Abstract
Thyroid cancer is now the most common endocrine malignancy and the effect of miR-429 in the development of thyroid cancer still need to be further investigated. The expression level of miR-429 was quantified by qPCR in both clinical samples and cultured cell lines. MTT, flow cytometry, migration analyses and Matrigel invasion assays were conducted to test the proliferation, apoptosis, migration and invasion of MiR-429 transfection in thyroid cancer cell lines. Luciferase activity assay and western blot were conducted to detect the direct effect of miR-429 on Zinc finger E-box-binding homeobox 1 (ZEB1) expression. In this study, it was found that miR-429 was frequently decreased in thyroid cancer tissues and cell lines. Transfection of miR-429 in thyroid cancer cell lines substantially suppressed cell proliferation, migration and invasion. Besides, miR-429 up-regulation would induce apoptosis in different cell lines. ZEB1 was identified as a direct target of miR-429 and miR-429 transfection could inhibit ZEB1 by direct binding to its 3’-untranslated region (3’-UTR). In conclusion, these data indicated that miR-429 could act as a tumour suppressor miRNA and contribute to the development and progression and metastasis of thyroid cancer.
Introduction
Thyroid cancer, which is the most common neoplasm of the endocrine system, has been a more and more important malignant in decades of years worldwide [Citation1,Citation2]. In general, the cases of thyroid cancer would be divided into three main categories: papillary thyroid cancer (PTC), follicular thyroid cancer (FTC) and anaplastic thyroid cancer (ATC) [Citation3]. Despite the development of diagnosis and treatments for thyroid cancer, the 5-year cancer-specific survival rate still remains unsatisfied [Citation4]. Emerging evidence suggests thyroid cancer was caused by genetic and epigenetic changes, and more researches were required in the pathogenesis of thyroid cancer to provide improvement management of thyroid cancer cases.
Micro RNAs (miRNAs), generally defined as short RNA molecules of approximately 22 nucleotides, have captured significant attention in carcinogenesis [Citation5,Citation6]. MiRNAs could demonstrate posttranslational regulation effect and thus inhibit of the expression of target gene through targeting the 3’-untranslated regions (3’-UTRs). It has been reported that miRNAs play a significant role in a wide range of physiological and pathological processes including tumourigenesis [Citation7]. Many miRNAs, such as miR-29b, miR-106 and miR-206, have been demonstrated as critical regulators of carcinogenesis and development of many cancers [Citation8]. It was also reported that miRNAs would also play important roles in the development of thyroid cancer. Several previous studies showed that miRNAs were dysregulated in thyroid cancer and cell lines and the dysregulated miRNAs would be associated with the diagnosis and prognosis of thyroid cancer [Citation9,Citation10].
Recent studies have suggested that miR-429 may act as a suppressor of different cancers, including lung, liver and breast cancers [Citation11–13]. Through in-vivo and in-vitro study, it was found that miR-429 could play a central role in tumour vascularisation as well as tumour invasion through targeting its target gene [Citation14,Citation15]. It was reported that zinc finger E-box-binding homeobox 1(ZEB1), which was involved in tumourigenesis of various malignancies, has been also one of the targets of miR-429 [Citation16,Citation17]. In a study, it was reported that miR-429 could inhibit the expression of ZEB1 and thus suppress the development of breast cancer [Citation18]. However, the detailed role of miR-429 in the development of thyroid cancer remained unclear. Herein, this study was conducted to investigate the expression pattern, biological function and underlying molecular mechanisms of miR-429 in thyroid cancer.
Materials and methods
Human tissue samples and ethics statement
A total of 59 thyroid cancer cases were enrolled in this study between June 2016 and December 2017. All the cases were hospitalized patients and received thyroid cancer surgical ablation in Sichuan Cancer Hospital. No patients had ever received any other therapy before admission. The specimens of thyroid cancer and matched non-neoplastic tissues were obtained in liquid nitrogen after surgery within 5 min of excision and then transported frozen to the laboratory and stored at −80 °C before usage. Informed consents were obtained from the patients or their guardians. This study was approved by the Ethics Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao University.
Cell culture and transfection
Human normal thyroid cell line Nthy-ori 3–1 and two human thyroid cancer cell lines (TCP-1 and NPA) were purchased from American Type Culture Collection (ATCC, Manassas, VA). The cells were routinely maintained in complete DMEM culture medium (Hyclone, Thermo, San Jose, CA), supplemented with 10% foetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin (both from Sigma-Aldrich, St. Louis, MO). The cells were plated into plates and incubated in a humidified incubator at 37 °C under 5% CO2. The cells were grown to an appropriate density and used at a passage of 3 ∼ 5.
Thyroid cancer cells were transfected with either 50 nM control miRNA or 50 nM pre-mir-429 in different groups. Transfections were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Both TCP-1 and NPA cells were divided into two groups and either miRNA control or miR-429 treatments were conducted. After reaching 80% confluence, the cells were incubated with different conditions for 24 h in these experiments.
RNA extraction and miRNA/mRNA detection
Total RNA was isolated from both tissue samples and cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The expression of miR-429 was determined using TaqMan MicroRNA Assay Kits (ABI, Foster City, CA) and the expression of ZEB1 was determined using SYGR green real-time PCR (TAKARA, Tokyo, Japan). The primers used in this study were as the following: miR-429: forward 5′-AGGTCT CTGAGGGTCAAGCA-3′, reverse 5′-CTGGTTGAAAAGCATGAGCA-3′, ZEB1: forward 5′-TGGAAGGGAAGGGAAGGGAGTC-3′, reverse 5′-AGGCAGGGCTACCATCAGTC-3′; GAPDH: forward 5′-ATGTCGTGGAGTCTACTGGC-3′, reverse 5′-TGACCTTGCCCACAGCCTTG-3′. All the PCR assays for miR-429 and ZEB1 were conducted with the 7300 PCR Assay System (Grand Island, NY). GAPDH was used as internal controls. Data were analysed by the −2ΔΔct method.
Cell proliferation detection
The cell proliferation was conducted by a MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide) method. Both TCP-1and NAP cell lines were spread on the 96-holes plate, and the cell density was as set to 3 × 104, and then the cells were treated in different groups, respectively. After transfection miR-429 precursor or miRNA control for 24 h, 20 μL of MTT dye was added and incubated for another 4 h at 37 °C. After removing MTT-containing PBS, and 150 μl of DMSO (Sigma-Aldrich, St. Louis, MO) was added to each well. After gentle mixing for 15 min, the reduced purple formazan crystals were dissolved. The 570 nm wavelength was selected for the measurement using microplate spectrometer (Bio-Rad Laboratories Inc., Hercules, CA). Each experiment was repeated for three times.
Apoptosis analyses
The apoptotic rates were detected by Flow Cytometry (Beckman Coulter, Brea, CA) using the Annexin V-FITC/PI kit (Roche, Penzberg, Germany), in which Annexin V binds to the exposed phosphatidylserine on the plasma membrane of apoptotic cells. The analyses were conducted according to the manufacturers. The percentage of early apoptosis was calculated from the proportion of cells that were Annexin V-positive and PI-negative, while the percentage of late apoptosis plus necrosis was calculated from the proportion of cells that were Annexin V and PI double positive.
Cell migration and invasion detection
In this study, transwell method was obtained for the cell migration and invasion assays. All the analyses were conducted using transwell chambers with 8 μm pore filters (Corning, New York, NY). Before analyses of migration detection, the TCP-1 and NPA cells in each group were grown to about 50% confluency and incubated in serum-free medium for 24 h. After that, cells were trypsinized before 5 × 104 cells suspended in serum-free medium were added to the upper chambers of transwell plates. Medium containing 10% FBS was added to the lower chamber. Cells were incubated for 24 h at 37 °C, and then completely removing the non-migrating cells. The migrated cells were then fixed in 4% paraformaldehyde and stained with 0.5% crystal violet. The stained cells were visualized under a microscope and quantified by calculating the cell numbers. For the invasion analyses, the transwell filters were coated on the lower side with 8 μg/μL Matrigel (BD Biosciences, Franklin Lakes, NJ) and placed on a 24-well plate containing medium. The other procedures were just like migration analyses. Each experiment was repeated for three times and the mean value was used for the statistical analyses.
Luciferase activity assay
Mutations were introduced in potential miR-429 binding sites using the QuikChange site-directed mutagenesis Kit (Stratagene, San Diego, CA). Co-transfection of indicated plasmids and 1 ng pRL-TK Renilla was performed using Lipofectamine 2000 Reagent (Life Technologies, Gaithersburg, MD) according to the manufacturer’s instruction. TCP-1 and NPA cells were seeded in triplicate in 24-well plates and allowed to settle for 24 h and cells were transfected with the pMir-Report vectors containing the 3’-UTR variants and miR-429 precursor, or control minics for 24 h. The pRL-TK vector (Promega, Madison, WI) carrying the Renilla luciferase gene was used as an internal control to normalize the transfection efficiency. Luciferase values were determined using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI).
Western immunoblotting
Both TCP-1and NPA cells in each group were homogenized and the cellular lysates were prepared. After quantified protein concentration by BCA, the extracted protein was used for the western blotting. The extracted total protein was separated by sodium dodecyl sulphate polyacrylamide gel (SDS-PAGE; 10% polyacrylamide gels), and the proteins were transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were then placed in a TBST solution containing 5% skimmed milk/FBS and were incubated at room temperature for 1 h. Then membranes were immunoblotted with primary antibodies, followed by HRP-linked secondary antibodies. Antibodies were purchased from Cell Signaling, Danvers, MA (ZEB1, GAPDH and secondary antibody). The expression level of ZEB1 protein was normalized to that of GAPDH protein.
Statistical analysis
Paired and unpaired t-test was performed on data of qRT-PCR and luciferase assays. All data are reported as means ± SEM unless otherwise stated. p < .05 was considered statistically significant.
Results
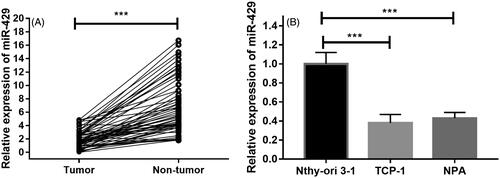
Down-regulated expression of miR-429 in thyroid cancer
To delineate the expression of miR-429 in the thyroid cancer cases, we analysed the expression pattern of miR-429 in 59 pairs of primary tumour and control nontumorous tissues. As shown in , it was found that the relative expression of miR-429 was significantly lower in thyroid cancer tissues. Moreover, advanced studies were conducted to detect the expression of miR-429 in normal thyroid cells and thyroid cancer cell lines. It was found that expression of miR-214 in normal thyroid cancer cell line Nthy-ori 3–1 and two cancer cell lines TCP-1 and NPA. Compared with the expression of miR-429 in Nthy-ori 3–1, the levels of TCP-1 and NPA were remarkably decreased ().
miR-429 suppressed cell proliferation of thyroid cancer cells
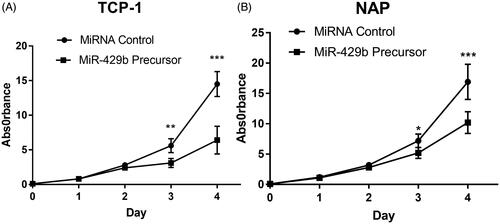
We also detect the effect of miR-429 in the cell proliferation of cultured thyroid cell lines. After treatment with miR-429, the cell proliferation effects in day 1–4 were recorded. It was found that the proliferation effects were significantly suppressed after the third day of transfection ().
Figure 2. Overexpression of miR-429 inhibits thyroid cancer cell proliferation. (A) The growth curve of TCP cells after miR-429 precursor transfection compared to miRNA control. (A) The growth curve of NPA cells after miR-429 precursor transfection compared to miRNA control. *p < .05, p < .01 and p < .001.
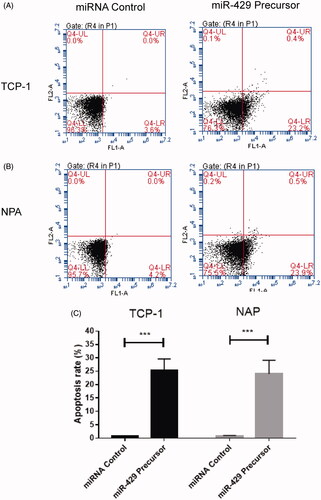
Effects of miR-429 on apoptosis of thyroid cancer cells
The flow cytometric analyses were conducted to detect the effects of miR-429 on apoptosis of thyroid cancer cells. It was demonstrated that transfection of TCP-1 and NPA cells with miR-429 for 24 h would result in a significant increase in the percentage of apoptotic cells comparing with the control group (p < .001), as shown in .
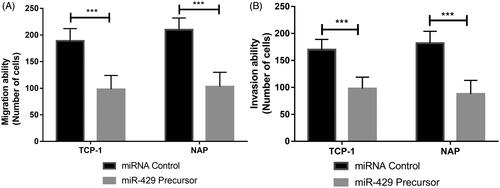
miR-429 reduces thyroid cancer cells invasion and migration
Advanced studies were also conducted to assess the invasion and migration ability of TCP-1 and NPA cells after the transfection of miR-429 using transwell assays. As shown in , it could be found that the up-regulation of miR-129-5p expression in both TCP-1 and NPA cells could inhibit the migration and invasion ability (p < .001).
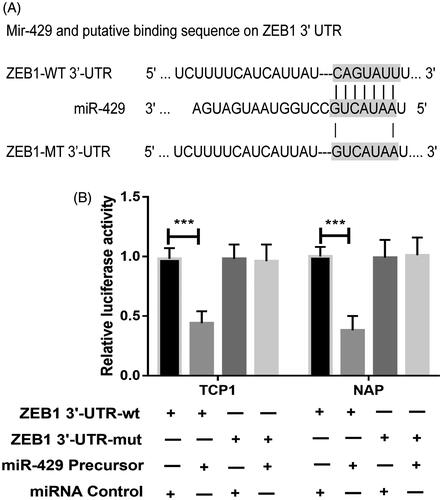
miR-429 directly targets ZEB1 in thyroid cancer cell lines
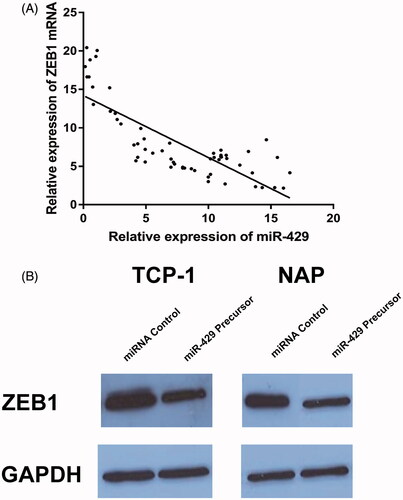
To identify the downstream targets of miR-429, TargetScan algorithm was used to predict targets of miR-429 in human. Among the numerous possible candidates, ZEB1 was chosen for further analysis in this study (). First, the expression pattern miR-429 and ZEB1 mRNA was detected in 59 thyroid cancer samples. As shown in , it was detected that the miR-429 expression was inversely correlated with ZEB1 mRNA. To validate the effect site of miR-429 and ZEB1, luciferase activity assay was conducted. In , it could be found that miR-429 significantly suppressed the WT 3’-UTR but not that of Mut 3’-UTR of ZEB1 luciferase activity in both TCP-1 and NPA cells. In advance, we also detect the effect of miR-429 up-regulation on the expression of ZEB1 protein. As showed in , overexpression of miR-429 significantly suppressed ZEB1 protein level in both TCP-1 and NPA cells.
Discussion
Emerging evidence has revealed that miRNAs participate in the development and progression of various cancers including thyroid cancer via regulation of the expression their target genes. In this study, the results demonstrated that miR-429 was down-regulated in thyroid cancer and functions as a tumour suppressor in through targeting its target gene, ZEB1.
In previous studies, miR-429 has been regarded as a tumour suppressor and was studied in different cancers. In a study based on 45 serum specimens and 78 colorectal cancer tissues, it was reported that miR-429 is an independent prognostic indicator for chemo-response to 5-FU therapy among colorectal cancer patients. High level of miR-429 expression was correlated with enhanced malignant potential and poor prognosis of colorectal cancer patients [Citation13]. A study based on clinical samples and in-vitro cultured cells showed that reduced expression of miR-429 in gastric cancer tissues and overexpression of miR-429 could inhibit the transcription and translation of the heparanase gene [Citation19]. When cervical cancer was considered, IKKβ was downregulated by miR-429, nuclear factor κB (NF-κB) pathway activation, interleukin-6 (IL-6) and interferon-β (IFN-β) production were decreased in cervical cancer cells. These findings indicated that miR-429 was involved in regulation of the NF-κB pathway by targeting IKKβ and functions as a tumour suppressor in cervical carcinogenesis [Citation20]. In this study, it was found that miR-429 was significantly lower in the thyroid cancer tissue samples compared with the normal tissues. However, we didn’t conduct the detection of miR-429 in the normal controls and thus it is now insufficient to conclude that miR-429 could be used as a diagnostic marker of thyroid cancer. In the following study, we would conduct advanced study involving the blood samples, healthy control and following-up data and that would help us to detect the diagnostic and prognostic effect of miR-429 for thyroid cancer cases.
There were different potential targets of miR-429, including LIM domain kinase 1, cyclin-dependent kinase inhibitor 2B and RAB23 were reported in previous studies [Citation21–23]. It has also been reported that ZEB1 was one potential target of miR-429. Through studying chronic constriction injury rat spinal cords and isolated microglials, it was reported that miR-429 was significantly decreased. Advanced study showed that ZEB1 was predicted as target of miR-429 and dual-luciferase reporter assays confirmed the correlation between them [Citation17]. ZEB1, which is significantly associated with epithelial-to-mesenchymal transition, has been reported to be dysregulated in different cancers [Citation24]. Epithelial-mesenchymal transition has been reported to be one of the factors that promotes tumoural progression and ZEB1 was regarded as one of its main activators [Citation25]. Thus several previous studies were conducted to detect the effect of ZEB1 in the tumourigenesis. For instance, in a study based on 76 fresh-frozen tissues and microarray analyses, it was found that ZEB1 in stromal cells were significantly correlated with degree of dysplasia. The hazard of death was significantly increased in patients with expression of epithelial ZEB1 and stromal ZEB1 [Citation26]. In another study, it was reported that ZEB1 expression correlates with Gleason score in prostate cancer samples and that expression of ZEB1 regulates epithelial-mesenchymal transition and malignant characteristics in prostate cancer cell lines [Citation27]. In this study, it was detected that ZEB1 was the target of miR-429 and miR-429/ZEB1 axis demonstrated important functions in the development of thyroid cancer. Considering the important role of ZEB1 in the tumourigenesis, it is quite valuable to conducted advanced study on the effect of ZEB1 in the thyroid cancer.
In conclusion, our results demonstrated that miR-429 was down-regulated in thyroid cancer clinical samples and cell lines. It could inhibit cell proliferation, invasion and promoting apoptosis. The activation of miR-429 suppressed tumourigenesis through targeting its target, ZEB1. Our results strongly support that miR-429 may be a new target for treatment of thyroid cancer.
Ethical approval and consent to participate
This study was approved by the Ethics Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao University. All the cases were hospitalized patients and received thyroid cancer surgical ablation in Sichuan Cancer Hospital. Informed consents were obtained from the patients or their guardians.
Disclosure statement
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Kim SC, Kim JH, Won JK, et al. Asymptomatic intrathyroidal pyriform sinus fistula mimicking thyroid cancer: a case report and literature review. Medicine (Baltimore). 2018;97:e0488.
- Fisher SB, Perrier ND. The incidental thyroid nodule. CA Cancer J Clin. 2018;68:97–105.
- Tiedje V, Stuschke M, Weber F, et al. Anaplastic thyroid carcinoma: review of treatment protocols. Endocr Relat Cancer. 2018;25:R153–R161.
- Roth MY, Witt RL, Steward DL. Molecular testing for thyroid nodules: review and current state. Cancer. 2018;124:888–898.
- Ren C, Liu Q, Wei Q, et al. Circulating miRNAs as potential biomarkers of age-related macular degeneration. Cell Physiol Biochem. 2017;41:1413–1423.
- Liu H, Lei C, He Q, et al. Nuclear functions of mammalian MicroRNAs in gene regulation, immunity and cancer. Mol Cancer. 2018;17:64.
- Curtale G. MiRNAs at the crossroads between innate immunity and cancer: focus on macrophages. Cells. 2018;7:pii: E12.
- Liu K, Zhao K, Wang L, et al. Prognostic value of microRNA-155 in human carcinomas: an updated meta-analysis. Clin Chim Acta Int J Clin Chem. 2018;479:171–180.
- Wang R, Ma Q, Ji L, et al. miR-622 suppresses tumor formation by directly targeting VEGFA in papillary thyroid carcinoma. Onco Targets Ther. 2018;11:1501–1509.
- Fang L, Kong D, Xu W. MicroRNA-625-3p promotes the proliferation, migration and invasion of thyroid cancer cells by up-regulating astrocyte elevated gene 1. Biomed Pharmacother. 2018;102:203–211.
- Yu L, Wu D, Gao H, et al. Clinical utility of a STAT3-regulated miRNA-200 family signature with prognostic potential in early gastric cancer. Clin Cancer Res. 2018;24:1459–1472.
- Wang Y, Xu YM, Zou YQ, et al. Identification of differential expressed PE exosomal miRNA in lung adenocarcinoma, tuberculosis, and other benign lesions. Medicine (Baltimore). 2017;96:e8361.
- Dong SJ, Cai XJ, Li SJ. The clinical significance of MiR-429 as a predictive biomarker in colorectal cancer patients receiving 5-fluorouracil treatment. Med Sci Monit. 2016;22:3352–3361.
- Zou J, Liu L, Wang Q, et al. Downregulation of miR-429 contributes to the development of drug resistance in epithelial ovarian cancer by targeting ZEB1. Am J Transl Res. 2017;9:1357–1368.
- Dong H, Hao X, Cui B, et al. MiR-429 suppresses glioblastoma multiforme by targeting SOX2. Cell Biochem Funct. 2017;35:260–268.
- Deng Y, Luan F, Zeng L, et al. MiR-429 suppresses the progression and metastasis of osteosarcoma by targeting ZEB1. EXCLI J. 2017;16:618–627.
- Yan XT, Zhao Y, Cheng XL, et al. Inhibition of miR-200b/miR-429 contributes to neuropathic pain development through targeting zinc finger E box binding protein-1. J Cell Physiol. 2018;233:4815–4824.
- Kong X, Ding X, Li X, et al. 53BP1 suppresses epithelial-mesenchymal transition by downregulating ZEB1 through microRNA-200b/429 in breast cancer. Cancer Sci. 2015;106:982–989.
- Sheng N, Zhang L, Yang S. MicroRNA-429 decreases the invasion ability of gastric cancer cell line BGC-823 by downregulating the expression of heparanase. Exp Ther Med. 2018;15:1927–1933.
- Fan JY, Fan YJ, Wang XL, et al. miR-429 is involved in regulation of NF-κBactivity by targeting IKKβ and suppresses oncogenic activity in cervical cancer cells. FEBS Lett. 2017;591:118–128.
- Xue H, Tian GY. MiR-429 regulates the metastasis and EMT of HCC cells through targeting RAB23. Arch Biochem Biophys. 2018;637:48–55.
- Yang J, Liu Y, He A, et al. Hsa-miR-429 promotes bladder cancer cell proliferation via inhibiting CDKN2B. Oncotarget. 2017;8:68721–68729.
- Li D, Wang H, Song H, et al. The microRNAs miR-200b-3p and miR-429-5p target the LIMK1/CFL1 pathway to inhibit growth and motility of breast cancer cells. Oncotarget. 2017;8:85276–85289.
- Larsen JE, Nathan V, Osborne JK, et al. ZEB1 drive epithelial-to-mesenchymal transition in lung cancer. Clin Invest. 2016;9:3219–3235.
- Orellana-Serradell O, Herrera D, Castellon EA, et al. The transcription factor ZEB1 promotes an aggressive phenotype in prostate cancer cell lines. Asian J Androl. 2018;20:294–299.
- Chang YR, Park T, Park SH, et al. Prognostic significance of E-cadherin and ZEB1 expression in intraductal papillary mucinous neoplasm. Oncotarget. 2018;9:306–320.
- Orellana-Serradell O, Herrera D. Castellon EA and contreras HR: the transcription factor ZEB1 promotes an aggressive phenotype in prostate cancer cell lines. Asian J Androl. 2017.