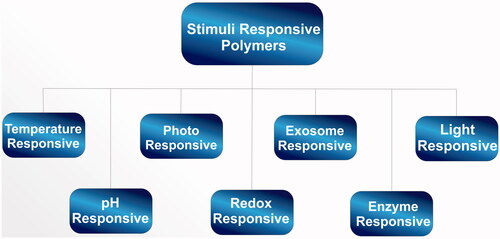
Over the last decade, there has been a rejuvenated focus on applications of polymeric materials which are stimuli-responsive, lipids, dendrimers or macromolecules for intracellular delivery of drugs [Citation11–15]. These materials give a local response to stimuli such as those of pH, temperature, light, specific biomarkers (e.g. glucose, urea), redox potential and any combination thereof (e.g. pH/temperature, light/pH/temperature, pH/magnetic) and undergo specific change(s) in their properties [Citation16–18]. In this review, discussions on mono-, dual- and multi-polymers which are stimuli-responsive have been extensively included with particular emphasis on cancer treatment.
Stimuli-responsive polymers
Synthetic polymers are being explored for their therapeutic applications. These polymers contribute most often to an improved pharmacokinetic profile with great potential for tissue targeting. Polymer therapeutics, a subclass of nanomedicines, can be classified as polymeric drugs, polymer–protein conjugates, polymeric micelles and also polyplexes which are buildings of polymers and poly-(nucleic acids) (). It involves a whole array of analytical tools, diagnostics, imaging methods, innovative delivery systems, therapeutics and also various systems for tissue recovery and repair. Other than its applications in a passive manner, synthetic polymers regularly embrace a more dynamic part, for example, discharging on an external stimulus, a medication particle, peptide or an oligo/poly-(nucleic corrosive). Such polymers are considered as stimuli-responsive.
Major interest and exploration of the responsive polymeric materials have been possible due to the advances in their preparation methods, including but not restricted to ‘click’ chemistry, polymerization reactions such as the reversible addition-fragmentation chain transfer polymerization, atom transfer radical polymerization, nitroxide-mediated radical polymerization and ring-opening metathesis polymerization [Citation16]. In addition to life and biomedical sciences, these multi-responsive materials have found their utility in catalysis, technique of tissue engineering and smart interfaces [Citation19]. The preparation of these materials is definitely synthetically challenging. One can be very creative or imaginative within the realms of synthetic feasibility for ‘designing’ these so-called ‘smart’ materials. The mimicry of biologically complex systems such as tumour microenvironment is feasible to some extent by these materials. The scientific community is greatly interested in these ‘wonder’ molecules for therapeutic, diagnostic and both (theranostic) purposes.
Multi-responsiveness in cancer therapy
Most of the stimuli-sensitive polymeric carrier systems deal with response to a single stimulus like pH or temperature. However, biological performance of macromolecules like nucleic acids and proteins is often due to a combination of different environmental changes. This is surely not due to response of a single factor or signal. The role of multiple stimuli in cancer therapy can be understood by the very fact that a single outcome in a biological procedure or process is due to multiple changes happening in the near surroundings simultaneously. Hence to mimic some biological processes, the complexity of multi-stimuli responsiveness is necessary to comprehend at the molecular level to manipulate the development of custom-designed molecules for targeting cancer cells. Multi-responsiveness is a strategy successfully being utilized for drug delivery to cancer tissues and cells. It results in the formation of tumour cell cytoplasm or sub-cellular organelles like those of nucleus or mitochondria. The mechanism of drug release at the tumour site seems to be crucial. The systemic toxicity caused by premature drug release can be avoided using the multi-responsiveness.
Certain tumours and inflamed or wound tissue display a pH different than physiological pH 7.4. For instance, constant injuries have been reported to have pH in the range of 5.4–7.4 [Citation20]. Cancerous tissues prefer acidic extracellular environment which eventually is crucial for metastasis [Citation21]. The liquid stage pinocytosis or receptor-intervened endocytosis helps polymers to be taken up by the cells. The pH drops from 6.2 to 5.0 within the early endosome which is towards lysosomes (by means recently matured endosomes). This results in an extensive change in [H+] inside the compartments. The steady decrease in pH (and also in lysosomal chemicals) has been used with a specific end goal to discharge drug molecules to the cytosol from lysosomes [Citation20].
Temperature sensitivity trends in responsiveness
Temperature as a response has been one of the most deliberate stimuli-responsive materials for various drug delivery applications. A significant change in temperature brings a quick and practically visible change in many characteristics like conformation, structure, solubility or hydrophilic-lipophilic balance. The convenience of using temperature as a stimulus and its economic feasibility have made it a popular tool for applications in stimuli-sensitive systems. In addition, temperature sensitivity has been easily fitted with a wide variety of multi-stimuli like pH, light or redox giving it a wider potential for diverse applications.
The use of polymers in multi-stimuli responsiveness has played an important role in mimicking ideal biological processes. Polymers undergo hydrophilic–hydrophobic phase transformation to help the formation, deformation or transformation of aggregates in response to variations in temperature. The minimum temperature at which the polymer undergoes phase transition is termed as lower critical solution temperature (LCST). The poly-(N-isopropyl acrylamide) (PNIPAM) has shown widespread use among all the polymers, due to its closeness of LCST to the human body. PNIPAM turns out to be an expanded coil form below the LCST and above it starts becoming more hydrophobic as it collapses to a globular state. PNIPAM is a very fascinating material due to its biocompatibility and LCST at 32–33 °C which is pretty much observed for the controlled release application. The oral conveyance of calcitonin and insulin has been primarily contemplated by PNIPAM copolymers. The polymeric beads help in immobilizing peptides or hormones thereby imparting stability during its passage through the stomach. The beads break down at the basic pH in the digestive tract and the drug is discharged. Some of the most commonly used polymers include poly(methyl vinyl ether) which has a characteristic temperature of 37 °C, making it exceptionally intriguing for the biomedical applications. Even though poly(N-vinyl caprolactam) PVCa has exceptionally fascinating properties both for therapeutic and biotechnological applications, it has not been contemplated as PNIPAM. Poly(N-ethyl oxazoline) is known to have a transition temperature of 62 °C, which is higher for any application of medication conveyance. The united polymerization of EtOx onto an altered PINPAM backbone results in an arranged twofold thermo-responsive framework. Unfortunately, the chemistry of poly(oxazoline) has the hindrance that it is not exceptionally tolerant against the functionalities that are unprotected. The LCST behaviour of the polypeptides is indicated when hydrophilic and hydrophobic deposits are adjusted and also the balance is adjusted. The pentapeptide GVGVP is made from a polymer and its repeating unit displays a phase transition of volume which is assembling in nature at 30 °C. The systems with upper critical solution temperature (UCST) conducted inside the biomedical setting can be explained using an interpenetrating system of poly(acrylic corrosive) and polyacrylamide which is just a handful of the whole.
The transition temperature is 25 °C. The UCST conduct originates from H-bonding amongst both of acrylic corrosives (AAc) and AAm units which takes place due to the cooperative effects. The similar phenomenon is exhibited by poly(N-vinyl caprolactam) PVCL, poly(ethylene glycol) (PEG) or poly(ethylene oxide) and poly(propylene oxide). The exceptional characteristic of minimal cost of acrylic polymers and their adhesion to the organic surfaces have added value to this class of polymers with long-standing interest in all pharmaceutical applications. The temperature-responsive polymers with pH in combination with light delicate behaviour empowers an exceptional set of ‘smart’ materials to be prepared [Citation22,Citation23].
Loh et al. [Citation24] prepared a triply-triggered supramolecular nanocontainer with potential chemotherapeutic applications. The PNIPAAm was used as a temperature-responsive block and poly(dimethylamino ethyl methacrylate) for controlling the release of loaded doxorubicin. It also acted as a pH-responsive segment. The temperature-responsive part was suitable for triggering Doxorubicin (DOX) release either by infrared irradiation or the pH-responsiveness which triggered the release within endosomal and lysosomal vesicles at an acidic of pH of 4.
Wang et al. [Citation25] reported that p-sulphonatocalix[4]arene and assymetric viologen share an assembly of host–guest complex formation for the development of a nanosupramolecular binary vesicles. The stimuli, namely, temperature, host–guest inclusion and redox got a response from the vesicle. The triple mechanism helped in the efficient release of DOX ().
Figure 2. Host–guest complex DOX release by triple mechanism (adapted from Wang et al. [Citation25]).
![Figure 2. Host–guest complex DOX release by triple mechanism (adapted from Wang et al. [Citation25]).](/cms/asset/23b7fe03-b812-4bf6-8d14-33d85efef45b/ianb_a_1559176_f0002_c.jpg)
The amphiphilic block copolymer was prepared with the help of three components, viz., an acid-sensitive hydrophobic core, a temperature-sensitive hydrophilic shell and a linker between the two, serving as a redox-sensitive interface [Citation26]. PNIPAM was yet again chosen as the temperature-sensitive block exhibiting a reversible thermos-sensitive phase transition in aqueous solution which changed from hydrophilic to hydrophobic above its LCST. The developed system showcased an efficient multiple stimuli response to pH, temperature and redox state. In addition, the controlled release kinetics of the encapsulated drug in the tumour cells was achieved by pH or the redox triggers.
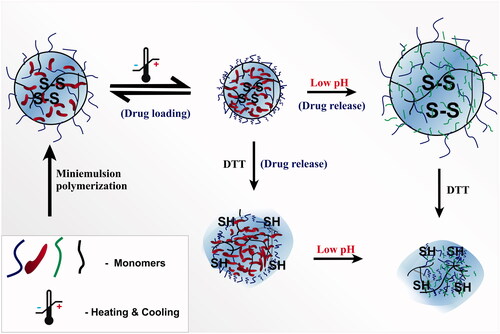
A mini-emulsion copolymerization method was used for the formation of thermoresponsive nanogel which showed triple-responsive behaviour for monomethyl oligo(ethylene glycol) acrylate and an ortho ester-containing acrylic monomer, 2–(5,5-dimethyl-1,3-dioxan-2-yloxy) ethyl acrylate [Citation27]. The nanogel exhibited good encapsulation of the hydrophobic anticancer drugs, DOX and paclitaxel (PTX), induced degradation along with acid- as well as reduction triggered hydrolysis ().
The elastin-like polypeptides were engineered and broadly explored for smart drug delivery and targeting systems [Citation28,Citation29]. The elastin pentapeptide materials repeat Val-Pro-Gly-Xaa-Gly sequence which were developed for showcasing LCST behaviour around 40 °C by the alteration of these repeat sequences and inclusion of both the oligoalanine and oligoglycine residues. The transition temperatures had the outline of the target that the particles might frame on ultrasound induction of hyperthermia, thereby locallytargeting drugs to an ELP backbone. The thermal transition of homopolypeptides took place over a narrow range and was completely reversible for a (Val5-Ala2-Gly3)150 polypeptide. The beginning of LCST was between 40 °C and 42 °C. The preparation of block co-polymers happened with more complex thermal transitions indicating a range of intermediate species formed as differential blocks aggregated. The particles of 40–100 nm were created for both homo- and co-polymers over the LCST with proposed favourable use in disease treatment that was inferable from the aggregation of particles of this size in tumour tissues. A similar group has detailed the utilization of ELP-doxorubicin conjugates for temperature which intervened tumour suppression. A promising start in drug targeting is the local aggression of the ELP-conjugates in tumour cells by responsive synthetic polypeptides.
pH-responsivity trends
The pH responsiveness tends to adapt to multiple environmental changes rather than a single change. The cationic polymers have been utilized for intracellular conveyance of oligo/poly(nucleic acids), which complexed the negatively-charged nucleic acids. Further, they tended to deprotonate inside the endosome and it triggered the endosome film interruption and led to discharge into the cytosol before achieving lysosome with its hydrolytic compounds. Hence, tailoring the protonation/deprotonation by the alteration of the polymer structure can largely allow fine-tuning of the response in a specific compartment. Some of the traditional monomers such as AAc, methacrylic corrosives, maleic anhydrides, and N,N-dimethylaminoethyl methacrylate (DMAEMA) and the polymers containing phosphoric corrosive subordinates are accounted [Citation30]. The intertwined and pH-responsive behaviour have been utilized for the initiation of controlled release of model mixes like caffeine and drugs like indomethacin or lysosomal cationic proteins [Citation31].
Dong et al. [Citation32] prepared light-, temperature- and pH-based polymeric micelles with controlled release of the entrapped drug. Preferably, such a system is appropriate for in vivo cancer microenvironment. From the formulation point of view, the segments of pyrene-quaternized were responsible for light-responsive shell while pH/temperature-responsiveness was dedicated to the unquaternized segment. With the subjecting of light, the micelles dissociated and above LCST, there was shrinking of the micelles. At pH 3, the micelles could either be swollen or dissociated at pH 10 ().
Polycations in non-viral gene therapy
The non-viral gene therapy involves and utilizes cationic polymers. The complex of polycations with nucleotides would be attributed to electrostatic cooperation. When the pH drops during cellular uptake, the responsive character of the polymer is evident. The polymer turns out to be increasingly charged and also triggers osmotic, endosomolytic or different events subsequently. Polyethyleneimine is known to be a smart standard against which each new polymer is being tested. Some different applicants are PAMAM and some of the dendrimers such as poly(DMAEMA), poly(amidoamine)s (PAA), poly(l-lysine) (PLL) or modified chitosan.
Acid triggering drug release in cancer targeting
The pH alteration is used for two kinds of routes with a specific target both to have a controlled release and a triggered release of the drug molecule [Citation33]. Firstly, with respect to extracellular tissue, tumour tissue has an extracellular pH of 6.5–7.2, which is marginally low than the physiological pH of 7.4. The drug, post-cellular uptake, experiences a pH of 4.5–5.0 in lysosomes. The hydrolytic chemicals, for example, cathepsin B, are used again and again for the discharge of drug load. Prodrugs are conjugates to a polymer which are very latent and inactive in nature. It adds on as an advantage to cytotoxic medications, for example, in cancer therapy, the incorporation of a targeting system helps in avoiding or minimizing adverse side reactions due to non-specific toxicity. Notwithstanding, prodrugs are given full advantage by just a proficient release of the medication at the site of activity [Citation30].
Polyanions and amphoteric polymers for endosomolytic delivery
On the gradual acidification of a polyanion, the carboxylate groups will be protonated, and the polymer backbone increasingly becomes hydrophobic and at one point, the deprotonated polymer transitions start becoming membrane active. This is accompanied by coil-to-globule transition of the polymer. The pH, at which this occurs, relies upon the hydrophilic/hydrophobic balance, for example, poly(acrylic corrosive) is protonated at pH 3. The addition of a hydrophobic alkyl deposit to the backbone shifts the transition to the higher values. When the transition is happening at endosomal pH (ca. pH 6.0), the drug load escapes to cytosol and later exert its action. As discussed before, amphoteric PAA demonstrate endosomolytic movement. Later, the change of pKa and the spatial position of the charges appear to have an impact on the transition point. Class of the poly(l-histidine)s with a pKa of 6.0 were effectively utilized as a part of quality conveyance in blend with PLL [Citation34].
Enzyme responsiveness in multi-stimuli settings
Enzymes are the fundamental proteins which have a significant role to play in most of the vital tasks in a biological molecule. Typically from the smaller chemical reactions in a cell to larger metabolic processes in a tissue or organ comprise of enzymes. It is nearly difficult to imagine a single biochemical reaction inside a human body without the use of enzymes. Enzymes are sharp biological triggers to react over minute changes inside the human body. Sometimes they may be just used as catalyst or to speed up a reaction. The changes in dysregulation of enzyme expression can be very well used for therapeutics. Inherent characteristics of the enzymes like ability to work like a catalyst, specificity, detectability and responsiveness make them important stimuli to work against cancer. For example, as compared to the healthy cells, there is a changed catalyst articulation observed in cancer cells. As cancer-affected cells display features which can be recognized by the enzyme-mediated delivery system. With a proper control over the enzymatic activity, many delivery systems can be used to exploit enzyme responsiveness to target cancer cells. This section describes some of these notable drug delivery systems.
MMPs responsive drug delivery
MMPs are a family of around 24 human endopeptidases which work collectively to degrade components of many proteins including the extracellular matrix. MMPs are also known as the matrixins. These zinc-containing proenzymes (zymogens) must be proteolytically activated to become a functional enzyme. These enzymes are apparent in many human diseases including cancers where they can be effectively used as biomarkers against tumours. MMPs action is visible at several stages of cancers including the regulation, expression, progression and metastasis formation [Citation35]. Particularly in the two cancer types, MMP-2 and MMP-9 are related to tumour attack, progression of cancer and their metastasis at different stages like the pro-metastatic or anti-metastatic.
Terada et al. [Citation36] were one of the firsts to report the use of the MMP-2 cleavable linker with the PEGylated liposome to target the hepatocellular carcinoma. After the enzymatic cleavage of PEG-lipid due to the presence of MMP-2, the liposomes were taken up in the carcinoma cells. Similarly, MMP-2/9-responsive micelles composed of TPGS were used to transport the anticancer drugs to the tumour site with an enhanced accumulation [Citation37]. Herein, the MMP-2/9 was inserted as the cleavable oligopeptide, spacer, between α-tocopherol succinate and methoxy poly(ethylene glycol) (MPEG). Shargh et al. [Citation38] recently exploited the degradability of these MMPs for formulating nanoparticles (NPs) using a combination of gelatin and albumin. These NPs showed MMPs trigged release of the encapsulated GNF-5837. In addition, these NPs were claimed to be better at targeting the breast cancer due to restoring or repairing of the apoptosis phenomenon.
Cathepsin B-responsive drug delivery
The lysosomal cysteine protease enzyme cathepsin B is known to be overexpressed in many cancerous endothelial cells and hence is a very useful trigger factor in responsive kind of drug delivery systems. In this regard, this substrate can be utilized as linker between the anticancer drug and the polymer to facilitate the endocytosis mechanism and allow the entrapped drug to have an intra-lysosomal release. Lee et al. [Citation39] synthesized dendrimers with a perspective of using the cleavable peptide cathespine B in doxorubicin conjugated with MPEG. The drug release was more than governed by the cleaving potential of the enzyme.
Recently, to deliver the anticancer drug gemcitabine (GEM), a dual enzyme sensitive nano delivery vectors were reported by exploiting both MMPs (MMP-9) and cathepsin-B for targeting [Citation40]. This complex had the benefit of PEG for providing long circulation with enhanced cellular uptake and at the same time, the cleavability of cathepsin-B provided desired GEM release. The role of MMP-9 came into the picture for exposing the targeting ligand to the pancreatic cancer cells. The responsiveness of cathepsin B has also been used with the chimeric peptides to provide a solution to multidrug resistance occurrence during chemotherapy [Citation41]. The nanomicellar system encapsulating DOX was able to restrict the efflux of DOX and provide higher drug concentration at the site of action.
Hyaluronidase-responsive drug delivery
Hyaluronic acid (HA) is a macromolecule which is extensively spread throughout the human body, particularly in connective and epithelial tissues. Their substantial role in malignant tumour cells is also dedicated to their active part in stages of proliferation and progression of cancer cells. Hyaluronidase (HAase) is a family of enzymes that are used in the degradation of HA. Some cancerous cells show an elevated level of HAase particularly in breast, brain and bladder cancers; while in pancreatic cancer the HAase level is lowered. The responsive HAase intervened degradation of HA has been exploited for developing mesoporous silica nanoparticles (MSN) for targeting colon cancer cells [Citation42]. Similarly, the MSN conjugated with HA were used for delivering the anticancer drug 5-fluorouracil by the virtue of having its release based on the enzyme-sensitivity [Citation43]. Applications of HA have lately been underlined for their role in targeting cancer cells due to their affinity to bind CD44 clusters. This principle was used to target the tumour microenvironment of drug-resistant breast cancer cells by HA to deliver DOX [Citation44].
Azoreductase-responsive medication conveyance
Azoreductase enzymes are collective groups which are generally involved in the biotransformation or degradation of azo-based or nitro-aromatic group based moieties. Their sensitivity to various substrates, pH and temperature make them promising responsive materials for drug delivery too. As the azorductase is made in the human colon, they are prominently used in the treatment of local conditions of colon, inflammation in the bowl and colon cancer. Some of the prodrugs of the anticancer segment were prepared to work as per the azo-based approach to target the colon [Citation45]. These prodrugs (example, methotrexate, GEM) were responsive to azoreductase enzymes and precisely delivered the anticancer drugs to the colon.
A micelle delivery system based on azoreductase responsiveness is reported to break in the presence of the azoreductase enzyme to deliver the loaded drug to the colon area [Citation46]. Recently, by the use of an azo derivative an antineoplastic drug was reported to have dual function of therapeutic and diagnostic (theranostic agent) with a possibility to enhance the cancer therapy [Citation47].
Redox responsiveness
Glutathione (GSH) is a thiol present in cells to regulate many reactions, act as antioxidant, act as buffer and control the redox state of many proteins within the human body. GSH acts as a trigger for the reduction of the disulphide bond linkage. This reducibility by triggering GSH can be used to formulate prodrugs or complexes wherein the entrapped drug’s release can be spatially and temporally controlled. The rationale of GSH with respect to the cancer cells comes out due to its presence in higher concentration within the tumour or the microenvironment of the cancer cells. When the drug delivery system comes in contact with the tumour microenvironment, the presence of GSH would breakdown the disulphide bonds and the loaded active moiety would be released at the right place to specifically destroy the cancerous cells [Citation48].
Tu et al. [Citation49] reported one of the first’s nanomotor systems comprising of redox responsive behaviour for enhancing cancer therapy. Herein, the disulphide bond was integrated amid the hydrophilic and hydrophobic block formed by PEG and polystyrene, respectively. When this system was exposed to the redox environment, the hydrophilic cover was removed, disabling its movement and eventually releasing the payload at the cancer site.
With an aim to intracellularly deliver the anticancer drugs, a micelle-based reduction responsive delivery system was prepared having the cleavable disulphide linkage at three positions like POEOMA-ss-(PLA-ss-PLA)-ss-POEOMA [Citation50]. Where, POEOMA represented the polymethacrylate pendant oligo(ethylene oxide) hydrophilic block and polylactic acid (PLA) as the hydrophobic part. There was a rapid drug (DOX) release from the micelles was triggered by the presence of greater concentration of GSH intracellularly. This eventually led to enhanced cytotoxicity thereby inhibiting higher cell proliferation.
Light responsiveness
Light is an external stimulus to trigger drug delivery. High biocompatibility and simplicity in case of application make the system very appealing. The major challenge for the light stimuli drug delivery system is the depth to which it is able to penetrate the tissue. The penetration depth is imperative for proficient and targeted drug release in mass tissue. The near infrared (NIR) light with wavelengths of range 650–900 nm have an alluring optical stimuli due to the negligible attenuation by blood and soft tissues. Accordingly, NIR light takes into consideration non-intrusive and profound tissue penetration. A novel light-responsive (PnP) AZO substituted poly(acrylic acid) templates has shown to trigger DOX delivery derivative system. The photocleavage response is likewise used to make light-activated polymeric nanocarriers. Numerous investigations demonstrated that o-nitrobenzyl and coumarin-subordinate consolidated copolymers demonstrate a productive mode for developing UV/NIR activated systems. The amphiphilic diblock copolymer micelles have worked as phototriggered medicate discharge and upgraded for attractive reverberation imaging (MRI) differentiate execution.
Huang et al. [Citation51] have classified light responsive systems into five groups as per the photo-reaction mechanisms; into photo-based isomerization, photo-induced rearrangement, photo-based cleavage, photo-induced crosslinking and photo-induced energy conversion.
A covalent Gd3+ OEGMA-b-P(NIPAM-co-NBA-co-Gd) light-responsive block copolymer was reported which upon UV irradiation, converted into hydrophilic derivative form hydrophobic NBA moieties [Citation52]. This light-triggered systems can be effectively used for cancer cells imaging and hence are suitable for next-generation novel theranostic carriers.
Polymer-based drug delivery systems exhibiting responsiveness in cancer
In the present section, we have identified the two of the most commonly used polymer-based drug delivery system – nanogels and micelles, which act as responsive systems in cancer therapy. However, there are other drug delivery systems too which show responsive behaviour.
Nanogels as a responsive carrier for cancer therapy
The crosslinked hydrophilic polymers forming a three dimensional gelled network in an aqueous medium are termed as nanogels or nano-sized hydrogels which have their globule sizes with diameters in the range of 1–1000 nm. The unique properties of responsiveness, high drug loading, ability to attach ligands, ability to load both hydrophilic and hydrophobic drugs, functionalization, etc. make them one of the most sought out delivery system for anticancer drugs [Citation23,Citation53].
Light-responsive nanogels for controlled release of DOX is recently reported by using infrared light (graphene emitting Red colour light) [Citation54]. The designing of such light sensitive nanogel was done by fabricating DOX with graphene through ester linkages and then conjugating them with HA. This linkage creates a complex which is cleavable at the site of tumour microenvironment to release the entrapped drug.
The tumour microenvironment is usually at a lower pH, higher temperature than the healthy cells, expresses certain enzymes and hence offers multiple responsive at the same time. In this context, Don et al. [Citation55] recently prepared a cationic nanogel using protamine-poly(AA-b-NIPAAm) copolymer. These nanogels showed multi-responsiveness as they were able to react to changes in temperature, pH, and presence of certain enzymes. This was said to be due to conformational changes in the protamine with the protein undergoing enzymatically hydrolyzed. The developed nanogels not only efficiently impaired the multidrug resistant cancer cells but also enhanced the chemotherapy by specifically destroying cancer cells.
Similarly, triple responsive nanogels have been recently reported to respond to temperature, pH and reduction by using the PNIPAM polymer [Citation56]. At higher temperature, these nanogels had greater uptake by the cancer cells and an enhanced drug release was observed at reducible microenvironment which is at a lower pH. Such synergistic delivery is needed for enhancing the anticancer efficacy. Nanogels, in fact, can be made multifunctional, be hybrid in nature, exploit the tumour microenvironment (e.g. using the reactive oxygen species) and load active moieties of diverse nature to enhance the cancer therapeutics [Citation57].
Micelles as a responsive carrier for cancer therapy
The formation of micelles is due to spontaneous self-assembly of amphiphilic polymers/co-polymers in water-based surroundings. The existence of both the hydrophilic and hydrophobic portions in the amphiphilic moieties allows good loading of both lipophilic and hydrophilic drugs. Micelles offer many distinctive features to be termed as versatile responsive carrier in cancer therapy. These features can be briefed as their capability to entrap/incorporate hydrophilic/hydrophobic drugs and also contrast agent or moieties for diagnostic applications; modify the release of the entrapped drug; efficient use of many responsive characteristics like temperature, light, pH or redox [Citation58].
Amphiphilic copolymers (PEG, PLA and poly amino esters) with pH sensitivity were utilized to form block co-polymers which self-assemble at the water environment to form micelles [Citation59]. These micelles were insoluble at pH 7.4, but were solubilized at lower pH due to protonation. The pH change also impacted the release of the entrapped drug (DOX) which was sustained for a longer duration and speeded at lower pH of 5 (tumour microenvironment pH) indicating good cancer cells targeting ability. A folate-targeted unimolecular micelle was proposed for delivery of DOX by use of aliphatic polyester (Boltorn H40) [Citation60]. Inside the cancer cells, this micelle being pH-sensitive were cleaved by the acidic pH and immediately released DOX. The anticancer drug DOX was again loaded in another unimolecular micelle with pH sensitivity with an co-delivery aspect of photothermal agents to enhance the cancer therapy [Citation61].
A redox-responsive micelle was prepared using the hydrophilic poly(oligo(ethylene glycol) methacrylate) (POEG) and dasatinib (Das) as the hydrophobic blocks for delivery of DOX [Citation62]. The redox environment at cancer site caused the cleavage of Das due to the disulphide bond in the micelle and this caused a triggered release of DOX specifically in the tumour cells. The novel micellar prodrug carrier was also reported to have enhanced cytotoxicity against prostate PC3 cell lines. The hydrophobic anticancer drug PTX was conjugated with disulphide bonds to react at the presence of GSH in the tumour microenvironment to utilize the redox responsiveness [Citation63]. The system made use of micelle with albumin-mediated cancer-targeted delivery for PTX drug. The in vivo studies in rats and cell line studies in vitro showed improved uptake of the PTX micelles formulated and the better cancer cell apoptosis was reported.
Expert opinion and future prospects
It is well understood now that the use of multi-responsive polymeric materials ensures the delivery at the site of action, but also the ablation of toxicity at off-sites (e.g. spleen, kidney, lungs and liver). To activate the system either all the stimuli may work together at the same time or may be activated sequentially. summarizes some of the important outcomes of the above-mentioned responsiveness in polymers to bring strategic change in cancer therapy.
Table 1. Some examples of stimuli responsiveness of polymers with their applications
Conclusion
Polymeric biomacromolecules are adaptable and biodegradable; subsequently, they also are utilized in controlled and targeted release of encapsulated biomolecules for efficacious cancer therapeutics. The shift from mono- to dual to multi-stimuli-responsive polymeric materials has strengthened the belief in their utility in delivering drugs intracellularly with overcoming the problems associated with mono- and dual responsive materials. The sensitivity of these molecules to multiple stimuli makes them quite versatile and increases their applicability domain. These responsive materials are able to mimic some biological processes and recognize at the molecular level itself to manipulate the development of custom-designed molecules for targeting cancer cells.
Acknowledgements
The authors are thankful to Dr. Prashant Kharkar, Professor at Shobhaben Pratapbhai Patel School of Pharmacy & Technology Management, SVKM's NMIMS, Mumbai for his critical inputs in this review and Mr. Viral Bakhai M. Pharm. (Pharmaceutical Technology) second year student for his valuable help in drawing the figures.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Salas-Vega S, Iliopoulos O, Mossialos E. Assessment of overall survival, quality of life, and safety benefits associated with new cancer medicines. JAMA Oncol. 2017;3:382–390.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
- Da Silva CG, Rueda F, Löwik CW. Combinatorial prospects of nano-targeted chemoimmunotherapy. Biomaterials. 2016;83:308–320.
- Liu D, Auguste DT. Cancer targeted therapeutics: from molecules to drug delivery vehicles. J Control Release. 2015;219:632–643.
- Hussain T, Nguyen QT. Molecular imaging for cancer diagnosis and surgery. Adv Drug Deliv Rev. 2014;66:90–100.
- Duncan R, Vicent MJ, Greco F, et al. Polymer–drug conjugates: towards a novel approach for the treatment of endocrine-related cancer. Endocr Relat Cancer. 2005;12:S189–S199.
- Safra T, Muggia F, Jeffers S, et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11:1029–1033.
- Soni G, Yadav KS. Communication of drug loaded nanogels with cancer cell receptors for targeted delivery. In: Suzuki J, Nakano T, Moore MJ, editors. Modeling, methodologies and tools for molecular and nano-scale communications. Cham: Springer; 2017. p.503–515.
- Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380.
- Cerchia L, de Franciscis V. Targeting cancer cells with nucleic acid aptamers. Trends Biotechnol. 2010;28:517–525.
- Tayo LL. Stimuli-responsive nanocarriers for intracellular delivery. Biophys Rev. 2017;9:931–940.
- Yang D-P, Oo MNNL, Deen GR, et al. Nano-star-shaped polymers for drug delivery applications. Macromol Rapid Commun 2017;38:1700410.
- Singh B, Khurana RK, Garg B, et al. Stimuli-responsive systems with diverse drug delivery and biomedical applications: recent updates and mechanistic pathways. Crit Rev Ther Drug Carrier Syst. 2017;34:209–255.
- Liu X, Yang Y, Urban MW. Stimuli-responsive polymeric nanoparticles. Macromol Rapid Commun. 2017;38:e1700030.
- van Elk M, Murphy BP, Eufrásio-da-Silva T, et al. Nanomedicines for advanced cancer treatments: transitioning towards responsive systems. Int. J. Pharm. 2016;515:132–164.
- Zhuang J, Gordon M, Ventura J, et al. Multi-stimuli responsive macromolecules and their assemblies. Chem Soc Rev. 2013;42:7421–7435.
- Su Z, Jiang X. Multi-stimuli responsive amine-containing polyethers: novel building blocks for smart assemblies. Polymer 2016;93:221–239.
- Shaikh RP, Pillay V, Choonara YE, et al. A review of multi-responsive membranous systems for rate-modulated drug delivery. AAPS PharmSciTech. 2010;11:441–459.
- Stuart MA, Huck WT, Genzer J, et al. Emerging applications of stimuli-responsive polymer materials. Nature Mater. 2010;9:101–113.
- Dissemond J, Witthoff M, Brauns TC, et al. PH-Wert des milieus chronischer wunden. Der Hautarzt. 2003;54:959–965.
- de Las Heras Alarcon C, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34:276–285.
- Soni G, Yadav KS. High encapsulation efficiency of poloxamer-based injectable thermoresponsive hydrogels of etoposide. Pharm Dev Technol. 2014;19:651–661.
- Soni G, Yadav KS. Nanogels as potential nanomedicine carrier for treatment of cancer: a mini review of the state of the art. Saudi Pharm J. 2016;24:133–139.
- Loh XJ, del Barrio J, Toh PPC, et al. Triply triggered doxorubicin release from supramolecular nanocontainers. Biomacromolecules. 2012;13:84–91.
- Wang K, Guo DS, Wang X, et al. Multistimuli responsive supramolecular vesicles based on the recognition of p-sulfonatocalixarene and its controllable release of doxorubicin. Acs Nano. 2011;5:2880–2894.
- Klaikherd A, Nagamani C, Thayumanavan S. Multi-stimuli sensitive amphiphilic block copolymer assemblies. J Am Chem Soc. 2009;131:4830–4838.
- Qiao ZY, Zhang R, Du FS, et al. Multi-responsive nanogels containing motifs of ortho ester, oligo (ethylene glycol) and disulfide linkage as carriers of hydrophobic anti-cancer drugs. J. Control. Release. 2011;152:57–66.
- Chilkoti A, Dreher MR, Meyer DE. Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery. Adv Drug Deliv Rev. 2002;54:1093–1111.
- Haider M, Megeed Z, Ghandehari H. Genetically engineered polymers: status and prospects for controlled release. J Control Release. 2004;95:1–26.
- Hussain M, Shchepinov MS, Sohail M, et al. A novel anionic dendrimer for improved cellular delivery of antisense oligonucleotides. J Control Release. 2004;99:139–155.
- Nakamae K, Nizuka T, Miyata T, et al. Lysozyme loading and release from hydrogels carrying pendant phosphate groups. J Biomater Sci Polym Ed. 1998;9:43–53.
- Dong J, Wang Y, Zhang J, et al. Multiple stimuli-responsive polymeric micelles for controlled release. Soft Matter. 2013;9:370–373.
- Yadav KS, Saxena R, Soni G. Nanogels as targeted drug delivery vehicles. In: Vashist A, Kaushik AK, Ahmad S, Nair M, editors. Nanogels for Biomedical Applications. Vol. 30. Royal Society of Chemistry; 2017. p.143.
- Putnam D, Zelikin AN, Izumrudov VA, et al. Polyhistidine-PEG:DNA nanocomposites for gene delivery. Biomaterials. 2003;24:4425–4433.
- Isaacson KJ, Jensen MM, Subrahmanyam NB, et al. Matrix-metalloproteinases as targets for controlled delivery in cancer: an analysis of upregulation and expression. J Control Release. 2017;259:62–75.
- Terada T, Iwai M, Kawakami S, et al. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. J Control Release. 2006;111:333–342.
- Zhang X, Wang X, Zhong W, et al. Matrix metalloproteinases-2/9-sensitive peptide-conjugated polymer micelles for site-specific release of drugs and enhancing tumor accumulation: preparation and in vitro and in vivo evaluation. Int J Nanomedicine. 2016;11:1643.
- Shargh VH, Hondermarck H, Liang M. Gelatin-albumin hybrid nanoparticles as matrix metalloproteinases-degradable delivery systems for breast cancer therapy. Nanomedicine (Lond). 2017;12:977–989.
- Lee SJ, Jeong YI, Park HK, et al. Enzyme-responsive doxorubicin release from dendrimer nanoparticles for anticancer drug delivery. Int J Nanomedicine. 2015;10:5489.
- Han H, Valdepérez D, Jin Q, et al. Dual enzymatic reaction-assisted gemcitabine delivery systems for programmed pancreatic cancer therapy. Acs Nano. 2017;11:1281–1291.
- Zhang C, Liu LH, Qiu WX, et al. A transformable chimeric peptide for cell encapsulation to overcome multidrug resistance. Small. 2018;14:1703321.
- Zhang M, Xu C, Wen L, et al. A hyaluronidase responsive nanoparticle-based drug delivery system for targeting colon cancer cells. Cancer Res. 2016;76(24):7208–7218.
- Jiang H, Shi X, Yu X, et al. Hyaluronidase enzyme-responsive targeted nanoparticles for effective delivery of 5-fluorouracil in colon cancer. Pharm Res. 2018;35:73.
- Dong X, Yin W, Zhang X, et al. Intelligent MoS2 nanotheranostic for targeted and enzyme-/pH-/NIR-responsive drug delivery to overcome cancer chemotherapy resistance guided by PET imaging. ACS Appl Mater Interfaces. 2018;10:4271–4284.
- Sharma R, Rawal RK, Gaba T, et al. Design, synthesis and ex vivo evaluation of colon-specific azo based prodrugs of anticancer agents. Bioorganic Med Chem Lett. 2013;23:5332–5338.
- Rao J, Khan A. Enzyme sensitive synthetic polymer micelles based on the azobenzene motif. J Am Chem Soc. 2013;135:14056–14059.
- Zhou Y, Maiti M, Sharma A, et al. Azo-based small molecular hypoxia responsive theranostic for tumor-specific imaging and therapy. J Control Release. 2018;288:14–22.
- Cai Z, Zhang D, Lin X, et al. Glutathione responsive micelles incorporated with semiconducting polymer dots and doxorubicin for cancer photothermal-chemotherapy. Nanotechnology. 2017;28(42):425102.
- Tu Y, Peng F, White PB, et al. Redox‐sensitive stomatocyte nanomotors: destruction and drug release in the presence of glutathione. Angew Chem Int Ed. 2017;56:7620–7624.
- Ko NR, Oh JK. Glutathione-triggered disassembly of dual disulfide located degradable nanocarriers of polylactide-based block copolymers for rapid drug release. Biomacromolecules. 2014;15:3180–3189.
- Huang Y, Dong R, Zhu X, et al. Photo-responsive polymeric micelles. Soft Matter. 2014;10:6121–6138.
- Li Y, Qian Y, Liu T, et al. Light-triggered concomitant enhancement of magnetic resonance imaging contrast performance and drug release rate of functionalized amphiphilic diblock copolymer micelles. Biomacromolecules. 2012;13:3877–3886.
- Sharma A, Garg T, Aman A, et al. Nanogel-an advanced drug delivery tool: current and future. Artif Cells Nanomed Biotechnol. 2016;44:165–177.
- Khatun Z, Nurunnabi M, Nafiujjaman M, et al. A hyaluronic acid nanogel for photo–chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale. 2015;7:10680–10689.
- Don TM, Lu KY, Lin LJ, et al. Temperature/pH/Enzyme triple-responsive cationic protein/PAA-b-PNIPAAm nanogels for controlled anticancer drug and photosensitizer delivery against multidrug resistant breast cancer cells. Mol Pharmaceutics. 2017;14:4648–4660.
- Xu X, Wang X, Luo W, et al. Triple cell-responsive nanogels for delivery of drug into cancer cells. Colloids Surf B Biointerfaces. 2018;163:362–368.
- Yang HY, Li Y, Lee DS. Multifunctional and stimuli‐responsive magnetic nanoparticle‐based delivery systems for biomedical applications. Advanced Therapeutics. 2018;1:1800011.
- Oerlemans C, Bult W, Bos M, et al. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. 2010;27:2569–2589.
- Zhang CY, Yang YQ, Huang TX, et al. Self-assembled pH-responsive MPEG-b-(PLA-co-PAE) block copolymer micelles for anticancer drug delivery. Biomaterials. 2012;33:6273–6283.
- Yang X, Grailer JJ, Pilla S, et al. Tumor-targeting, pH-responsive, and stable unimolecular micelles as drug nanocarriers for targeted cancer therapy. Bioconjugate Chem. 2010;21:496–504.
- Jia T, Huang S, Yang C, et al. Unimolecular micelles of pH-responsive star-like copolymers for co-delivery of anticancer drugs and small-molecular photothermal agents: a new drug-carrier for combinational chemo/photothermal cancer therapy. J Mater Chem B. 2017;5:8514–8524.
- Sun J, Liu Y, Chen Y, et al. Doxorubicin delivered by a redox-responsive dasatinib-containing polymeric prodrug carrier for combination therapy. J Control Release. 2017;258:43–55.
- Zhang Y, Guo Z, Cao Z, et al. Endogenous albumin-mediated delivery of redox-responsive paclitaxel-loaded micelles for targeted cancer therapy. Biomaterials. 2018;183:243–257.
- Zhang T, Huang S, Lin H, et al. Enzyme and pH-responsive nanovehicles for intracellular drug release and photodynamic therapy. New J Chem. 2017;41:2468–2478.
- Wang F, Klaikherd A, Thayumanavan S. Temperature sensitivity trends and multi-stimuli sensitive behavior in amphiphilic oligomers. J Am Chem Soc. 2011;133:13496–13503.
- Knipe JM, Strong LE, Peppas NA. Enzyme-and pH-responsive microencapsulated nanogels for oral delivery of siRNA to induce TNF-α knockdown in the intestine. Biomacromolecules. 2016;17:788–797.
- Jaiswal MK, De M, Chou SS, et al. Thermoresponsive magnetic hydrogels as theranostic nanoconstructs. ACS Appl Mater Interfaces. 2014;6:6237–6247.
- Sadighian S, Rostamizadeh K, Hosseini MJ, et al. Magnetic nanogels as dual triggered anticancer drug delivery: toxicity evaluation on isolated rat liver mitochondria. Toxicol lett. 2017;278:18–29.
- Fan T, Li M, Wu X, et al. Preparation of thermoresponsive and pH-sensitivity polymer magnetic hydrogel nanospheres as anticancer drug carriers. Colloids Surf B Biointerfaces. 2011;88:593–600.
- Yang H, Wang Q, Huang S, et al. Smart pH/redox dual-responsive nanogels for on-demand intracellular anticancer drug release. ACS Appl Mater Interfaces. 2016;8:7729–7738.
- Zhang X, Achazi K, Haag R. Boronate Cross‐linked ATP‐and pH‐Responsive Nanogels for Intracellular Delivery of Anticancer Drugs. Adv Healthcare Mater. 2015;4:585–592.
- Li R, Feng F, Wang Y, et al. Folic acid-conjugated pH/temperature/redox multi-stimuli responsive polymer microspheres for delivery of anti-cancer drug. J Colloid Interface Sci. 2014;429:34–44.