Abstract
In recent times, Gold nanoparticles (AuNPs) synthesized from plant extracts and their anticancer activity have attracted significant attention. We report the green approach for the synthesis of AuNPs using extract from Abies spectabilis plant. In this study, the reaction parameters were optimized to control the size of the nanoparticle, which was confirmed by Transmission Electron microscopy (TEM). Various characterization technique such as SAED pattern, UV visible spectroscopy, EDX, FTIR, and AFM were employed to analyze the synthesized AuNPs obtained from A. spectabilis plant extract. Furthermore, we investigated the anticancer activities using T24 cell lines. Interestingly, the results of extensive screening on the applications of newly synthesized AuNPs were tested for their cytotoxicity effects on anticancer activity against T24 cells by MTT assay. The cell apoptosis was studied using TUNEL, DAPI, caspase activity, cell invasion and migration. Nanoparticles at different concentrations ranging from 1 to 25 μg/ml exhibited a dose dependent cytotoxicity for 24 h. Condensation and DNA fragmentation are characteristic of apoptosis by DAPI, TUNEL staining, and the significant up regulations of Beclin-1, Bax and caspase 3, whereas the expressions of anti-apoptotic Bcl-2 and Bid were down regulated. However, this study, therefore attempts to report the synthesis, characterization, and anticancer activity of gold nanoparticles of A. spectabilis plant extract beneficial for cancer therapeutics.
Introduction
Currently, nanoparticles play a major role in the medical sector as a major tool in nanomedicine drug delivery system for cancer therapy. Gold nanoparticles (AuNPs) are used in modern medical and biology studies, as well as drug delivery, photothermal cancer therapy, chemical and biochemical sensing, imaging and catalysis [Citation1,Citation2]. Gold nanoparticles are widely used for medical applications such as diagnostics, therapy, prevention and hygiene [Citation3]. AuNPs synthesized from microorganisms [Citation4], algae [Citation5], fungi [Citation5], and plant materials [Citation6,Citation7] have been reported. There are several methods for the synthesis of diverse nanoparticles, such as physical and chemical as well as biological methods. Physical and chemical methods employ high pressure, temperature and toxic chemicals which may be risky to both the environment and humans. Therefore, to avoid the above risks, an environment eco-friendly technique for synthesis of nanoparticle is required.
Green synthesis of AuNPs using plant extract has been reported for biological applications such as antibacterial [Citation8,Citation9], antiparasitic [Citation10,Citation11], antioxidant [Citation9,Citation12], and anticancer activities [Citation13,Citation14]. The synthesis of metal nanoparticle using plant extracts is popular because of the comparatively easy to control synthesis of mono dispersed nanoparticles, and it’s non-toxic, eco-friendly and biocompatibility properties [Citation15]. Recently, Yahim et al. [Citation16] reported that AuNPs has become significant in medical research field owing to their nontoxic nature, self-assembly and improved drug delivery. Cancer is a state complex disease in which a normal cell of the body transforms into impaired cell exhibiting uncontrolled growth, hence form an abnormal tissue called tumour. Moreover, they do not spread to other parts of body but could be malignant as the tumour invades nearby tissues through the blood or lymph system in a process called metastasis and destroys the other healthy tissues.
In context, cancer is one of the highest cause of death worldwide and according to a report, cancer causes 8.2 million deaths and about 32.6 million people worldwide are living with cancer as at 2012. By the year 2030 it is expected there will be 26 million new cancer cases and 17 million cancer deaths per year [Citation17]. However, bladder cancer is one of the common type of urinary tract cancer affecting both women and men [Citation18]. It is the fourth of the eleven most commonly diagnosed cancer in both men and women [Citation19]. During the last three decades, the incidence rate of bladder cancer significantly increased in the last years with the highest rates in USA and European countries while lower rates were recorded in many African and Asian countries [Citation20]. About 25–30% of bladder cancers are diagnosed as muscle invasive bladder cancer and 70–85% of patients with bladder cancer have superficial and non-invasive tumours at the time of diagnosis [Citation21]. Bladder cancer has now become a global problem; the fight against cancer by the development of therapies for severely multiplying tumours, including surgery on trans-urethral resection are the most common treatment for these patients [Citation22].
However, the recurrence rate of the tumour is high after the surgery, hence chemotherapy is used to prevent it, but the specificity is restricted by dose limiting toxicity. However, the use of these drugs are limited due to its severe side effects such as haemorrhagic cystitis [Citation23]. It is a challenge to discover the natural drug therapy to treat the different types of cancer. Therefore, conservative methods that combine prohibited technology and target drug delivery, which is more efficient and less harmful are required. Hence, nanomaterials are becoming more effective to revolutionizing cancer therapy and diagnosis [Citation24,Citation25].
Therefore, novel innovations are needed to address and eliminate bladder cancer cells with less side effects. A number of nanoparticle-mediated beneficial and diagnostic agents have been developed for treatments, including cancer, diabetes, pain, asthma, allergy, infections, among others [Citation26]. However, some reports suggest that the endocytosed AuNPs may cause cytotoxic effects which could be beneficial for the treatment of cancer [Citation27]. Currently, an in vitro cytotoxic assay, AuNPs have been used in anticancer therapy for several types of cancers, including colon carcinoma HCT-116, HT-29 cell lines [Citation28], hepatocellular carcinoma HepG-2 [Citation29] and breast adenocarcinoma MCF-7 [Citation30]. In this study, the plant extract of A. spectabilis, a conifer species in the family of Pinaceae was used for the synthesis of AuNPs. This is the first time that the A. spectabilis plant extract is investigated for the synthesis of AuNPs and its anti-cancer activity also reported.
We investigated the synthesis of AuNPs using plant extract as reducing agent. The synthesized of AuNPs was characterized by Transmission Electron Microscopy (TEM) to determine the size, shape, and morphology. Also, the elemental composition of AuNPs was analyzed by energy dispersive X-ray analysis (EDX). Furthermore, the phytochemical property of the synthesized AuNPs was analyzed by Fourier transform infra-red spectroscopy (FT-IR). The A. spectabilis mediated AuNPs was analyzed for its cytotoxic and anticancer activity on T24 cell lines. T24 cell lines for cell viability, intracellular reactive oxygen sepsis (ROS) generation, nuclear fragmentation and condensation of cells was monitored by TUNEL and DAPI staining. Morphological changes in T24 cells, were observed by crystal violet staining, and caspases activities and determined by ELISA & apoptotic pathways including Becline-1, Bax, Bcl-2, Bid & Caspase-3 by western blotting.
Materials and methods
A. spectabilis plant extraction
Ten grams of the coarsely powdered air-dried aerial parts of A. spectabilis sample was mixed in 100 ml of distilled water and boiled for 5 min. After which, the extract was cooled to room temperature and filtered using Whatmann number one filter paper. The resulting filtrate was centrifuged at 10,000 rpm for 10 min to remove the supernatant particles. Then the collected supernatant was stored at 20° C and use for the synthesis of eco-friendly AuNPs.
Synthesis of AuNPs using plant extract
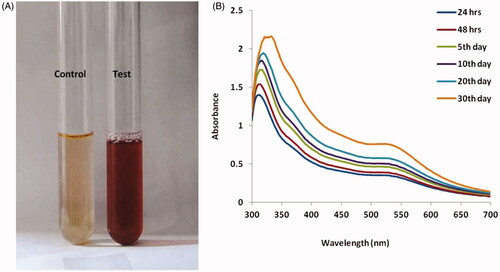
Two millilitres of the extract was added to 50 ml of HAuCl4.3H2O (10−4 M) aqueous solution and incubated at 29 °C for 24 h. The reduction process of gold ions to AuNPs kicked off after 5 min, then after 15 min, a pale yellow colour was observed in the mixed solution which turned ruby red indicating the complete formation of AuNPs. Hence, the reduction of the metal ions was continuously monitored by visual inspection as well as with UV-visible spectrometer analysis in the wavelength range of 300–800 nm.
Characterization of gold nanoparticles
The synthesized AuNPs were characterized by UV-Visible spectrometer for their absorption properties, the absorbance spectra was obtained using Shimadzu UV-1800 connected to a computer, the extract was poured into a 10 nm optical path length quartz cuvettes of 1 nm resolution, and scanned at wavelength of 200–1100 nm. The structure and size of the synthesized AuNPs were analyzed by X-ray Diffraction (Shimadzu XRD– 6000/6100 model) and Dynamic light scattering method using Malvern Zetasizer nanosizer respectively. The morphological studies including size, shape and distribution was analyzed using TEM, and selected area electron diffraction (SAED) (JEOL JEM-2000 EX microscope) patterns with an accelerated voltage of 200 KV. A drop of aqueous re-dispersed suspension of AuNPs were placed on a carbon coated copper grid and air-dried before the TEM measurement. Energy dispersive X-ray spectroscopy (EDX) was performed using FESEM, ZEISS Supera 40VP system with an accelerated voltage of 5 KV. The FT-IR spectra of AuNPs were used to determine the functional biomolecules. The FTIR absorption spectrum was measured at room temperature in the range of 4000–400 cm−1 for the aqueous plant extract using FT-IR (Shimadzu-8400) spectrometer. Atomic force microscopy (AFM) was used to characterize the nanoparticle film with or without heat treatment, using an Agilent 5500 AFM with silicon cantilever model and typical resonance frequency 2.7 N/m and 80 kHz, respectively, and tip radius <10 nm. Experiments were made in non-contact mode (topography and phase contrast). Gwyddion 2.10 software was used to analyze the AFM images.
Cell viability by MTT assay
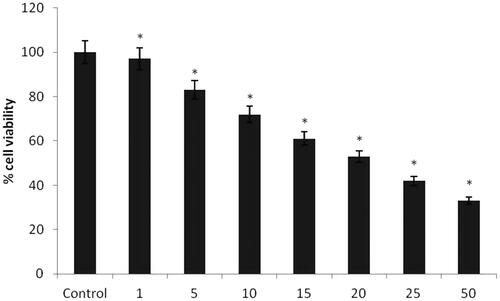
The cytotoxicity test of A. spectabilis extract AuNPs were performed on bladder cancer T24 cell lines using 3-(4,5-dimethylthiazol-2-yl-)-2,5-diphenyltetrazolium bromide (MTT) assay. The T24 cells were seeded into a 96 well plate with the density of (5 × 103 cells/well) for 24 h. Subsequently, cells were treated with AuNPs from the A. spectabilis plant extract suspension with different concentrations (1, 5, 10, 15, 20 and 25 μg/ml) added to each well. After the 24 h treatment, the medium was replaced with fresh DMEM complete medium and cells were incubated with MTT reagents (5 mg/ml in PBS) at 37 °C for 4 h. After the 24 h incubation, the medium was removed and DMSO was added to each well to dissolve the formazan crystals. Finally, the absorbance of the formazan reduced product was read from ELISA plate reader (Bio-Rad, Model 680, Hercules, CA) with a test wavelength of 570 nm. Cell viability was expressed as a percentage of the value in the control.
Cell apoptosis detection by TUNEL and DAPI staining
Cell slides 20 mm in diameter were placed into the wells of a 12 well plate [Citation31]. T24 cells seeded with density of 4 × 105 cells/well were treated with AuNPs (15 and 20 µg/ml) and incubated at 37 °C for 0 h, 12 h and 24 h with untreated cells. After 24 h of treatment, 80–90% of the cells in the culture medium was removed and replaced with fresh medium. Then the cells were incubated for 3 h at 37 °C in 5% CO2 incubator. After 3 h, the medium was removed and cells were placed on cell the cover slip of each group, washed and then fixed. Furthermore, the in-situ cell death detection kit: TER red and DAPI staining reagent were used as well as TUNEL stains and DAPI staining. Apoptosis of cells was analyzed by counting positive cell of TUNEL staining or counting cells with positive DAPI staining and compared between different groups at 10 randomly selected fields at ×400 magnification in a blinded manner using a fluorescence microscope (IX71; Olympus Corporation).
Intracellular ROS measurement
The intracellular ROS generation levels was measured using fluorescence dye DCFH-DA that can penetrate into the intracellular matrix of cells where it is oxidized by ROS to fluorescent dichlorofluorescence (DCF) [Citation32]. First, isolated T24 cells (5 × 105 cells/well) were made up to a final volume of 2 ml in normal PBS. 1 μl of DCFH-DA (1 mg/ml) was added to 1 ml aliquot of cells and incubated at 37 °C for 30 min under dark condition. The fluorescence of DCFH-DA was measured using multimode reader (BioTek Instrument) with excitation and emission at 485 ± 10 and 530 ± 12.5 nm.
Assays for caspase-3 and caspase-9 activity by ELISA
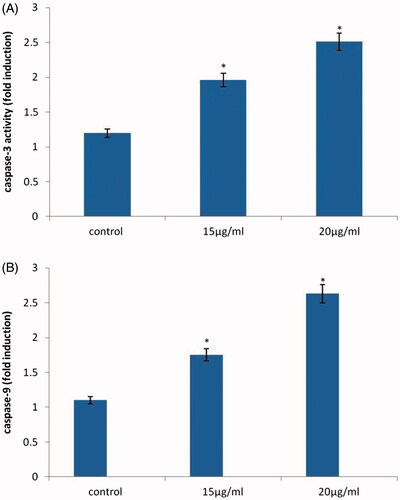
The T24 cells were seeded into a 96 well plate and cells were treated with or without AuNPs in 15 and 20 μg/ml for 24 h. Subsequently, cells were isolated and homogenated according to the caspase 3 and 9 colorimetric assay substrate kit utilized for the apoptotic process. Cells lysate containing 50 μg proteins were then incubated for 1 h at 37 °C with specific caspase-3 and 9 substrate. A spectrofluorometer was used to measure fluoresence excitation wavelength read at 485 20 nm, emission wavelength read at 528 20 nm.
Scratch wound healing assay
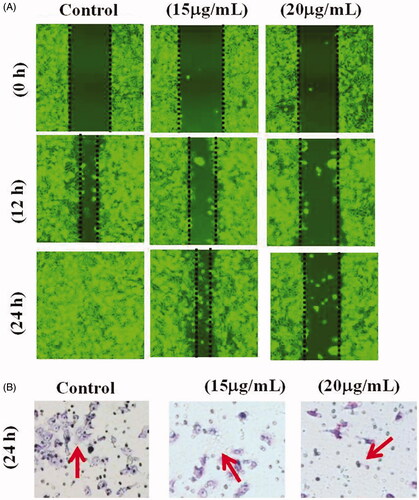
The effect of AuNPs on cell migration was examined using a scratch wound healing assay according to Luo et al. [Citation33]. The wound healing assay was used to determine the effect on cell migration induced by the AuNPs in the T24 cells. T24 cells were seeded in 12 well plates with density of 4 × 105 cells/well till it reached a confluence of 100. The original medium was replaced by PBS buffer to make a scratch using a 1 ml tip. Then the cells were washed with PBS and treated with 15 μg/ml and 20 μg/ml of AuNPs at 37 °C in a 5% CO2 humidified atmosphere for 0 h, 12 h and 24 h with untreated cells used as control group. Wound healing was observed and photographed using an inverted microscope (Olympus) at 0 h, 12 h and 24 h of treatment period.
Transwell migration assay with cells at 2 × 105/ml were added in the upper transwell migration chamber and cultured in 200 µl 1% v/v FBS medium containing different concentrations (15 and 20 µg/ml) of AuNPs at 37 °C in 5% CO2 atmosphere. Then complete culture DMEM medium was added to the 24 well plates. Moreover, cells were incubated for 24 h at 37 °C in 5% CO2 incubator, then fixed with fixative methanol and stained with 0.1% crystal violet. The stained images were observed under microscope (Olympus 1X73). The migrated cells were quantified by manual counting methods and represented as a percentage of control group.
Immunoblotting
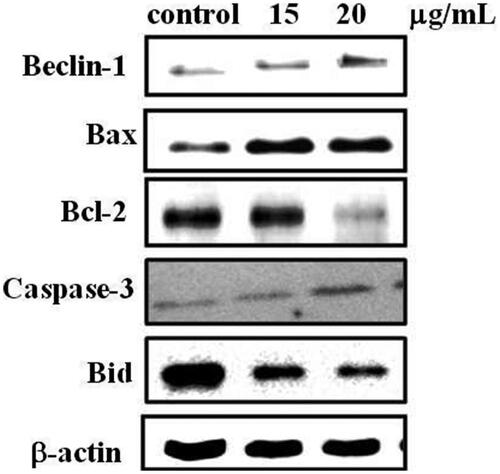
Western blot analysis was performed based on the methods previously described [Citation34,Citation35]. T24 cells (1 × 105) in six well plates, were treated with AuNPs for 24 h; cells were harvested and lysed in lysis buffer (50 mMTris-HCL, 0.5 mM EDTA, 1% SDS, 1 mM DTT) in ice. Then the supernatant was collected and protein concentrations were determined by nanodrop spectrophotometer. 50 µg of each protein samples was added to 10% SDS-polyacrylamide gel and then transferred to PVDF membranes (Millipore, USA). Membranes were blocked with 5% BSA membrane for 1 h, and incubated with primary antibody (dilution at 1:1000) at 4 °C overnight. After washing with TBST, the membranes were incubated with secondary antibodies at room temperature for 1 h, then washed with TBST for three times and detected with chemiluminescent detecting kit (Bio-Rad).
Statistical analysis
All the experiments’ data were analyzed as Mean ± standard deviation (SD). The results were analyzed by one-way ANOVA followed by Duncan analysis using SPSS. A value of p < .005 was considered as statistically significant.
Results
Analysis of absorption spectra of AuNPs by characterized by UV-Visible spectroscopy
The AuNPs absorption properties was determined by UV-visible spectroscopy which showed surface Plasmon resonance peak at 510–550 nm, as well as the maximum range of approximately 530 nm as shown in .
XRD and DLS pattern of AuNPs synthesised from A.spectabilis
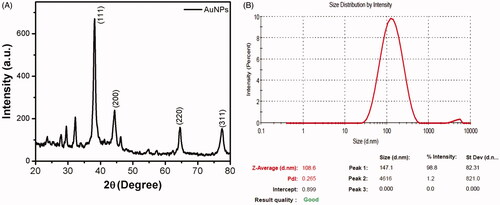
The X-ray diffraction pattern and zeta potential of the synthesized AuNPs were represented in . AuNPs showed four Bragg’s diffraction peaks positioned at 2θ angles: 36.75°, 44.13°, 64.39°, 77.47° which corresponded to (111), (200), (220), (311) planes, exhibiting the crystalline nature of synthesized AuNPs, which is consistent with the standard database file (JCPDS card No 04–0783). The size of the synthesized nanoparticle was measured by Dynamic light scattering analysis and the size distribution curve is shown in . The AuNPs had an average particle size of 108.6 nm and PDI value of 0.265.
TEM-SAED pattern of AuNPs synthesised from A. spectabilis
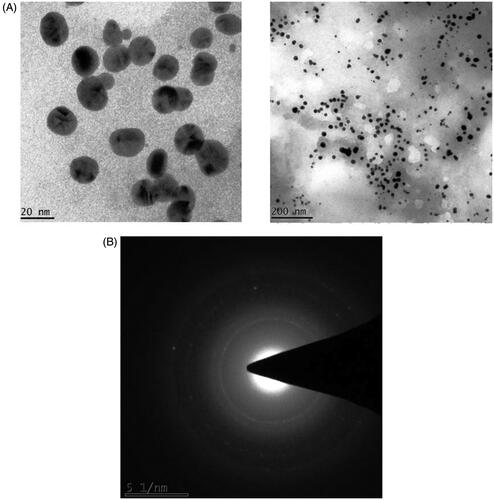
TEM-SAED images confirmed the formation AuNPs and the characterization determined the morphology, particle size, and crystal structure of AuNPs. TEM result showed the image size distribution histogram of the AuNPs obtained at magnification of 150,000x. The AuNPs possess spherical morphology with a diameter of 20–200 nm, and plant extract showed closely spherical AuNPs which dominated a wide spectrum of the particle size and confirmed by TEM. Furthermore, SAED analysis showed a single spherical particle, hence confirmed the AuNPs formation as shown in . The rings corresponded to (200) and (220) which indicated that the synthesized AuNPs crystallized in a face centred cubic phase.
Elemental analysis of AuNPs using EDX
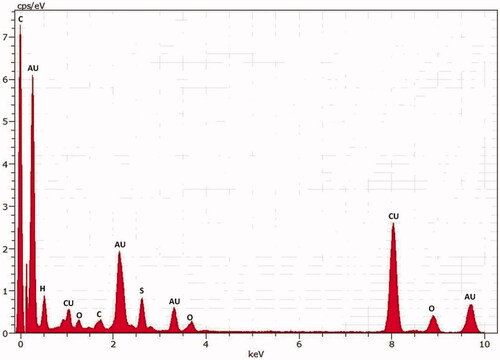
The spot profile EDX spectra of the AuNPs suspensions showed strong gold signals characteristic peaks showed () and corresponds to the elemental AuNPs. Additionally, AuNPs EDX spectra exhibited weaker carbon and oxygen peaks which represented the organic elements of the plant extract.
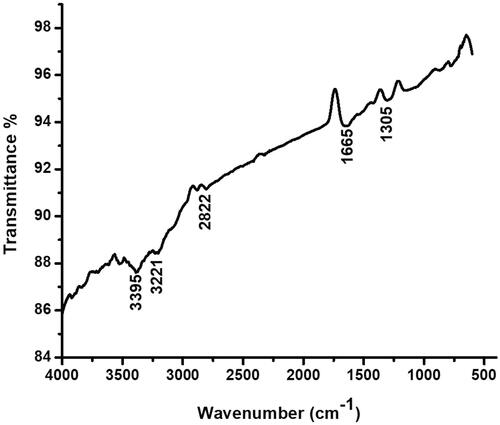
FTIR analysis
The FTIR spectrum of synthesized AuNPs was shown in the . The maximum peak range of AuNPs was seen between 1500–3500 cm−1, with characteristic peaks at 1305 (S = O strong stretch), 1665 (C = C), 2822 (C – H), 3221 & 3395 cm−1 (O – H stretch or H bond) which is due to the reduction of gold ions by AuNPs. The peak at 3313 cm−1 indicated the presence of hydroxyl group of polyphenols from AuNPs, the stretch at 2822, 1665 cm−1 specified the presence of alkanes and 1305 cm−1 stretch indicated the sulphone group.
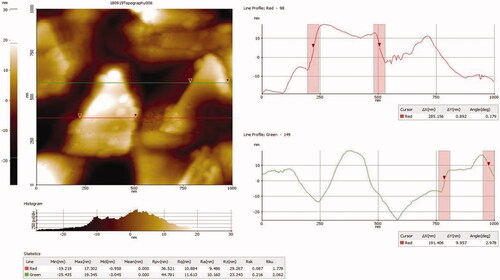
AFM of AuNPs synthesized from A. spectabilis
AFM results revealed the results () represent how the AuNPs size distribution are generated. The AFM analysis of AuNPs average scanning area was found by 5 × 5 µm tapping mode and both two dimensional and three dimensional images were generated. Moreover, AuNPs images were confirmed to have uniform distribution the particles of approximately 50 nm consistent with the TEM analysis.
Cytotoxicity effect of AuNPs from A. Spectabilis
To evaluate the cytotoxic effect of AuNPs from A. spectabilis plant extract was examined on bladder cancer T24 cell line by MTT assay. In the cytotoxic effect of the AuNPs plant extract was found to reduce the viability of bladder T24 cancer cells in a dose dependent manner. The 50% inhibitory concentration effect (IC50) values of AuNPs was 15 and 20 μg/mL respectively.
Figure 7. Cytotoxicity effect of AuNPs from A. spectabilis. Values are expressed as the mean ± SD (n = 3). Significant at *p < .05; compared with untreated control cells.
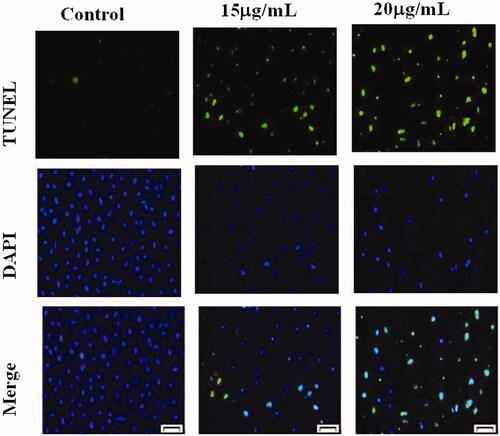
The effect of AuNPs treatment on induction of apoptosis in bladder cancer cells
TUNEL and DAPI staining were used to determine the apoptosis in the cells after AuNPs treatment. TUNEL assay can detect DNA strand breaks that are mainly induced by ROS and abortive apoptosis. confirmed that AuNPs TUNEL stained cells showed strong green fluorescence spots which indicated DNA fragmentation. These findings were further corroborated with DAPI staining which revealed cell shrinkage in the cells treated with 15 μg/ml of AuNPs, and apoptosis features, cell morphological alterations such as chromatin condensation, cell membrane blebbing were found in cells treated with 20 μg/ml of AuNPs.
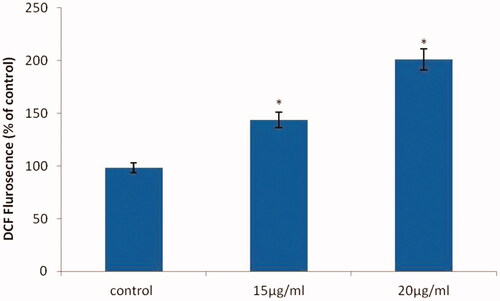
ROS generation by AuNPs from A. spectabilis
ROS formation is one of the events that take place at the onset of apoptosis. As shown in , bladder T24 cancer cells with 15 μg/ml AuNPs significantly increased 150% in the DCF fluorescence compared to the control, cells treated with 20 μg/ml AuNPs had a significant increase by 200% in the DCF fluorescence compared to low dose of 15 μg/ml. It suggests that ROS level is enhanced by many anticancer drugs causing oxidative stress.
Effect of AuNPs from A. spectabilis on cell migration in T24 cell lines
Untreated control cells showed increased cell proliferation at 0–24 h incubations. Whereas, treatment with AuNPs decrease cell migration in T24 cells in a concentration dependent manner (). In this study, 15 μg/ml concentration of AuNPs effectively inhibited cell migration and wound healing in T24 cell lines. Furthermore, AuNPs mediated cell death was analyzed by crystal violet assay. We found that AuNPs induced cell death in T24 cell lines when compared with control cells. Evidently, the 15 μg/ml concentrations of AuNPs effectively induced cell death in the T24 cell lines.
Effect of AuNPs from A. spectabilis on caspase-3 and 9 activity
shows the caspase 3 and caspase 9 signalling cascade in AuNPs induced apoptosis against bladder cancer T24 cell lines. The levels of caspase 3 and 9 in AuNPs treatment with T24 cell line significantly increased caspase 9 more than one-fold activity of caspase 3 at concentration of 20 µg/mL in a dose dependent manner compared to the control group. The results suggested that AuNPs significantly inhibited apoptosis.
Modulation of pro and anti-apoptotic protein expressions after AuNPs treatment
The protein expression of pro and anti-apoptotic markers including Beclin-1, Bax, Bcl-2, caspase3 and Bid was analyzed by western blotting on T24 cell lines. The T24 cells were treated with AuNPs which significantly increased the protein expression of Beclin-1, Bax and Caspase-3 and diminished the expression of anti-apoptotic Bcl-2 and Bid when compared to untreated control cells shown in . Whereas, there are no significant changes in the 15 and 20 µg/mL AuNPs concentrations.
Discussion
In the last few decades, advances in nanotechnology in particularly AuNPs have been allowed the development of many biosciences for biomarkers detection based on the characteristic optical properties of colloidal AuNPs. AuNPs are generally synthesized from plant extract of A. spectabilis to use to reducing agent in to the solution which promotes reduction of gold ions and Au atoms aggregation and grow as AuNPs. In the present study, we examined plant extract to synthesis AuNPs was used to aqueous extract of A. spectabilis. Zuber et al. [Citation36] reported that the formation of AuNPs was evident from the change in solution colour from orange-blue to ruby-red as well as from the presence of the typical Plasmon peak in the range of 520–540 nm with a peak is a distinctive characteristic of Spherical AuNPs with a diameter of 10–20 nm. Similarly, our results founded via UV-vis spectroscopy confirmed the completion of the reaction after 24 h as evidenced from the stability of the plasmonic peak ()
In the present study, we examined a plant extract to synthesize AuNP’s. Phytochemical based synthesis of AuNPs from A. spectabilis. From the images, TEM studies were revealed that the morphology of AuNPs were mostly spherical with an average particle size measured for the colloids are observed to be 20–200 nm respectively. This shows that higher temperature during synthesis leads to increase in particle size. Whereas, SAED analysis confirmed their particle size of crystalline nature, AuNPs of A. spectabilis have shown 51 nm for the colloids respectively which is corresponding between the fcc gold. The SAED patterns shown the diffraction ring inner to outer which can be indexed as (111), (200), and (220), (311) Braggs reflections respectively of fcc structure of metallic gold. This is interesting to note that almost all particles in the images are not in physical content and they are separated from each and other by a uniform inter particle distances [Citation2,Citation37,Citation38]. This study helped us to support our present findings.
Although the rising attention being synthesis of AuNPs from natural plant extract, because the bio-reduction mechanism remains to have been elucidate. El-Batal et al. [Citation39] demonstrated that AuNPs synthesized from Antigonon leptopus leaf extract containing metabolites and biomolecules observed through FTIR analysis were responsible for the bio-reduction of Au ions as well as in soybean-garlic aqueous extract have been reported. The different functional groups with their corresponding stretches obtained in the presently synthesized AuNPs are in agreement with the previous reported of Varshney et al. [Citation40]. And these functional groups could be related to the recorded bio-reduction process, as they might have acted as capping agents [Citation41]. The observed differences in size of nanoparticle could have been possibly due to the reduction of ions to gold being formed at different times [Citation42]. Consistent with this, the FTIR data reported () were consistent with the involvement of the multiple compounds in the AuNPs synthesis. Additionally, EDX pattern signalled the traces of Cu, which could have occurred due to the grid employed during analysis [Citation43]. The size of the NPs may be smaller at higher concentration of extract owing to the phytochemicals (present in the extract) that can effectively stabilize the NPs [Citation44].
With consistent a strong clear peak found of gold atom was seen in type spot EDX spectrum of all the AuNPs. Furthermore, the formation of different size and shapes are due to aggregates of plant extracts. The DLS and XRD data provides accuracy of particle size and distribution. The size of images were observed by AFM were larger than those measured in TEM analysis (20 nm) were found in AuNPs. The cold welding phenomena of the AuNPs on the AFM size has discrepancy between the TEM and AFM size measurement [Citation45]. Similarly our results on AFM analysis showed that the particle size were measured 50 nm for AuNPs from the enhanced AFM images and with narrow size distribution.
Anticancer effects of AuNPs from plant extract A. spectabilis against T24 cancer cell line were examined. The results were shown the cytotoxic activity against bladder cancer cells. AuNPs play significant role in the anticancer activity. The AuNPs are having good stability against T24 in the 20 μg found good results followed by 15 μg, 10 μg, 5 μg and 1 μg respectively. The lowest inhibitory action was observed from the concentration of 25 and μg/mL. Liu et al. [Citation46] reported that identified the AuNPs shows the fight against cancer cells. Similarly, AuNPs synthesized from Corchorus olitorius extract show good in vitro cytotoxic assay of the biocompatible against three human cancer cell lines, such as human laryngeal cancer cell lines (Hep-2), breast cancer cell lines (MCF-7), and colon cancer cell lines (HCT-116) [Citation1]. In previous reports synthesized the AuNPs and reported their great potential to inhibit the cell viability of bladder cancer cell lines. In the present findings also reported that concordant with this previous report.
In order to understand the mechanism of apoptosis involved by AuNPs against bladder T24 cancer cell lines. Oxidative stress which is response for increased generation or elimination of ROS which cause cellular injury in cellular levels. Increased generation of free radicals induces by lipid peroxidation which is the major consequences of oxidative stress and antioxidant depletion in T24 cell lines. Moderate concentrations of ROS are involved in physiological condition as part of defence mechanisms and signalling process [Citation47]. Zorov et al. [Citation48] demonstrated that the overproduction of ROS levels by cellular disruption in many cellular process that occurs during cell proliferation, inflammation and apoptosis, further increased ROS generation triggers cell death have been reported. This study suggested that the cytotoxic effect exerted by AuNPs is associated with generation of ROS were untreated control cells significantly decreased ROS levels. Whereas, in our study, we observed increased ROS generation was found in AuNPs 15 and 20 μg/mL concentration as compared to control cells.
The ability of AuNPs to induce apoptosis is also supported by measuring the DNA damage in cells. TUNEL positive cells were detected by immunofluroscence indicates apoptotic cells with the presence of DNA fragments. During the apoptosis the DNA strands breaks. Moreover, AuNPs inhibits the cell migration at 24 h incubation in T24 cells. These results are closely related to TUNEL and DNA fragmentation assay. Mata et al. [Citation49] reported that the anticancer activity of green synthesized gold nanoparticles through DNA damage and ROS mediated apoptosis in HT-29 cells. Further, our results of extensive screening on identifying the newly formed AuNPs application anticancer activity has been explored in T24 bladder cancer cells. The AuNPs exposed and these effects were determined by 15 and 20 μg/mL concentration. AuNPs induced apoptosis in T24 cells showed DNA fragmentation occurred as showed in .
Moreover, apoptosis is cell intrinsic machines helps to abolish potentially injurious cells, generally regulated by Bcl-2 family proteins. In this study we found the protein expression of Beclin-1, Bax, Bcl-2, caspase-3 and Bid in response to AuNPs exposed in T24 cells because apoptotic cells is controlled through these pathways. Protein expression results showed that AuNPs up-regulated protein expression protein Beclin-1 and pro-apoptotic bax and caspase-3. Expression of anti-apoptotic Bcl-2 and Bid was down-regulated in cells exposed to AuNPs concentration of 15 and 20 μg/mL. Furthermore, caspase activities of apoptotic caspase-3 and 9 were increased in AuNps treated bladder cancer T24 cells. Manar et al. [Citation50] reported that gold nanoparticles may induce apoptosis in MCF-7 cells via p53, Bax/Bcl-2 and casapase pathways. Furthermore, we found pro-apoptotic members of Bcl-2 family, such as bax induces permeabilization of the outer mitochondrial membrane, which releases soluble proteins from the inter-membrane space into the cytosol, where they promote caspase activation. Previous studies reported that induction of apoptosis by AuNPs through activation of caspase-3 and caspase-9 in cervical cancer cells [Citation51]. Similarly, Sellappa et al. [Citation52] reported that AuNPs from Argemone mexicana leaf against HepG2 cells inducing apoptosis in part by modulating the expression of caspase-3 levels. Our study also reported that AuNPs from A. spectabilis inducing apoptosis consistence with previous report.
Conclusions
In conclusion, our results confirmed that the AuNPs was synthesized from A. spectabilis plant extract as a reducing source. A synthesized AuNP’s morphological study was confirmed spherical, oval shaped with a size of 20–200 nm by TEM and SAED analysis. The crystalline nature of AuNPs was established by EDX analysis respectively. FT-IR analysis confirmed the presence of pytochemicals interaction in reduction and stabilization of nanoparticles.
Furthermore, AuNPs produces significant cytotoxicity on bladder T24 cancer cells, which was confirmed by MTT cytotoxicity assay. Moreover, AuNPs induces apoptosis by increasing the nuclear fragmentation and DNA damage in bladder T24 cell. AuNPs also inhibits the bladder T24 cancer cells through inhibiting intrinsic apoptotic pathway. Overall our findings, A. spectabilis plant extract based synthesis of AuNPs are easier, faster and eco-friendly, it has been proven its potent anticancer activity. The study extends further in near future for the determination of the exact mechanism behind its anticancer agent in the molecular studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ismail EH, Saqer AMA, Assirey E, et al. Successful green synthesis of gold nanoparticles using a corchorus olitorius extract and their antiproliferative effect in cancer cells. Int J Mol Sci. 2018;19:2612–2618.
- Bonigala B, Kasukurthi B, Konduri VV, et al. Green synthesis of silver and gold nanoparticles using Stemona tuberosa Lour and screening for their catalytic activity in the degradation of toxic chemicals. Environ Sci Pollut Res Int. 2018;25:32540–32548.
- Dykman LA, Khlebtsov NG. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae. 2011;3:34–55.
- Mukherjee P, Ahmad A, Mandal D, et al. Bioreduction of AuCl(4)(-) ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew Chem Int Ed. 2001;40:3585–3588.
- Ramakrishna M, Babu DM, Gengan RM, et al. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J Nanostruct Chem. 2016;6:1–13.
- Elia P, Zach P, Hazan S, et al. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int J Nanomedicine. 2014;9:4007–4021.
- Dzimitrowicz A, Jamroz P, di Cenzo GC, et al. Preparation and characterization of gold nanoparticles prepared with aqueous extracts of Lamiaceae plants and the effect of follow-up treatment with atmospheric pressure glow microdischarge. Arab J Chem. 2016. [Epub ahead of print]. doi: 10.2174/1574891X13666180828115543.
- Rad MR, Kazemian H, Yazdani F, et al. Antibacterial activity of gold nanoparticles conjugated by aminoglycosides against A. baumannii isolates from burn patients. Recent Pat Antiinfect Drug Discov. 2018. [Epub ahead of print]. doi: 10.2174/1574891X13666180828115543.
- Vijayan R, Joseph S, Mathew B. Eco-friendly synthesis of silver and gold nanoparticles with enhanced antimicrobial, antioxidant, and catalytic activities. IET Nanobiotechnol. 2018;12:850–856.
- Soni N, Prakash S. Green nanoparticles for mosquito control. Sci World J. 2014;2014:1.
- Suryawanshi R, Patil C, Borase H, et al. In vitro antiparasitic activity of microbial pigments and their combination with phytosynthesized metal nanoparticles. Parasitol Int. 2015;64:353–356.
- Lakshmana A, Umamaheswari C, Nagarajan NS. A facile phytomediated synthesis of gold nanoparticles using aqueous extract of Momordica cochinchinensis rhizome and their biological activities. J Nanosci Technol. 2016;2:76–80.
- Patil MP, Kim G-D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl Microbiol Biotechnol. 2017;101:79–92.
- Patil MP, Ngabire D, Thi HHP, et al. Eco-friendly synthesis of gold nanoparticles and evaluation of their cytotoxic activity on cancer cells. J Clust Sci. 2017;28:119–132.
- Abid Ali Khan MM, Jain DC, Bhakuni RS, et al. Occurrence of some antiviral sterols in Artemisia annua. Plant Sci. 1991;75:161–165.
- Yahia-Ammar A, Sierra D, Mérola F, et al. Self-assembled gold nanoclusters for bright fluorescence imaging and enhanced drug delivery. ACS Nano. 2016;10:2591–2599.
- Thun MJ, DeLancey JO, Center MM, et al. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31:100–110.
- Patafio FM, Robert Siemens D, Wei X, et al. Is there a gender effect in bladder cancer? A population-based study of practice and outcomes. Can Urol Assoc J. 2015;9:269–274.
- Ferlay j, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592.
- Wong MCS, Fung FDH, Leung C, et al. The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep. 2018;8:1129.
- Isharwal S, Konety B. Non-muscle invasive bladder cancer risk stratification. Indian J Urol. 2015;31:289–296.
- Tseng WH, Liao AC, Shen KH, et al. Role of second-look transurethral resection of bladder tumors for newly diagnosed T1 bladder cancer: experience at a single center. Urol Sci. 2018;2:29–95.
- Rajeshkumar S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J Genet Engin Biotechnol. 2016;14:195–202.
- Rosarin FS, Arulmozhi V, Nagarajan S, et al. Antiproliferative effect of silver nanoparticles synthesized using amla on Hep2 cell line. Asian Pac J Trop Med. 2012;29:1–10.
- Bae KH, Chung HJ, Park TG. Nanomaterials for cancer therapy and imaging. Mol Cells. 2011;31:295–302.
- Zhang L, Gu FX, Chan JM, et al. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–769.
- Huang K, Ma H, Liu J, et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012;6:4483–4493.
- Rezaee Z, Yadollahpour A, Bayati V, et al. Gold nanoparticles and electroporation impose both separate and synergistic radiosensitizing effects in HT-29 tumor cells: an in vitro study. Int J Nanomed. 2017;12:1431–1439.
- Fadel M, Kassab K, Abd El Fadeel DA, et al. Comparative enhancement of curcumin cytotoxic photodynamic activity by nanoliposomes and gold nanoparticles with pharmacological appraisal in HepG2 cancer cells and Erlich solid tumor model. Drug Dev Ind Pharm. 2018;44:1809–1816.
- Hasanzadeh M, Tagi S, Solhi E, et al. Immunosensing of breast cancer prognostic marker in adenocarcinoma cell lysates and unprocessed human plasma samples using gold nanostructure coated on organic substrate. Int J Biol Macromol. 2018;118:1082–1089.
- Changxing C, Houxuan L, Jun J, et al. Inhibiting PHD2 in bone marrow mesenchymal stem cells via lentiviral vector-mediated RNA interference facilitates the repair of periodontal tissue defects in SD rats. Oncotarget. 2017;8:72676–72699.
- Hafer K, Iwamoto KS, Schiestl RH. Refinement of the dichlorofluorescein assay for flow cytometric measurement of reactive oxygen species in irradiated and bystander cell populations. Radiat Res. 2008;169:460–468.
- Luo KW, Lung WY, Chun-Xie, et al. EGCG inhibited bladder cancer T24 and 5637 cell proliferation and migration via PI3K/AKT pathway. Oncotarget. 2018;9:12261–12272.
- Su CC, Su JH, Lin JJ, et al. An investigation into the cytotoxic effects of 13-acetoxysarcocrassolide from the soft coral Sarcophyton crassocaule on bladder cancer cells. Mar Drugs. 2011;9:2622–2642.
- Su TR, Lin JJ, Chiu CC, et al. Proteomic investigation of anti-tumor activities exerted by sinularin against A2058 melanoma cells. Electrophoresis. 2012;33:1139–1152.
- Zuber A, Purdey M, Schartner E, et al. Detection of gold nanoparticles with different sizes using absorption and fluorescence based method. Sens Actuators B. 2016;227:117–127.
- Aromal SA, Vidhu VK, Philip D. Green synthesis of well-dispersed gold nanoparticles using Macrotyloma uniflorum. Spectrochim Acta A Mol Biomol Spectrosc. 2012;85:99–104.
- Balasubramani G, Ramkumar R, Krishnaveni N, et al. Structural characterization, antioxidant and anticancer properties of gold nanoparticles synthesized from leaf extract(decoction)of Antigonon leptopus Hook. & Arn. J Trace Elem Med Biol. 2015;30:83–89.
- El-Batal AI, Hashem AM, Abdelbaky NM. Gamma radiation mediated green synthesis of AuNPs using fermented soybean-garlic aqueous extract and their antimicrobial activity. Springer Plus. 2013;2:129–138.
- Varshney R, Bhadauria S, Gaur MS. Biogenic synthesis of silver nanocubes and nanorods using sundried Stevia rebaudiana leaves. Adv Mater Lett. 2010;1:232–237.
- Gopinath K, Venkatesh KS, Ilangovan R, et al. Green synthesis of gold nanoparticles from leaf extract of Terminalia arjuna, for the enhanced mitotic cell division and pollen germination activity. Ind Crops Prod. 2013;50:737.
- Rajasekhar RG, Jayakumar C, Antony BM, et al. Green synthesis, characterization and in-vitro antibacterial activity of polyshaped gold nanoparticles by using Senna siamea (Lam.) plant leaf extract. Int J Green Chem Bio. 2012;2:1–5.
- Das S, Roy P, Mondal S, et al. One pot synthesis of gold nanoparticles and application in chemotherapy of wild and resistant type visceral leishmaniasis. Colloids Surf. 2013;107:27–34.
- Paul K, Bag BG, Samanta K. Green coconut (Cocus nucifera Linn) shell extract mediated size controlled green synthesis of poly shaped gold nanoparticles and its application in catalysis. Appl Nanosci. 2013;4:3.
- Choi Y, Kang S, Cha SH, et al. Platycodon saponins from Platycodi Radix (Platycodon grandiflorum) for the green synthesis of gold and silver nanoparticles. Nanoscale Res Lett. 2018;13:23.
- Liu Z, Wu Y, Guo Z, et al. Effects of internalized gold nanoparticles with respect to cytotoxicity and invasion activity in lung cancer cells. PLoS One. 2014;9:e99175.
- Espinosa-Diez C, Miguel V, Mennerich D, et al. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197.
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-Induced ROS release. Physiol Rev. 2014;94:909–950.
- Mata R, Nakkala JR, Sadras SR. Polyphenol stabilized colloidal gold nanoparticles from Abutilon indicum leaf extract induce apoptosis in HT-29 colon cancer cells. Colloids Surf B Biointer. 2016;143:499–510.
- Selim ME, Hendi AA. Gold nanoparticles induce apoptosis in MCF-7 human breast cancer cells. Asian Pac J Cancer Prev. 2012;13:1617–1620.
- Baharara J, Ramezani T, Divsalar A, et al. Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells. Avicenna J Med Biotechnol. 2016;8:75–83.
- Sellappa S, RafiqKhan M, Selvaraj V, et al. Cytotoxic effect of green synthesized gold nanoparticles using Argemone mexicana leaf against HEPG2 cells. Indo Amer J Phar Res. 2015;5:3394–3398.