?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nanotechnology has emerged strongly in most of the field of sciences at a tiny scale. At this size, atoms and molecules work differently and present a diversity of amazing and appealing applications. Pharmaceutical nanocarriers comprise nanoparticles, nanospheres, nanocapsules, nanoemulsion, nanoliposomes and nanoniosomes. The major objectives in designing nanocarriers are to manage particle size, surface properties as well as drug release in order to fulfil specific objectives. Hence, characterizations of nanocarriers are very critical to control their desired in vitro and in vivo behaviour. Nanocarriers are characterized by their size, morphology and surface charge, using highly advanced microscopic techniques as scanning electron microscopy, transmission electron microscopy and atomic force microscopy. Surface morphology and size are measured by electron microscopy while dynamic light scattering and photon-correlation spectroscopy are used to determine the particle size and size distribution. Colloidal stability is ascertained through zeta potential which is an indirect measure of the surface charge and differential scanning calorimetry is used to characterize particles and drug interaction. Further, binding and internalization of targeted carriers to the specific cells could be determined by cell uptake study. Biodistribution study of targeted nanocarriers is carried out and intracellular uptake and subcellular localization of the nanocarrier could be confirmed using confocal microscopy. This review covers all the aforementioned aspect related to in vitro and in vivo characterization of pharmaceutical nanocarriers.
Introduction
Nanotechnology has emerged strongly in most of the field of sciences in the last few decades. Nanotechnology is the science of small particles at a minute scale. On nanoscale, atoms and molecules work in a different pattern and present a diversity of amazing and appealing applications. Therefore, formulation or drug delivery scientists also entered into this new era of science and research related to pharmaceuticals nanocarriers in full swing to overcome the limits of conventional formulations. Pharmaceutical nanocarriers are described as submicron size (>200 nm in diameter) drug carriers with or without biodegradable characteristics. The term nanocarriers comprises nanoparticles, nanospheres, nanocapsules, nanoemulsion, and nano-sized vesicular carriers, that is, liposomes and niosomes. Nanospheres are matrix particles in which drugs have been dispersed in uniform pattern, whereas in nanocapsules, drug is surrounded by a different polymeric membrane. During last two decades, noteworthy research initiatives have been taken for the development of nanotechnology as the selective carriers for the delivery of small drug molecules to macro size such as protein, vaccine and genes to the target site. A detailed list of nanocarriers based marketed formulation is presented in . Because of numerous advantages, nanocarriers are popular in formulation and development; among them crucial parameter is the ratio of surface atoms/molecules with the total number of atoms/molecules improved drastically, hence effective surface area multiplied exponentially [Citation1]. Eventually, nanocarriers with very high count and tiny size could reach to inaccessible sites which mainly includes tumour cells and inflamed tissue. Because of superior permeability and retention effect (EPR; Enhanced permeability and retention) along with altered lymphatic drainage, could be beneficial for the selective delivery of drugs, proteins and vaccines [Citation2]. These carriers in most cases could achieve site-specific delivery of the therapeutics to enhance bioavailability aspects, improving solubility, prolong duration of action as well as protection from various degrading enzyme .to produce optimal therapeutic efficacy.
Table 1. A Summary of some polymeric nanoparticles prepared by several techniques to deliver APIs/proteins.
The employment of an appropriately designed nanocarrier for the sustained and targeted delivery of pharmaceuticals offers numerous advantages over plain drug administration, that is, enhancement in quantity of drug that travelled to the targeted site, avoidance of drug degradation and superior transportation method. Despite large number of advantages, nanoparticles possess drawbacks which mainly includes modified physical properties allowing particle – particle aggregation as well as difficulty in handling of nanoparticles in liquid and dry state due to their tiny size and huge surface area. Moreover, the tiny size of the carriers restricts drug loading in addition to burst release of entrapped molecules, is usually observed with these particles. These experimental difficulties should be countered prior to successful clinical and commercial use of nanoparticles [Citation3]. The key aspects in engineering of nanocarriers as a carrier is to deal with particle size, surface properties over and above of drug release in order to fulfil the specific objectives. Hence, characterizations of these nanocarriers are very critical to control their desired behaviour in vitro as well as in vivo.
Advantages
Nanoparticles as a drug delivery system possess numerous advantages over conventional therapies such as [Citation4]:
Easy to alter the size and surface charge of nanoparticles, hence, could be used for both passive and active drug targeting after parenteral administration.
Altering the property of the matrix offers the chance of controlled release of medicaments.
Nanocarriers are generally made of biodegradable substances, therefore, do not remain in the body.
Greater drug encapsulation can be obtained into the carriers without any chemical interaction; therefore, drug activity is fully retained compared to chemically modified conjugates.
Biodistribution of therapeutics could be changed as per outer surface characteristics of the nanoparticles by late clearance of the drug in order to get the highest therapeutic potency with diminished undesired effects.
Targeting moieties can be anchored to particles surface or guidance through magnetic based targeting.
Nanocarriers are designed for oral, nasal, parenteral and ocular delivery.
Due to their smaller size, they can penetrate through smaller capillaries; hence, allowing optimal dug deposition at the target site.
Classification of nanocarriers
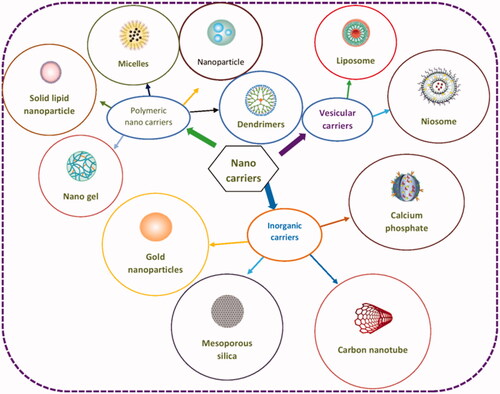
Nanoparticles have been extensively studied as targeted delivery systems in last three decades. The word “nanoparticle” is commonly used for the depiction of nearly all pharmaceutical carriers; hence, added differentiation is required for clarity. A cluster of nanocarriers, that is, polymer–drug conjugates, colloidal carriers of polymers developed using techniques such as emulsion polymerization, colloidal gold, gold nanoshells, crosslinked nanogel matrices, dendrimers, quantum dots and carbon nanotubes. A schematic diagram of various types of nanocarriers used for drug delivery is depicted in .
Vesicular nanocarriers
Nanoliposomes
Liposomes are composed of lipid bilayers made of phospholipid having enclosed aqueous compartment which can be used for the delivery of smaller molecular weight therapeutics, imaging agents, peptides, proteins and nucleic acids [Citation5]. The particle size can range from 25 nm to several micrometers depending on the number of bilayers as well as method of preparation. Liposomes are proved to possess adaptable properties in terms of particle size, bilayer charge, bilayer composition and their encapsulation ability, makes these carriers useful for drug delivery. Smaller nano size liposomes can sustain the release of an encapsulated agents, resulting in prolonged exposure at the target site and enhanced efficacy. Nevertheless, unlike liposome nanoliposome does prone to faster degradation and taken up by liver macrophages and could be used for active targeting [Citation6].
Niosomes
Niosomes are lamellar structures created by combining cholesterol and non-ionic surfactant of the alkyl or dialkylpolyglycerol ether class and followed by their hydration in order to form [Citation7] closed bilayer vesicle by means of providing some energy such as physical agitation. These bilayer structures are spontaneous arrangement of surfactant monomer in such a way so that hydrophilic heads remain in contact with the aqueous solvent whereas hydrophobic tails are oriented away from the aqueous solvent. The configuration of niosomes involves an amphiphilic monomer composed of two key parts, a hydrophilic or polar head group and a hydrophobic tail or non-polar. The size, charge, lamellarity and tapped volume of the niosomes could be altered by changing the surfactant composition. Niosomes are multilamellar or unilamellar and having aqueous compartment capable for encapsulation of hydrophilic drugs while hydrophobic drugs are entered into the hydrophobic bilayer [Citation8]. Since, niosomes are composed of surfactants, hence, having greater stability as compared to liposomes. The surface active agents used for the preparation of niosomes should be biodegradable, biocompatible and non-immunogenic. The main non-ionic surfactant classes used for noisome preparation are alkyl esters alkyl ethers, alkyl amides, fatty acids and amino acids among them, the most commonly used are sorbitan monoesters (Spans) composed of 12–18 carbon atoms (C12–C18).
Particulate carriers
Polymeric nanoparticles
Nanoparticles are submicron sized (10–1000 nm) made up of solid polymeric carriers. Polymeric nanoparticles hold noteworthy assurance for the effective treatment of disorders as they have striking physicochemical properties, that is, size, surface charge, hydrophilicity and hydrophobicity, hence, they have been considered as prospective carriers for drugs and pharmaceuticals. An overview of current state of development of drugs using nanotechnology is presented in [Citation9]. Actually, nanoparticles offer several advantages over free drugs, such as protection from unwanted interaction with biological moieties and degradation, improving the absorption into the desired organ (tumours), and escalating the pharmacokinetics of the therapeutics. Additionally, release of the encapsulated agents through the nanoparticles can be altered with intended duration of action in selected target site.
Table 2. Overview of current state of development of drugs using nanotechnology.
Solid lipid nanoparticles (SLNs)
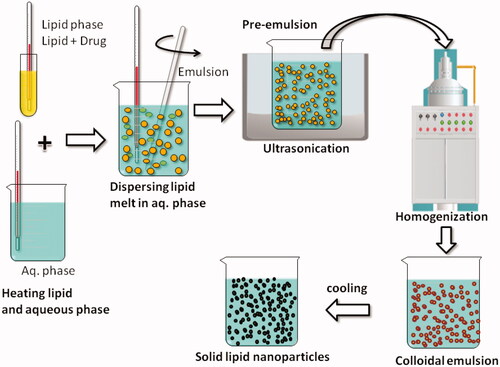
SLNs are made of solid lipids and stabilized with emulsifying agents in an aqueous dispersion. They resemble with nanoemulsion only replacing liquid lipid with a solid lipid. shows common method of preparation of SLNs using hot melt technique. Controlled drug release can be attained in an outstanding manner by replacing solid lipids with oils, since solid lipid lower mobility of drug significantly, as compared to oil phase. SLNs are associated to possess some advantages and circumvent few disadvantages of many other carrier systems such as polymeric nanoparticle, liposome, ethosomes and lipid emulsion. Recently major challenges, applications and safety aspects of lipid-based nanocarriers have been reviewed in an excellent manner [Citation10].
Dendrimers
These are novel category of controlled-structure polymers having nanoscale dimension with structural surface functionality. These have demonstrated site-specific programmed delivery of therapeutics along with usefulness in imaging studies. Usually, dendrimers have hyperbranched structures with a core in which therapeutics and imaging agents have been trapped.
Inorganic nanocarriers
Current progression in nanotechnology has led to the application of various inorganic nanoparticles such as calcium phosphate, carbon nanotubes, graphene oxide nanoparticles, mesoporous silica nanoparticles (MSNs) and gold nanoparticle in drug delivery [Citation11]. Day by day inorganic nanoparticles are gaining importance among them, carbon nanotubes, gold nanoparticles and nanospheres have been widely investigated as drug carrier, as their nanometer size enables them to move easily inside the body. The major advantages of inorganic nanoparticles attributed due to their hydrophilic nature, low toxicity profile, biocompatibility and resistant to microbial growth and higher stability.
Silica nanoparticles and calcium phosphate nanoparticles (CaP)
Mesoporous silica is one of the widely employed inorganic material produces surface template nanoparticles via combination of surfactant along with silicates. MSNs are comparatively biocompatible, making them suitable for biological applications [Citation12,Citation13], however, they are not bioresorbable. Pore size and pore structure could be easily controlled by selection of surfactant and co-assembly conditions. CaP having diameter of 40–50 nm were also used for the delivery of therapeutic agents. CaP were developed and their surface was altered by PEG hence these modified nanoparticles had zeta potential very close to zero.
Gold nanoparticles
Gold nanoparticles have shown applications in biomedical use since they are bio-inert, biocompatible, low toxicity, having flexibility of surface modifications and cellular imaging ability. Gold nanoparticles of 2.5 nm size are explored as useful transporter for intracellular delivery of β-galactosidase into the various cell lines and found that β-galactosidase was effectively reached inside the cell membrane of HeLa cells [Citation14].
Characterization of nanoparticles
The key objectives in designing nanocarriers as a delivery system are to manage particle size, surface properties as well as release of drugs in order to fulfil the specific objectives. Hence, characterizations of these nanocarriers are very critical to control their desired behaviour in vitro as well as in vivo. Pharmaceutical nanocarriers are characterized by various sophisticated techniques based on size, surface structure and charge potential. The surface properties along with dimensions alter the stability as well as in vivo behaviour of these carriers. Surface morphology and size are measured by electron microscopy (EM) while dynamic light scattering (DLS) and photon-correlation spectroscopy are used to determine the particle size and size distribution.
Size
It is a crucial parameter that differentiates nanoparticles from other drug delivery system and bulk powder. There are quite a few methods available which can be used to determine the nanocarriers size and most useful are discussed as follows. It is worth to mention here that every procedure has its merits and demerits, those should be looked carefully while characterizing nanocarriers. The choices of a specific technique depend on various parameters, such as expected size, and population of the nanoparticles. As diameter of the particles has an effect on the release of encapsulated agents hence, smaller particles provide larger surface area [Citation14,Citation15]. Consequently, the majority of the entrapped material will be revealed to the release medium results in faster drug release whereas, therapeutics little by little diffused out from inner layer of the macroparticles. However, finer particles have a propensity to aggregate while shipping and storage of nanoparticles suspension. Hence, there is a negotiation between a finer size and improved stability of nanoparticles [Citation16].
Dynamic light scattering
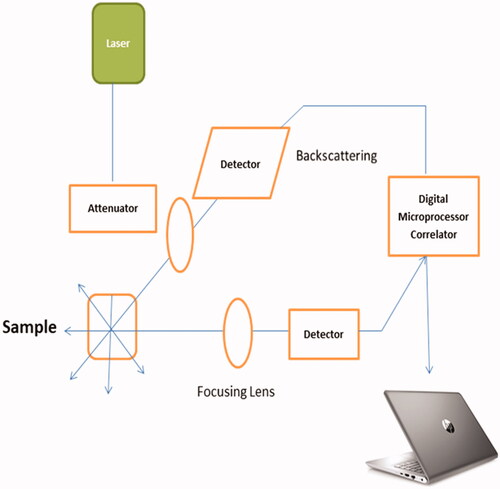
Most frequently used method for particle size determination is DLS. It is a technique that computes particle size in suspension and is commonly referred to as photon correlation spectroscopy (PCS). When the size of the particles is less than the 1/10th of incident light wavelength (i.e. λ/10), the scattered beam bears similar energy (elastic scattering) to the incident light and also is angle independent (Rayleigh scattering) [Citation17]. On the other hand, once diameter of particles crosses the limit of λ/10, Rayleigh pattern is substituted by Mie scattering it means scattered beam does not carry the similar energy (inelastic scattering) to the incident light and angle-dependent [Citation18]. DLS is usually used to carry out the size analysis of nanocarriers having size range of 1–500 nm, even though many instruments insist working range of 0.3 nm–10 μm. Particle size analysis of polydisperse nanoparticles suspension is tricky using DLS irrespective of its wide applications. Because of the fact that the signals are based in the existence of a low portion of large particles as the signal strength is proportional to its diameter to the power of six for particles having spherical shape. Therefore, DLS is most suitable technique to determine the size of unimodal nanoparticles. DLS instrument, detects scattered beam of laser light with a photon detector and intensity of the scattered light is proportional to the size of the nanoparticles which are monitored. A number of light scattering instruments Brookhaven (NanoDLS® series), Microtrac (Wave II® series)] [e.g. Malvern (Zetasizer® series), have emerged recently. On the whole, the instruments contain three key components – laser source, sample holder and light detector (. Latest DLS equipments are attached with APD (avalanche photo diode) detectors which have limited quantum efficiency ∼65% for red wavelength hence mostly lasers of 633 nm are utilized. In recent equipments, the detectors are placed at angle of 173° C in order to measure backscattering while earlier models (Nano S90, ZS90) still made at angle of 90° C. Finer silver NPs produces a superior apoptotic response against few cell lines whereas silica NPs having size of 20 nm demonstrated higher toxicity compared to 100 nm particles which were negatively charged [Citation19,Citation20].
Laser diffraction spectroscopy
LD is a valuable technique that covers a much broader detection range (20–2000 μm). It is also known as laser light scattering, which can be used alone and in combination with PCS to obtain a total population size range from tiny to macroparticles. LD depends on the fact that once a laser beam is passed through a liquid having suspended particles, the bigger particles scatter light at narrow angles whereas the minor ones scatter light at broader angles. The results produced by LD is utilized to estimate the correspondent spherical radius of particles as per the Mie scattering solution (also known as the “Mie theory”). Red lasers are responsible for estimating larger particles whereas blue lasers are used for the analysis of tiny ones. However, it is not recommended for colloidal suspensions having notably lesser diameter compared to the laser wavelength.
Although, laser diffractometer offers a fair estimation of polydispersity of particles as it covers a broader size range from nanometer to 100 of micron. However, its uses are limited to multi components nanocarriers because knowledge of refractive index at the measurement wavelength is critical as particle size distribution is extremely dependent on these optical parameters. The evolution of PIDS (polarization intensity differential scattering) markedly boosted the sensitivity of LD to finer particles [Citation21]. This technology merges wavelength dependence and polarization effects together hence drastically amplified the sensitivity of LD towards tiny particles. Still, regardless of advances in technology, it is greatly advocated to use PCS and LD concurrently.
Microscopic techniques
Microscopic techniques [(EM and atomic force microscopy (AFM)] are also used to study the particle size of the nanocarriers. Contrary to light scattering based techniques, individual particles are measured by the Coulter Counter and microscope techniques hence, these techniques provide the advantage of a direct close measurement. The data obtained by PCS and LD could be also prejudiced by the anisometric shape of the particles. Hence, the data generated by PCS and LD could be corroborated with electron microscopic studies to assess the real size of the particles. Electron beams are used for visual examination of nanocarriers in EM. Application of EM is precious as it offers the details of size and shape (morphology) in single examination through straight visual inspection but failed to supply population size and its average.
Differential centrifugal sedimentation
The principle behind differential centrifugal sedimentation (DCS) is that larger particles sediment faster than smaller particles if they have the same density. Though most of the nanocarriers do not settle under gravity itself (because of minute size), hence settling can be encouraged by centrifugation. A hollow disc introduced into a centrifuge tube which is optically clear and having a whole in the middle of it, revolves at 600–24,000 rpm. Disc compartment is somewhat filled with fluid, which allows liquid rings to fall against density difference. The sample is injected through central opening for measurement. DCS provides an exceptionally great resolution and various nanocarriers having of <5% variation in size can be resolved fully. In recent times, alteration in the size of Au nanoparticles after surface modification/functionalization usually is studied using DCS and found a swing of 0.5 nm in the size of the nanopartilcles after modification and this shift amounted to 2.1 nm after NPs modification with an entity having high molecular weight such as single-stranded DNA [Citation22]. The application of DCS in measuring the size of PEG-alkane thiol-modified gold NPs was also reported by Krpetic et al. [].
Nanoparticle tracking analysis (NTA)
NTA allows concurrent visual examination and poly-grade measurement of the nanocarriers by co-relating the nanoparticle diameter with speed of Brownian motion. The nanoparticles are observed depending on the scattered light upon illumination by the laser beam. Contrast to DLS, NTA has the unique capability to observe each and individual particles present in the dispersion by tracking each scattering independently. Measuring individual entity is having great importance particularly upon handling of multimodal dispersion of nanocarriers. However, this technique is also not capable to segregate portions of particles having narrow variation in the size of >50%. Impartial peak resolution of multimodal dispersion with NTA is of great importance which is very difficult to accomplish using DLS [Citation24].
Surface morphology
Electron microscopy
Overall morphology of nanoparticles is ascertained using EM techniques which may establish their toxicity profile. The most important application of pharmaceutical nanocarriers is modulation of drug release and drug targeting. As diffraction effects curb the resolution of optical microscopy it is very difficult to observe smaller size particles having diameter >1 µm using light. Consequently, greater resolution is desired such as electromagnetic radiation of shorter wavelength.
Scanning Electron Microscopy (SEM). For studying the nanocarriers using SEM, first they have to be transformed to a dry powder, later on sprinkled on a sample holder, followed by coated using a conductive metal such as gold, platinum, graphite osmium, iridium, tungsten, osmium, chromium or gold/palladium alloy, using a sputter coater. Next, a high-energy beam of electrons is passed to the sample to produce a diversity of signals on the surface of the objects specimens. The instrument includes an electron gun, condenser lenses and a vacuum system. SEM mainly produces three types of principal images: external X-ray maps, backscattered electron images and secondary electron images. The sample surface emitted the secondary electrons used to acquire the surface properties of the particles. Nanocarriers must be capable to hold on vacuum, as the electron rays can destroy the particles. Although, the average diameter generated by SEM is analogous to the DLS, however, these methods of analysis are more time taking, expensive and repeatedly supplemented with size distribution pattern. A scanning electron microscopic image of starch nanoparticles containing insulin is shown in . Also, the majority of the EM techniques, inclusive of SEM, restricted their use with the drawback of a destructive sample preparation, limiting its analysis by other modalities [Citation25]. An advantageous technique is environmental SEM (ESEM) that provides an opportunity to scan the samples in their natural state without modification or preparation [Citation26] as ESEM is having a sample compartment which is maintained at a low-pressure gaseous environment of 10–50 Torr and also coating of particles with conductive substances is no longer required.
Transmission Electron Microscopy (TEM). Electrons emitted out from the sample surface, whereas electrons transmitted through the sample is used for image formation in SEM and TEM respectively. TEM shows a greater resolution in contrast to SEM hence generally used to study NPs morphology. TEM obtained direct images and could also provide chemical information of nanomaterials at a spatial resolution down to the level of atomic dimensions (<1 nm) [Citation27,Citation28]. In the conventional TEM mode, an incident beam of electrons is transmitted through a thin foil of sample, transformed to unscattered electrons, elastically scattered electrons or inelastically scattered electrons. The ratio of the distance between objective lens and the specimen and the distance between objective lens and its image plane determines the resolution power of TEM. A line of electromagnetic lenses focus and project the scattered or unscattered electrons on a screen to generate the electron diffraction, a phase-contrast image, amplitude-contrast image or a shadow image of altering darkness as per the density of unscattered electrons [Citation29]. Additionally, TEM needs vacuum of high level and thin specimen sections in order to penetrate electron-beam through the sample. Specimen treatment and drying might change the physicochemical condition of the nanomaterials or induce artifacts. Also, in order to withstand the vacuum pressure of the microscope and facilitate proper handling, the nanocarriers are preset by means of a negative staining solution (phosphotungstic acid) or derivatives (e.g. uranyl acetate) [Citation30]. Later on, the particles are dried under a mercury lamp and observed under a monochromatic beam of electrons that penetrates the sample and generate an image. Small particles can be observed and the crystallographic structure of a sample can be imaged at an atomic scale. Local microstructures such as lattice fringe, glide plane, lattice vacancies and defects, screw axes, and the surface atomic arrangement of crystalline nanoparticles can be analyzed using high-resolution TEM [Citation31]. Surface morphology of PMMA/PEI nanoparticles is determined by field emission scanning electron microscope (FESEM) and found that the nanoparticles were evenly sphere-shaped and around 230 nm size in a dry state [Citation32].
Atomic force microscopy
AFM force spectroscopy allows the examination of the nano-mechanical properties of every molecules and particles under closer physiological conditions. Extreme-high resolution can be achieved in particle size measurement using AFM which physically scans the particles at sub-micron level through a probe tip of atomic scale. The instrument offers a topographical chart of objects depends on the forces between the tip and sample surface. Scans are obtained in contact or noncontact mode depending on their properties. The greatest advantage offered by the AFM over to TEM/SEM is its capability to scan non-conducting nanocarriers without any special sample preparation, hence delicate biological and polymeric nano and microstructures could be imaged [Citation33]. The AFM is equally suitable for characterization of pharmaceutical nanocarriers as it provides the opportunity of 3D visualization with both qualitative and quantitative information about physical qualities such as size, surface texture morphology and roughness. In addition, a broad variety of particle sizes from 1 nm to 8 μm can be characterized in the same scan. Moreover, the AFM can characterize the nanomaterials in multiple mediums such as ambient air, controlled environments and even liquid dispersions versus AFM analysis can be carried out in both liquid and gas medium. Such ability can be highly beneficial for nanoparticle characterization.
Nanoparticles classically divide into two subclasses first one tightly adhered to a solid structure whereas second one weakly adhered to the substrate, such as dispersions of nanocarriers in liquid or dry mediums. First category comprises solid matrix imbedded nanoparticles, as in the case of nanocomposites or nanoprecipitates which are classically scanned through cross-section of the composite material in order to find out average particle size and spatial allocation. Second category is dispersion of colloidal nanocarriers which may by chance adhered to the probe as a result, images obtained have decreased resolution hence, it is very much required to fix nanoparticles to an adhesive substrate such as double-sided sticky tape or any other suitable methods commonly used by microscopists. The AFM scans are more time consuming than an SEM but an entire measurement session inclusive of specimen treatment, image acquisition and analysis of the image consumes much reduced time (around one-fourth of the time to generate the data through an AFM compared to SEM/TEM). Moreover, an AFM is a highly cost-effective instrument available for nanocarriers imaging compared to other EM techniques. In addition, the AFM requires considerable smaller laboratory space than an SEM/TEM and also it is much simpler to operate AFM compared to SEM/TEM which requires a specially trained operator.
Surface charge
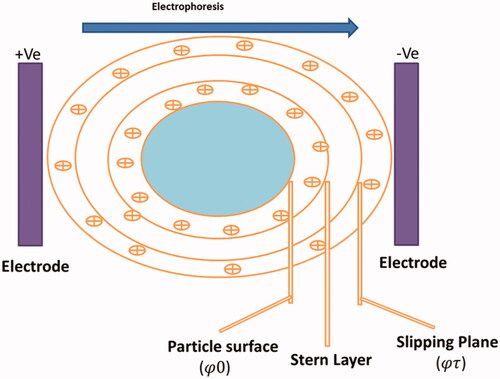
Once a charged particle is present in disperse state, an adsorbed double layer usually called an electrical double layer builds up around the surface of the nanocarrier (. The inner layer primarily composed of ions/molecules of opposite charge to that of the particle (Stern layer). Away from the stern layer, the electrostatic effects because of the surface charge associated with the particles decrease as per Debye’s law. The physical stability and redispersibility of the nanocarriers dispersion as well as their in vivo performance depends on the surface charge of the nanoparticles. The determination of surface charge sanctions forecasts about the long-term storage stability of nanocarriers. Usually, particle aggregation takes place slightly for charged particles (high zeta potential) because of electric repulsion between them. Determination of the Zeta potential (ζ) potential gives idea about the net charge of nanoparticles hence, provides a sign of the electrical repulsion or attraction among the particles as per their charge in a liquid suspension. Zeta potential is frequently determined by laser doppler anemometry which follows the principle of Doppler shift. A weak electric field is passed through the diluted suspension of nanoparticles and scattered light is observed for a frequency shift that is utilized to calculate the electrophoretic mobility (μ, particle velocity/strength of electric field). In addition, electrophoretic light scattering is employed to ascertain particle velocity under an electric field which permits calculation of zeta potential. Zeta potential is altered by the ionic strength, pH and the varieties of ions present in the dispersion liquid. Generally, zeta potential greater than ±30 mV stabilize the nanocarriers dispersion by blocking the contact between the particles because of electric repulsion [Citation34]. Also, the surface electrical potential of NPs is extremely essential particularly in formulation science as it modifies the interaction with adjacent particles (including neighbouring NPs) and the biological system. Moreover, it is suggested that ζ potential determination of nanoparticles is carried out in appropriate simulated biological media/solutions in which they interact with the biological systems.
Figure 5. Image indicating the Electrical Double Layer on a negatively charged particle. Right on above the particle surface there is a strongly bounded layer (Stern layer) containing opposite charge ions (positive ions). Further than Stern layer another diffuse layer deposits develops containing of both negative and positive charges. During electrophoresis study, the particle with bounded Electrical Double Layer moves towards the electrodes with the slipping plane becoming the interface between the mobile particles and dispersant. The ZP is the electrokinetic potential at this slipping plane.
Differential scanning calorimetry (DSC)
DSC is an eminent method based on measurement of structural modifications of materials in pharmaceutical research which are escorted by heat exchanges such as heat uptake (involved in melting) or heat emission (involved in crystallization). This technique is intended to compute the heat exchanges which takes places under controlled temperature programs and permits to illustrate findings on the physical qualities of a material. Depending on the sensitivity of the instrument, DSC is capable to scrutinize and compute even microscopic thermal activities which taking place in the material and able to recognize the temperatures at which these incidents take place but does not straight away disclose the reason of a thermal incident [Citation35]. Hence, the precise nature of the thermal transitions has to be resolute with corresponding methods such as thermogravimetry, microscopic observations or X-ray diffraction (XRD) to discriminate, among melting, polymorphic transitions, loss of water from hydrates or decomposition of the substance. In general, DSC analysis is all about the heating or cooling of the respective sample at a programmed rate and the heat absorption or emission is observed in a quantitative manner. Theoretically, isothermal transitions are rarely measured because such analysis typically requires extremely sensitive equipment (microcalorimetry).
Mainly two types of apparatus namely, heat flux DSC and power compensation DSC used for DSC investigations (. Both of the instruments contain two similar sample chambers which are heated (or cooled) generally in a linear way. One chamber holds a crucible containing sample, the other holds a reference crucible and both are kept under perfectly symmetric conditions. No variation in temperature between sample and reference is observed until and unless structural change occurs in the sample upon increasing the temperature of both crucibles at the similar rate. As soon as the sample exhibits a structural change, such as a melting transition, it absorbs energy which is supplied by the system in form of heat. Under the transition phase, all energy supplied by heating is consumed, the temperature of the sample no more enhance which brings to a temperature variation among the two crucibles containing the sample and the reference. In heat flux DSC, the changes in the temperature are calculated and transformed into a heat flow signal via a calibration method. On the other hand, power compensation DSC supply the heat through individual heaters, the finding of any temperature variation among the two furnaces brings out an instant correction of the heating power in such a manner that the temperature is kept (almost) similar in both the crucibles. The added (or shortened) heating power is directly linked with the heat absorption by the sample or emission by the sample which is determined using adequate calibration. In principle, after the calibration, there are, no noteworthy variation in the results has been found from the two different measurement techniques. However, endothermic transitions are exhibited as upward peak in power compensation DSC and as downwards peak in heat flux DSC. The thermal stability and the quantity of the nanocarriers and their conjugates can be calculated by numerous other thermal techniques [Citation26]. The temperature-dependent weight measurement of nanocarriers can be monitored using thermal gravimetric analysis.
Figure 6. Schematic representation of the thermal characterization instruments: (a) DTA, and (b) DSC. Reproduced with kind permission from Taylor and Francis [Citation66].
![Figure 6. Schematic representation of the thermal characterization instruments: (a) DTA, and (b) DSC. Reproduced with kind permission from Taylor and Francis [Citation66].](/cms/asset/72cabfa0-fdeb-40b8-b2b6-cb7780e2dc88/ianb_a_1561457_f0006_c.jpg)
X-ray diffraction
XRD is a versatile and widely used characterization tool for materials science and now gaining more and more importance in nanotechnology for characterization of crystalline size, shape and lattice distortion [Citation26]. It is based on the wide-angle elastic scattering of X-rays when a monocromatic radiation passes through a gap that has the same length than its wavelength. The diffraction of X-ray is elucidated as the reflection of a collimated beam of X-rays incident on the crystalline planes of an object. Even though XRD is widely explored to find out the sample structure at the atomic level but complexity in growing crystals and its capability of obtaining results from single conformation/binding state of the material restrict its uses [Citation26]. Sample preparation and experimental procedure are to be performed for liquid phase, by extracting out the solid nanocarriers by centrifugation followed by drying of pellets in an oven to eliminate all the moisture.
Small angle X-ray scattering (SAXS)
Opposite to XRD, which has restricted application for crystalline structure, SAXS offers knowledge of numerous properties by scanning either crystalline or amorphous materials from polymers, proteins to nanomaterials [Citation26]. In SAXS, a segment of an incident beam elastically scattered from the material makes a scattering pattern on a two-dimensional flat X-ray detector in perpendicular direction to the incident X-ray beam. By determining the strength of the scattered beam within the scattering angle, ranging from 0.1 to 3 degrees, SAXS can examine the shape, size and size distribution of the nanocarrier population, and structure of a diversity of polymers as well as of nanomaterial bioconjugates. The unique feature of SAXS does not require a perfect crystallized structure hence, make things easier in terms of sample treatment and provide an advantage of a non-destructive method. However, SAX does not provide high resolution but the same could be achieved by introducing synchrotron as the high-energy X-ray source [Citation36].
Drug encapsulation and loading capacity
Drug loading
Preferably, a viable nanocarrier should have high encapsulation efficiency. In this manner, nanocarriers decrease the amount of matrix polymers required for drug delivery. Drugs can be loaded to the nanoparticles mainly by two ways.
Usually, API is added at the time of nanoparticles preparation (incorporation technique)
Other one is by absorbing the drug into the preformed nanoparticles by saturating the particles with a concentrated drug solution (adsorption/absorption).
Encapsulation efficiency and % drug loading are mainly much varies as per the solid-state drug solubility in polymeric matrix (uniformly soluble state or discreted state), which depends on the molecular weight of polymer, its composition, its interaction with drug and functional groups found on outer polymer chain (ester or carboxyl) [Citation37]. Also, entrapment efficiency (EE) mainly depends on the type of nanocarriers, method of preparation used for that particular carrier and specific properties of the drug as well as inherent properties of polymer. Amount of encapsulated drug into the nanocarriers influences the release kinetics hence it is of utmost important to determine the encapsulation efficiency. The quantity of drug encapsulated per unit weight of nanocarriers is estimated after removal of the free drug. Separation of free drug can be carried out by ultracentrifugation, extensive dialysis, ultrafiltration, gel filtration or centrifugal ultrafiltration and using size exclusion chromatography for vesicular nanocarriers such as liposomes and niosomes. The amount of drug after separation of free drug is estimated using standard analytical technique such as UV spectroscopy or high performance liquid chromatography (HPLC). Alternatively, encapsulation efficiency can be assessed by dissolving nanocarriers into a suitable solvent or extracting out the drug into suitable solvent and subsequent analysis using standard analytical techniques. Loading efficiency is usually expressed as the amount of the drug entrapped into the particles divided by total amount present in the formulation called EE. EE is generally expressed in %:
Drug entrapment efficiency
The quantity of drug adhered per unit mass of matrix (generally moles of drug per mg of matrix or mg drug per mg of matrix) is called entrapment efficiency of the nanocarriers and commonly expressed as percentage relative to the polymer. A very large variety of drugs have been incorporated into the nanocarriers used for drug and vaccine delivery. A highly important parameter to adjudge the appropriateness of a drug carrier is its loading capacity which is usually denoted as percent related to the polymer phase. The various factors that affect the loading capacity of drug in nanocarriers depend on the type of nanocarriers and other aspects specific to that particular for nanocarriers. For instance, in case of SLN, the drug loading capacity depends upon the inherent solubility of drug into the melted lipid, miscibility of lipid and drug melt, chemical and physical structure of solid lipid matrix, polymorphic state of lipid material. In order to determine drug loading capacity nanocarriers have to be dissolved in an appropriate solvent followed by ultracentrifugation to separate out the drug from supernatant. A small volume of the supernatant is taken out, appropriately diluted with analytical method compatible solvent. Absorbance is determined at required wavelength spectrophotometrically against a blank and the drug levels in the supernatant are determined with help of a standard curve earlier constructed. The quantity of drug encapsulated in the nanocarriers is determined by reducing the amount of drug in the supernatant from the total amount taken initially.
Drug localization and drug release
An important aspect of nanoparticles as a carrier for drug delivery is to understand the way and level to which the therapeutics molecules are released. Release profile studies are performed in quite analogous manner to encapsulation efficiency determination assays which is measured for a time interval to find out the release mechanism [Citation38]. Numerous techniques are reported to determine in vitro release pattern of the encapsulated material from nanoparticles, few of them are as follows:
Compartment diffusion cells with artificial or biological membranes.
Stirring chased by centrifugation/ultracentrifugation.
Ultra-filtration or centrifugal filtration method
Diffusion through dialysis bag.
Reverse dialysis bag method.
Generally, drug release study is determined under controlled stirring and centrifugation. However, as the study is lengthy and having technical problems to be overcome in removal of nanoparticles from release buffer, the dialysis method is usually favourite. Mainly, release of drug from nanoparticles take place via five probable mechanisms for: (a) detachment of API adhered to the outer layer, (b) diffusion through the polymer matrix, (c) membrane controlled diffusion, (d) erosion of nanoparticles matrix or (e) combination of diffusion and erosion process [Citation37].
The kinetics of release pattern from nanocarriers could be assessed using a biexponential formula
In which C is the drug levels inside the nanocarriers at time t, A and B are constants depends on matrix properties (A represents for diffusion control and B for erosion control matrices) and α, β are rate constants could be determined using semi-logarithmic plots [39]. Overall release pattern of the drug depends on the inherent solubility of the drug, diffusion through the matrix and its biodegradation. The exact method to be utilized will depend on the drug and nanoparticles formulation but here is a method I have used for polymeric nanoparticles. In order to determine drug release from nanoparticles, lyophilize, weigh and re-suspend in buffer followed by incubation on a water bath at 37 °C with slight stirring. At scheduled time intervals (between 15 min and 10 days) an aliquot of dissolution medium should be taken for quantification; with the replacement of same quantity of fresh buffer in order to maintain sink conditions. Quantify the drug by determining the absorbance using a spectrometry or HPLC to calculate drug release with time. Always take placebo nanoparticles (devoid of any drug) as a control.
Drug release pattern largely depends on the polymer matrix used and type of nanocarriers prepared. Major concern with nanocarriers particularly for SLNs is the burst release observed with these carriers. Even it is probable to alter the release pattern as a function of lipid matrix, stabilizer concentration and production condition (e.g. temperature). SLN could be useful in achieving in vitro drug release for a period up to 5–7 weeks. Moreover, release profiles could be altered to avoid any burst release however this burst effect could be exploited to deliver an initial loading dose when desired. Even though the drug is loaded into the SLN it is not all the time uniformly solubilized in the lipid matrix [Citation40] and can be localized in various parts of the particles as well as in associated structures, that is, micelles, liposomes, drug nanocrystals. Regrettably, release experiments are carried out through several ways such as separation by centrifugation, dialysis hence, it is difficult to compare the results. Each method has its own merits and demerits. For instance, one of the widely selected methods is the use of a dialysis membrane, however, dialysis bag can occasionally restrict the diffusion and/or interact with drug molecules. The nanoparticle suspension is filled in a dialysis bag and hermetically sealed. Immerse properly sealed bags in an appropriate dissolution fluid under gentle stirring at 37 °C. The samples are withdrawn at predetermined intervals, centrifuged and analyzed for drug content using a suitable analytical method against the respective blank. The release patterns also varies as per the release conditions (sink or non-sink conditions, release medium etc).
In situ/Vivo characterization of nanoparticles
Nanoparticles may be evaluated in biological mediums, that is, blood, plasma, cells or primary culture. Cho et al. [Citation41] have reviewed various in vitro and in vivo characterization parameters in detail. Few of the in vitro tests which may be carried out are given in the following sections.
Phagocytic uptake
Phagocytic uptake is most widely used technique used to extrapolate in vivo behaviour of nanoparticles and measured by calculating phagocytic uptake via macrophages of fluorescently-labeled nanoparticles as a function of time and concentration [Citation42,Citation43]. Topical characteristics and size play a vital role in the process of phagocytosis [Citation44]. A study carried out with microparticles demonstrated that their morphology at the time of interaction with macrophage is important for phagocytosis [Citation45,Citation46]. For instance, linear polymeric micelles circulated for prolonged period compared to micelles of similar structural characteristics because of their confrontation to phagocytosis [Citation47]. Also, phagoctyic uptake by macrophages of PEG-coated rod-shaped gold nanorods were smaller and hence, showed a higher circulation time compared to spherical particles of same composition after in vivo injection to the mice [Citation48]. However, major drawback of the macrophage uptake studies is that due to variations in the composition of cell culture media comparable to biological fluids as a result phagocytosis along with the degree of protein adsorption are generally underpredicted.
Complement activation
Surface association of dissolved serum proteins (complement system) on the nanocarriers outer layer starts a biochemical pathway which leads to removal of nanocarriers from the serum through complement dependent receptor-mediated phagocytosis [Citation49]. The extent of complementary system could be monitored in order to forecast the capability of nanocarriers to escape the phagocytic removal. Any strange particles could activate dissolved protein components, C3, which cleave to the C3b and C3a. Hence, the relative proportion of C3b and C3a predict the degree of nanoparticles mediated complement activation using immunoelectrophoresis of nanocarriers in plasma. The surface modification of nanocarriers surface with a polymer manipulate the plasma proteins binding ability depending on the variable chain length of polymer and its orientation and electric potential [Citation44,Citation50].
Cellular uptake of nanocarriers
In addition to physical and chemical characterization of nanocarriers their biological responses are also measured in animal cell culture studies before initiation of in vivo administration. Animal cells are usually maintained in a cell culture flask and supplied with nutrients using medium at 37 °C in a CO2 incubator. Nanocarriers uptake study by the cells are usually carried out with monolayer cell culture model and it is proved to be extremely beneficial for ascertaining the translocation of nanoparticles to the cells, the therapeutic potential of drug released through nanocarriers and side effects of the carriers. This particles translocation is determined mostly using flow cytometry and confocal microscopy. However, nanocarriers labelling with a fluorescent marker is a prerequisite of these techniques, which can be carried out by simple incubation or covalent conjugation. Whilst simple incubation is a quick and easy process but always has a risk of leaching out of lipophilic dye from the carrier which might lead to false positive uptake [Citation51]. Superlatively, the carrier and therapeutics should be co-labelled with different dye so that their fate could be found independently. Confocal microscopy helps to ascertain the nanocarriers localization inside the cells whereas, amount of nanoparticles translocation is measured using flow cytometry. In flow cytometry, cells in the suspended state are separately scanned by a laser beam and response is obtained as florescent intensity to find out the ratio of dead cells to marker internalized cells [Citation52]. The intracellular delivery of various detoxified pertusis toxoid to CHO cells has been investigated using confocal microscopy (). Alexa Fluor 488antibody against pertusis toxoid has been used to visualize the toxin inside the cells and found that internalization initiated within 2 h and complete internalization of toxins took place in 12 h time [Citation53].
Figure 7. Confocal images of CHo cells treated with different ptx concentrations for 12 h. (A) Negative control; (B) ptx at 50 ng/ml; (C) ptx at 100 ng/ ml; D: ptx at 200 ng/ml, to exhibit the effect of ptx concentration on toxin translocation (ptx at 20 ng/ml). Green fluorescence represents ptx, blue fluorescence represents nucleus, and red fluorescence represents F-actin of cytoskeleton. Scale bar = 20 μm. Reproduced with kind permission from Taylor and Francis [Citation54].
![Figure 7. Confocal images of CHo cells treated with different ptx concentrations for 12 h. (A) Negative control; (B) ptx at 50 ng/ml; (C) ptx at 100 ng/ ml; D: ptx at 200 ng/ml, to exhibit the effect of ptx concentration on toxin translocation (ptx at 20 ng/ml). Green fluorescence represents ptx, blue fluorescence represents nucleus, and red fluorescence represents F-actin of cytoskeleton. Scale bar = 20 μm. Reproduced with kind permission from Taylor and Francis [Citation54].](/cms/asset/5c868020-9ff8-4904-9b49-8b5445f1e1ff/ianb_a_1561457_f0007_c.jpg)
Mechanisms of cellular uptake
The mechanism involved behind the nanoparticles entry into the cells is of equal importance as the successive pathways to be followed inside the cells depend on it. Physicochemical characteristics of NPs decide the mechanism of particle translocation such as phagocytosis, macropinocytosis, clathrin- or caveolae-mediated endocytosis [Citation54]. Clathrin-mediated endocytosis takes place through clathrin proteins which cover the NPs inside a vesicle (∼100 nm) and transferred to an early endosome [Citation55]. Particularly, clathrin- or caveolae-mediated endocytosis involve cellular receptors for specific ligands [Citation56], such as folic acid [Citation57], transferrin [Citation58] or albumin [Citation59], which facilitates endo or transcytosis of these molecules.
In order to investigate the uptake mechanisms, cells are subjected to precise inhibitors of particular internalization mechanisms before treatment with fluorescently tagged NPs. Chlorpromazine is an inhibitor of clathrin-mediated endocytosis, filipin and methyl-β-cylcodextrin are inhibitors of caveloae-mediated endocytosis [Citation60]. Macropinocytosis and phagocytosis can be inhibited by pretreatment with amiloride (inhibitor of Na-K exchange) or cytochalasin D (F-actin-depolymerizing drug) [Citation61]. Pretreated cells are subjected to NPs internalization and subjected to flow cytometry or confocal microscope equipped with software which support quantitation. KB cells (folate receptor overexpressing cell line) and A549 lung cancer cells (folate receptor-deficient cell line) are treated with polylactide-co-glycolide nanoparticles decorated with folic acid and showed that the particles are taken up in higher quantity by KB cells compared to A549 cells [Citation59]. In addition, cells are pretreated or co-incubated with large excess of pure ligands in order to prove that the particle uptake is competitively inhibited. The size of the nanocarriers is critical for receptor-mediated, cellular uptake, hydrodynamic diameters of NPs near to the vesicle size formed during clathrin- or caveolae-mediated endocytosis, that is 100 or 60 nm, correspondingly. However, macropinocytosis showed a superior elasticity in the higher limit of particle size and capable of uptake of 300 nm size particle [Citation54]. Apart from the particle size, particle shape and surface charge also affect the uptake of the nanocarriers.
Bioactivity of nanocarriers
Highest care should be taken to fully retain the strength and effectiveness of a drug to be encapsulated into the nanocarriers. Once an anti-cancer agent is targeted via NPs, different techniques based on tetrazolium salts such as MTT, MTX, XTT assays determines the metabolic action or integrity of cell could be explored to ascertain the cell viability. For instance, test based on colourimetry, that is, MTT, MTS, and XTT assays determine the capability of mitochondria of live cells to reduce tetrazolium salts to deeply coloured formazan dyes [Citation62]. Luciferase activity in terms of bioluminescence strength is determined which is proportional to ATP generated by live cell assays due to the conversion of luciferase into luciferin [Citation62]. Lactate dehydrogenase is a constitutive cytoplasmic enzyme, which is released when the cell membrane is disturbed which could be measured as an indication of the presence of unviable cells.
In vivo evaluation of nanocarriers
Animal models of tumour
After nanocarriers reveal preliminary effectiveness in vitro, these carriers are subjected to further evaluation in terms of their toxicity profile and response in biological species. A suitable animal model which can closely represent pathophysiology of human disorder is a priceless way to extrapolate healing potential in men. A proven in vivo effectiveness of nanocarriers paves a way for clinical trials. However, animal studies and selection of animal model is highly specific and selected on the basis of drug of investigation as well as the proposed route of administration. In vivo evaluation could supply vital knowledge of fate of the nanocarriers inside the biological system. Few in vivo evaluations which might be carried out such as dose–therapeutic response study, biodistribution of nanocarriers among the various body organs, acute and multidose efficacy studies as well as safety and pharmacokinetic parameters, that is, absorption, distribution, metabolism, and excretion (ADME). The eventual goal of in vitro and in vivo evaluation is to match the physicochemical aspects of the nanocarriers to its biological function.
To ascertain the therapeutic efficacy of nanocarriers containing cytotoxic drugs, allograft mouse model or human xenograft tumours are reported. In brief, cancer cells are injected (typically subcutaneously) to the immune-deficient mice which allow to grow visible tumours. In this regard, Wang et al. have reviewed the various strategies based on nanoparticles for vaccination against cancer [Citation63]. Further, investigational therapeutics are administered into the nanocarriers and scrutinize the pharmacokinetics, biodistribution and the pharmacodynamic responses. For instance, PEGylated liposomes of doxorubicin were evaluated for pharmacodynamic response against cancer using animal species by subcutaneously injecting colon carcinoma cells to the C-26 mouse. The effect of the therapy was examined by determining the tumour size after scheduled time [Citation64] and found that free Doxorubicin minutely reduced the tumour size whereas liposomal doxorubicin reduced the size up to an extent which is not measurable. Also, mean survival time was more than 120 days (duration of the experiment) compared to 50 and 49 days for saline and plain doxorubicin, respectively [Citation64].
Genetically engineered mouse models
Genetic sequence alteration in genetically engineered mouse (GEM) models through silencing of tumour suppressor genes or activation of correspondent oncogenes could lead to tumour formation. The major benefit of GEM models is that they truly produce tumours of specific type truly represent tumour host interactions hence proven to be best for dissecting the roles of oncogenes. However, GEMs showed limited application compared to other in vivo models in regular assessment of nanocarriers because they are costly and violation of IPR.
Streptozotocin-induced diabetes model
Diabetes is generally induced by intraperitoneal dosing of streptozotocin (STZ) to animals. STZ is a glucosamine–nitrosourea compound derived from Streptomyces achromogenes that are used clinically as a chemotherapeutic agent in the treatment of pancreatic β cell carcinoma. STZ damages pancreatic β cells, resulting in hypoinsulinemia and hyperglycemia. STZ can induce diabetic state in two ways, depending on the dose. Animals showing blood glucose more than 250% (200 mg/dL) of fasting levels is used in the subsequent study. Vitamin B12-NPs conjugates containing are administered orally and plasma glucose levels were estimated in order to test the oral effectiveness of these Nanoconjugates.
Conclusion
Due to an exponential increase in surface area at nanometer levels, nanocarriers could have the capability to alter physiological interactions from the molecular level to the systemic level, creating the in vivo delivery of nanomaterial a fascinating research topic. The scope of nanomedicine has gone wider and wider in the last two decades. Nanocarriers are now made up of different types depends on the type of matrix used such as organic versus inorganic with an extraordinary control over the particle diameter, morphology, surface characteristics, drug encapsulation and its release. However, their clinical transformation is comparatively slow and only a few commercial products such as liposomes or micelles. Regulatory guidelines for robust techniques of nanocarriers characterization are essential for assuring safety of nanomaterials. Novel nanocarriers are usually evaluated in terms of surface charge and ligand density, later on which decide their interactions with the cell surface. Conversely, in blood or other biological fluids, nanaocarriers are easily covered with protein aura, which eventually dictates in vivo fates and therapeutic response. In order to recognize the discrepancy between in vitro characteristics and in vivo behaviours, a lot of research groups shifted to models which can generate preliminary in vivo data. Furthermore, clinical extrapolative results obtained in animal species are freshly reconsidered, in terms of human diseases progression. In a nutshell, it is vital for the researchers to understand the restrictions and challenge of present technologies and discover a novel path for nanocarriers evaluation, that could forecast the clinical findings at the initial level of product development with a superior reliability.
Disclosure statement
The authors declare no conflict of interest.
References
- Ravichandran R. Nanoparticles in drug delivery: potential green nanobiomedicine applications. Int J Green Nanotechnol Biomed. 2009;1:B108–B130.
- Jong WHD, Borm PJA. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3:133–149.
- Mohanraj VJ, Chen Y. Nanoparticles a review. Trop J Pharm Res. 2006;5:561–573.
- Jawahar N, Meyyanathan SN. Polymeric nanoparticles for drug delivery and targeting: a comprehensive review. Int J Health Allied Sci. 2012;1:217–223.
- Patel S, Bhirde AA, Rusling JF. Nano delivers big: designing molecular missiles for cancer therapeutics. Pharmaceutics. 2011;3:34–52.
- Kumar A, Badde S, Kamble R, et al. Development and characterization of liposomal drug delivery system for nimesulide. Int J Pharm Sci. 2010;2:87–89.
- Malhotra M, Jain NK. Niosomes as drug carriers. Indian Drugs. 1994;31:81–86.
- Udupa N. Niosomes as drug carriers. In: Jain NK, editor. Controlled and novel drug delivery. 1st ed. New Delhi, India: CBS Publishers and Distributors; 2002.
- Amoabediny G, Haghiralsadat F, Naderinezhad S, et al. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: a comprehensive review. Int J Polym Mater Polym Biomater. 2018;67:383–400.
- Mishra DK, Shandilya R, Mishra PK. Lipid based nanocarriers: a translational perspective. Nanomedicine. 2018;14:2023–2050.
- Malmsten M, Zauscher S. Colloids and surfaces in biology. Curr Opin Colloid Interface Sci. 2013;18:468–480.
- Radin S, Falaize S, Lee MH, et al. In vitro bioactivity and degradation behaviour of silica xerogels intended as controlled release materials. Biomaterials. 2002;23:3113–3122.
- Lai C-Y, Trewyn BG, Jeftinija DM, et al. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J Am Chem Soc. 2003;125:4451–4459.
- Konwar R, Ahmed AB. An overview of preparation, characterization and application. Int Res J Pharm. 2016;4:47–57.
- Ghosh P, Yang X, Arvizo R, et al. Intracellular delivery of a membrane-impermeable enzyme in active form using functionalized gold nanoparticles. J Am Chem Soc. 2010;132:2642–2645.
- Redhead HM, Davis SS, Illum L. Drug delivery in poly(lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: in vitro characterisation and in vivo evaluation. J Control Rel. 2001;70:353–363.
- Fan X, Zheng W, Singh DJ. Light scattering and surface plasmons on small spherical particles. Light Sci Appl. 2014;3:e179.
- Ross DJ, Sigel R. Mie scattering by soft core-shell particles and its applications to ellipsometric light scattering. Phys Rev E Stat Nonlin Soft Matter Phys. 2012;85:056710.
- Kim TH, Kim M, Park HS, et al. Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res A. 2012;100:1033–1043.
- Park Y-H, Bae HC, Jang Y, et al. Effect of the size and surface charge of silica nanoparticles on cutaneous toxicity. Mol Cell Toxicol. 2013;9:67–74.
- Jores K, Mehnert W, Drechsler M, et al. Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J Control Rel. 2004;95:217–227.
- Krpetic Z, Singh I, Su W, et al. Directed assembly of DNA- functionalised gold nanoparticles using pyrol- imidazole polyamides. J Am Chem Soc. 2012;134:8356–8359.
- Krpetic Z, Davidson AM, Volk M, et al. High-resolution sizing of monolayer-protected gold clusters by differential centrifugal sedimentation. ACS Nano. 2013;7:8881–8890.
- Filipe V, Hawe A, Jiskoot W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm Res. 2010;27:796–810.
- Gmoshinski IV, Khotimchenko SA, Popov VO, et al. Nanomaterials and nanotechnologies: methods of analysis and control. Russ Chem Rev. 2013;2:48–76.
- Sapsford KE, Tyner KM, Dair BJ, et al. Analyzing nanomaterial bioconjugates: a review of current and emerging purification and characterization techniques. Anal Chem. 2011;83:4453–4488.
- Patri A, Dobrovolskaia M, Stern S, et al. Preclinical characterization of engineered nanoparticles intended for cancer therapeutics. In: Amiji M, editor. Nanotechnology for cancer therapy. Boca Raton (FL): CRC Press; 2006. p. 105–138.
- Wang H, Chu PK. Surface characterization of biomaterials. In: Bandyopadhyay A, Bose S, editors. Characterization of biomaterials. Oxford: Academic Press; 2013. p. 105–174.
- Williams DB, Carter CB. Transmission electron microscopy. 2nd ed. New York: Springer; 2009.
- Pal SL, Jana U, Manna PK, et al. Nanoparticle: an overview of preparation and characterization. J Appl Pharm Sci. 2011;01:6228–6234.
- Brice-Profeta S, Arrio M-A, Tronc E, et al. Magnetic order in γ-Fe2O3nanoparticles: a XMCD study. J Magn Magn Mater. 2005;288:354–365.
- Wu A, Jia J, Luan S. Amphiphilic PMMA/PEI core–shell nanoparticles as polymeric adsorbents to remove heavy metal pollutants Colloids Surf A Physicochem Eng Asp. 2011;384:180–185.
- Shi HG, Farber L, Michaels JN, et al. Characterization of crystalline drug nanoparticles using atomic force microscopy and complementary techniques. Pharm Res. 2003;20:479–484.
- Saupe A, Gordon KC, Rades T. Structural investigations on nanoemulsions, solid lipid nanoparticles and nanostructured lipid carriers by cryo-field emission scanning electron microscopy and Raman spectroscopy. Int J Pharm. 2006;314:56–62.
- Heike B, Tobias U. Characterization of lipid nanoparticles by differential scanning calorimetry, X-ray and neutron scattering. Adv Drug Del Rev. 2007;59:379–402.
- Rao CNR, Biswas K. Characterization of nanomaterials by physical methods. Annu Rev Anal Chem (Palo Alto Calif). 2009;2:435–462.
- Govender T, Stolnik S, Garnett MC, et al. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Control Rel. 1999;57:171–185.
- Kreuter J. Physicochemical characterization of polyacrylic nanoparticles. Int J Pharm. 1983;1:43–58.
- Hedley M, Curley J, Urban R. Microspheres containing plasmid-encoded antigens elicit cytotoxic T-cell responses. Nat Med. 1998;4:365–368.
- Ren J, Zou M, Gao P, et al. Tissue distribution of borneol-modified ganciclovir-loaded solid lipid nanoparticles in mice after intravenous administration. Eur J Pharm Biopharm. 2013;83:141–148.
- Cho EJ, Holback H, Liu KC, et al. Nanoparticle characterization: state of the art, challenges, and emerging technologies. Mol Pharm. 2013;10:2093–2110.
- Bocca C, Caputo O, Cavalli RB, et al. Phagocytic uptake of fluorescent stealth and non-stealth solid lipid nanoparticles. Int J Pharm. 1998;175:185–193.
- Zhang W, Liu J, Li S, et al. Preparation and evaluation of stealth Tashinone IIA-loaded solid lipid nanoparticles: influence of Poloxamer 188 coating on phagocytic uptake. J Microencapsul. 2008;25:203–209.
- Vonarbourg A, Passirani C, Saulnier P, et al. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials. 2006;27:4356–4373.
- Champion J, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4934.
- Longmire MR, Ogawa M, Choyke PL, et al. Biologically optimized nanosized molecules and particles: more than just size. Bioconjugate Chem. 2011;22:993–1000.
- Geng Y, Dalhaimer, P, Cai, S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nano. 2007;2:249–255.
- Arnida J-AMM, Ray A, Peterson CM, et al. Geometry and surface characteristics of gold nanoparticles influence their biodistribution uptake by macrophages. Eur J Pharm Biopharm. 2011;77:417–423.
- Dobrovolskaia M, Aggarwal P, Hall J, et al. Preclinical studies to understand nanoparticles interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharmaceutics. 2008;5:487–495.
- Vauthier C, Persson B, Lindner P, et al. Protein adsorption and complement activation for diblock copolymer nanoparticles. Biomaterials. 2011;32:1646–1656.
- Xu P, Gullotti E, Tong L, et al. Intracellular drug delivery by poly(lactic-co-glycolic acid) nanoparticles, revisited. Mol Pharm. 2009;6:190–201.
- Brown M, Wittwer C. Flow cytometry: principles and clinical applications in hematology. Clin Chem. 2000;46:1221–1229.
- Tan Y, Fleck RA, Asokanathan C, et al. Confocal microscopy study of pertussis toxin and toxoids on CHO-cells. Hum Vaccin Immunother. 2013;9:332–338.
- Anirudhan TS, Sandeep S. Synthesis, characterization, cellular uptake and cytotoxicity of a multifunctional magnetic nanocomposite for the targeted delivery and controlled release of doxorubicin to cancer cells. J Mater Chem. 2012;22:12888–12899.
- Rejman J, Oberle V, Zuhorn IS, et al. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169.
- Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908.
- Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Orchestra Cell. 2006;124:897–900.
- Sabharanjak S, Mayor S. Folate receptor endocytosis and trafficking. Adv Drug Deliv Rev. 2004;56:1099–1109.
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608.
- Ranjan A, Pothayee N, Seleem MN, et al. In vitro trafficking and efficacy of core-shell nanostructures for treating intracellular Salmonella infections. Antimicrob Agents Chemother. 2009;5:3985–3988.
- Ivanov AI. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol. 2008;440:15–33.
- Kim SH, Jeong JH, Chun KW, Et al. Target-specific cellular uptake of PLGA nanoparticles coated with poly (L-lysine)-poly(ethylene glycol)-folate conjugate. Langmuir. 2005;21:8852–8857.
- Wang J, Hu X, Xiang D. Nanoparticle drug delivery systems: an excellent carrier for tumor peptide vaccines. Drug Deliv. 2018;25:1319–1327.
- Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;15:61–69.
- Pal K, Singh VK, Anis A, et al. Hydrogel-based controlled release formulations: designing considerations, characterization techniques and applications. Polym Plast Technol Eng. 2013;52:1391–1422.