Abstract
Objectives
The study aimed to evaluate the prognostic value of systemic inflammation markers [neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), Prognostic Nutritional Index (PNI)] and hepatic inflammation markers [aspartate aminotransferase-to-platelet ratio index (APRI), γ-glutamyl transferase (γ-GT)/alanine aminotransferase (ALT)] in patients with hepatitis B virus (HBV)-associated hepatocellular carcinoma (HCC) and further to develop a novel prognostic score model.
Methods
A total of 401 cases with HBV-associated HCC who underwent hepatectomy as initial therapy were included in the analysis. Kaplan–Meier was performed to construct survival curves and receiver operating characteristic (ROC) analysis was used to detect the optimal cut-off value of markers. The prognostic score model was constructed using significant inflammation markers in the Cox model. Each factor was given a score of 1 and patients were stratified according to the scores.
Results
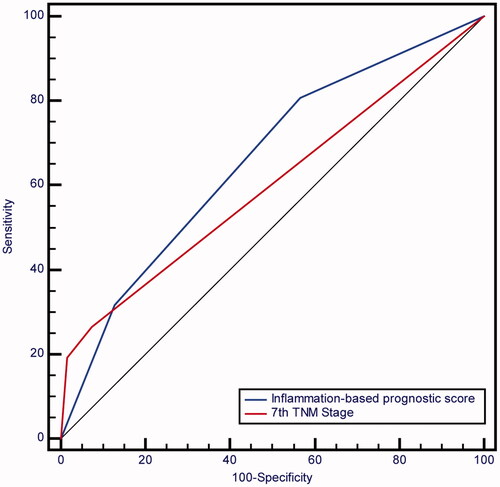
In the Cox model, α-fetoprotein (AFP), ALT, tumour differentiation, maximum size of tumours, TNM stage, PNI and γ-GT/ALT were independently prognostic factors. We established a preoperative inflammation-based prognostic scoring model combining PNI and γ-GT/ALT. The novel preoperative inflammation-based prognostic score was superior (area under the curve [AUC], 0.659) to 7th tumour-node-metastasis (TNM) stage (AUC, 0.600) despite no statistical significance (p = .1036).
Conclusion
PNI and γ-GT/ALT are independent predictors for prognosis. The novel prognostic score model based on systemic and hepatic inflammation markers is suitable for the prognosis evaluation in patients with HBV-associated HCC.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent malignancy, representing the third most common cause of cancer death in the world [Citation1,Citation2]. Currently, hepatectomy and transplantation are potentially curative treatments for HCC. However, the long-term outcome remains far from satisfactory due to the high incidence of post-operative recurrence. Therefore, it is important to identify the prognostic factors in order to help clinicians to adopt preventive and therapeutic strategies for risk patients.
In the past decades, the relationship between tumour and inflammation has been recognized. A large number of epidemiological studies have identified tumours often arise at sites of chronic inflammation including lung cancer, gastric cancer, colorectal cancer and liver cancer [Citation3]. Infiltrations of inflammatory cells are frequently observed and cytokines are unregulated in tumours, suggesting the strong associations between inflammation and tumorigenesis. Inflammation has been added as the seventh hallmark of cancer [Citation4]. It is well known that the pathogenesis of HCC is based on inflammation. Hepatitis B virus (HBV) infection plays a major aetiological role in the development of HCC in China. The body has formed a state of chronic inflammation in the long-term stimulation of the HBV infection. It is favourable to promote the occurrence, development and metastasis of HCC under this background. Therefore, it seems a reasonable approach to predict HCC prognosis with inflammatory biomarkers.
In HCC, the host inflammatory response to tumours consists of systemic alterations as well as changes in the liver microenvironment. C-reactive protein (CRP) [Citation5] is the first serum marker of systemic inflammation which is most commonly used in clinical practice. It has been confirmed as an independent risk factor for prognosis in patients with HCC undergoing hepatectomy. Moreover, some other haematological factors of the systemic inflammatory response have been scrutinized for prognosis evaluation including neutrophil-to-lymphocyte ratio (NLR) [Citation6], platelet-to-lymphocyte ratio (PLR) [Citation7] and Prognostic Nutritional Index (PNI) [Citation8]. Kinoshita et al. [Citation9] reported that NLR, PLR and PNI were significantly associated with clinical outcomes of patients with HCC. The data revealed the predictive values of systemic inflammatory indexes in the prognosis of HCC. In addition, α-fetoprotein (AFP), the aspartate aminotransferase (AST)-to-platelet ratio index (APRI) and the ratio of serum γ-glutamyl transferase (γ-GT) to alanine aminotransferase (ALT) (γ-GT/ALT) reflect inflammation disturbance in the liver. AFP has been employed as a serum biomarker for HCC detection, and its elevated level is significantly correlated with poor tumour differentiation, tumour burden, and early post-operative recurrence, and unfavourable survival. However, the sensitivity of AFP for HCC diagnosis displays a wide range, especially for early stages of HCC. Moreover, AFP level could be easily influenced by active hepatitis [Citation10]. Thus, AFP is not suitable for prognosis estimations among patients with HBV-associated HCC. APRI represents the ratio of AST to platelet which is initially identified for staging fibrosis in patients with chronic hepatitis [Citation11]. It is reported that serum APRI could be used in evaluating the degree of fibrosis and liver function reserve, as well as predicting prognosis for patients with chronic hepatitis [Citation12]. APRI is also a predictor for postoperative outcomes of HCC [Citation13]. In HBV-related HCC, Huang et al. had reported that serum APRI might be employed as a biomarker for assessing liver cirrhosis and predicting both recurrence and survival of the patients [Citation14]. ALT and γ-GT activity have been mainly used for the assessment of chronic hepatitis activity and liver function. Min-Jie Ju et al. [Citation15] found that the elevated level of preoperative serum γ-GT/ALT ratio was an independent risk factor for poor prognosis of Child-Pugh A HCC patients after liver resection. However, the reliability and reproducibility of these biomarkers for prognosis prediction remained unsatisfactory. In order to enhance the prognostic significance of inflammatory biomarkers for HCC in the clinic, we hypothesized that the combined application of the inflammatory markers might be a promising approach. To our knowledge, no previous researches have investigated the combined application value of the five inflammatory markers in predicting prognosis for HBV-associated HCC patients after curative resection. Further, controversy exists concerning the optimal cut-off points for these inflammatory markers to predict overall survival.
In the present study, we aimed to evaluate the combined prognostic value of systemic inflammation markers (NLR, PLR, PNI) and hepatic inflammation markers (γ-GT/ALT and APRI) for patients with HBV-associated HCC undergoing hepatectomy as initial treatment, propose an optimal cut-off point for the meaningful markers and develop a novel preoperative prognostic factors-based risk stratification model that directly predict HCC using only preoperative clinical parameters to select the patients with poor prognosis.
Patients and methods
Patients
The present study was a retrospective investigation. Data were collected from Chinese PLA General Hospital for all 401 patients who were diagnosed with HCC and underwent curative hepatectomy between January 2004 and December 2006. The diagnosis of HBV-associated HCC was based on the European Association for the Study of the Liver criteria [Citation16]. The diagnosis was confirmed by histopathology or radiological imaging [computed tomography (CT)/magnetic resonance imaging (MRI) scans]. Furthermore, the blood samples collected from the patients were positive to hepatitis Be antigen (HBeAg). Patients who met the following criteria were included in the present study: (1) All patients were only HBV-positive; (2) Child-Pugh A; (3) No portal or hepatic vein involvement and invasion; (4) Without lymph node or distant metastasis; (5) No anticancer treatment for HCC before surgery; (6) R0 resection; (7) Complete clinical, laboratory and follow-up data; (8) Patients had survived for at least 30 days postoperatively. Patients were excluded from the study if they had one or more of the followings: (1) Patients who underwent palliative surgery; (2) Missing blood records; (3) Patients who had any evidences of active concomitant infection or inflammatory disease such as sepsis, human immunodeficiency virus (HIV), inflammatory bowel disease or rheumatoid arthritis; (4) Emergency surgery for ruptured HCC patients; (5) Receiving blood transfusion within the last 2 months; (6) Patients who had other malignancies. Based on these criteria, a total of four hundred and one consecutive patients were enrolled. The study complied with the standards of the Declaration of Helsinki and current ethical guideline and was approved by the Research Ethics Committee of Peking University International Hospital.
Factors analyses
Routine assessment was carried out on the day of admission within one week before surgery, which consisted of a complete physical examination and laboratory tests, abdominal ultrasound and CT or MRI. CT and MRI scans of HCC diagnosed by histopathology or typical vascular pattern according to the 2005 AASLD criteria were blindly reviewed by an abdominal radiologist. Hepatectomy was performed on all patients with intent to cure. Before surgery, peripheral blood of all patients were collected and pre-operative demographic, laboratory data and pathological data were recorded for these patients including gender, age, serum level of AFP, ALT, AST, Albumin, alkaline phosphatase (ALP), γ-GT, total bilirubin (TB), tumour differentiation, number of tumour nodules, maximum size of tumours, liver cirrhosis, micro-vascular tumour thrombus (MVTT), capsular invasion and the 7th tumour node metastasis (TNM) stage. NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. Similarly, PLR was defined as the absolute platelet count divided by the absolute lymphocyte count. The PNI was calculated using the following formula: 10 × serum albumin value (g/dl)+0.005 × lymphocyte count in peripheral blood. APRI was calculated as the ratio of [(AST/upper limit of normal value: 45 IU/L)/platelet counts (109/L)] × 100. γ-GT/ALT was defined as serum γ-GT counts by ALT counts as the name shown.
Follow-up
All patients received basic physical examination, liver function test, AFP, ultrasound or dynamic contrast-enhanced CT or MRI at intervals of 3 to 6 months after surgery. Chest X-ray was also performed in each visit. Diagnosis of recurrence (intra-hepatic or systemic) was based on radiological evidence including dynamic contrast-enhanced CT or MRI, contrast-enhanced ultrasonography. Disease-free survival (DFS) was measured from the date of hepatectomy until tumour recurrence. Overall survival (OS) was defined as the duration from the date of hepatectomy to death or the last follow-up visit. Patients who died of HCC were categorized as tumour-related deaths. The last follow-up visit was up to 1 December 2016.
Statistical analysis
SPSS for Windows program (version 19.0) and MedCalc statistical software v13.1.2.0 were used to analyse the data. An optimal cut-off value for NLR, PLR, PNI, γ-GT/ALT and APRI was selected using the receiver operating characteristic (ROC) curve analysis. Categorical variables were reported as frequencies (percentages) and analysed using Chi-squared test or Fisher’s exact test (two-tailed). Continuous data were expressed as mean ± standard deviation or median (range). The Mann–Whitney U-test and Kruskal–Wallis test were used to compare continuous variables between groups. Spearman’s rank correlation coefficient was used to assess the correlation between the two parameters. Kaplan–Meier method combined with Log-rank test was performed for plotting overall survival curves. Cox proportional hazard model was used in univariate and multivariate regression analyses. Variables to be entered into the regression analysis were chosen on the basis of the results of univariate analysis.
To evaluate the sensitivity and specificity of the constructed prognostic model for the 5-year OS, ROC curve was plotted based on the peroperative inflammatory scores. The score was selected as the cut-off value for pre-operative NLR, PLR, PNI, γ-GT/ALT and APRI that was closest to the point with both maximum sensitivity and specificity by using MedCalc statistical software. These cut-off values were used to categorize the high and low groups. In brief, death within 60 months was recorded as an event; patients who survived longer than 60 months were recorded as non-events; patients who had a shorter follow-up were dropped from these analyses. The area under the ROC curve (AUC) for the score model was measured for comparison of two AUCs by the z-statistic. All statistical testings were two-tailed at the 5% level and a p value of less than .05 was considered to be statistical significant.
Results
Baseline characteristics and outcomes
The baseline characteristics of the total 401 patients are shown in . They were predominantly males (88.3%) and the mean age was 52.06 ± 10.48 years (median, 51 years; ranged from 21 to 77 years). 167 (41.6%) patients had an AFP level below 20 μg/L, 81 (20.2%) patients had an AFP level between than 20 and 200 μg/L, 48 (12.0%) patients had an AFP level between 200–1000 μg/L, and 105 (26.2%) patients had an AFP level greater than 1000 μg/L. In this study, the upper normal limits for serum ALT, AST, albumin, ALP, γ-GT, TB values were 40, 40, 35, 130, 50 and 34, respectively and were taken as the cut-off value. 36.2%, 30.2%, 13.0%, 51.6% and 3.2% patients had an ALT, AST, ALP, γ-GT, TB level more than their marginal value. 25 (6.2%) cases had an albumin level under 35 g/L. Concerning the tumour characteristics, 342 (85.3%) patients had single tumour nodule, 33 (8.2%) had two or three and 26 (6.5%) had more than three. 113 (28.2%) patients had a tumour size smaller than 3 cm, 125 (31.2%) patients had a tumour size between 3–5 cm, 113 (28.2%) patients had a tumour size between 5–10 cm, and 50 (12.5%) had a tumour size larger than 10 cm. Poor differentiation was observed in 33 (8.2%) cases, moderate differentiation was observed in 328 (81.8%) cases and well differentiation was observed in 40 (10.0%) cases. Underlying liver cirrhosis was found in 322 (80.3%) patients. 21 (5.2%) patients had a microvascular tumour thrombus. According to the 2010 American Joint Committee on Cancer (AJCC) staging classification, 337 (84.0%) patients were in stage I, 30 (7.5%) in stage II and 34 (8.5%) in stage III. The Barcelona Clinic Liver Cancer (BCLC) stage classification demonstrated that 350 (87.3%) patients were divided into stages 0–A, while stages B–C included 51 (12.7%) patients. At the end of the follow-up period, 166 (41.40%) patients had died and 235 (58.60%) patients were still alive. The 1-, 3- and 5-year OS rates were 81%, 64%, and 56%. The median OS was 95 months.
Table 1. The characteristics of the 401 HCC patients grouped by PNI and γ-GT/ALT.
An optimal cut-off value
The mean value of NLR, PLR, PNI, APRI and γ-GT/ALT were 1.85, 93.98, 49.49, 0.45 and 1.48, respectively. According to the ROC curve, the optimal cut-off value of preoperative NLR, PLR, PNI, APRI and γ-GT/ALT was 1.90, 83.80, 48.50, 0.39 and 1.52, respectively, as shown in . These cut-off values were used to categorize the high and low groups.
Table 2. An optimal cut-off value for NLR, PLR, PNI, γ-GT/ALT and APRI was selected by the ROC curve analysis.
Prognostic factors
The univariate analysis showed that gender (p = .024), serum AFP level (p < .001), ALT (p < .001), AST (p < .001), albumin (p = .026), ALP (p < .001), γ-GT (p < .001), TB (p = 0.025), tumour differentiation (p = .002), number of tumour nodules (p < .001), maximum tumour size (p < .001), MVTT (p = .014), TNM stage (p < .001), NLR (p = .002), PNI (p = .042), APRI (p < .001), γ-GT/ALT (p < .001) were associated with overall survival (). A multivariate regression performed on these significant factors showed that only the serum AFP level (p = .009), ALT (p = .001), tumour differentiation (p = .030), maximum tumour size (p = .001), TNM stage (p < .001), PNI (p = .047), γ-GT/ALT (p = .005) were independent prognostic predictors of overall survival (). Patients with PNI ≤ 48.50 had a median overall survival of 73.0 months, compared to the 111.8 months median overall survival of patients with PNI > 48.50 (). Patients with γ-GT/ALT ≥ 1.52 had a median overall survival of 55.8 months while patients with γ-GT/ALT < 1.52 had a median survival of 111.8 months (). Unexpectedly, BCLC stages had no significant effects on the overall survival of the included patients (log rank test, p = .084) (). In addition, the predictive values of the two biomarkers for DFS were also estimated. The median DFS time of patients with PNI ≤48.50 was obviously shorter than those with PNI > 48.50 (51 vs. 84 months, p = .004) (). Meanwhile, the patients with γ-GT/ALT ≥ 1.52 had a median overall survival of 51 months and those with γ-GT/ALT < 1.52 had a median survival of 84 months (p = .003, ).
Figure 1. Kaplan–Meier survival curves for overall survival in 401 patients undergoing hepatectomy for HCC. Significance was tested with Log-rank test. (A). Overall survival rates stratified by PNI; (B). Overall survival rates stratified by γ-GT/ALT; (C). Overall survival rates of the patients according to their BCLC stages; (D). Overall survival rates stratified by inflammation-based prognostic score.
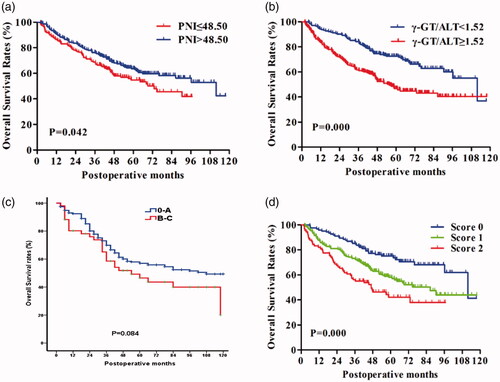
Figure 2. The curves for DFS in 401 patients with HCC. Significance was calculated using log rank test. (A). DFS rate according to PNI; (B). DFS rates stratified by γ-GT/ALT.
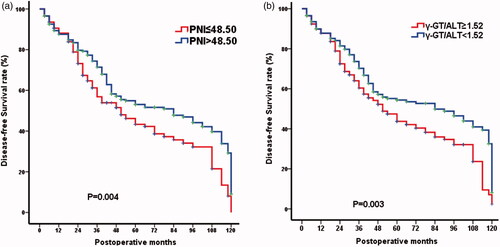
Table 3. Prognostic factors for overall survival in patients with HCC by univariate and multivariate analysis.
Comparison of variables between patients with different PNI and γ-GT/ALT group
We divided the total of 401 patients into different groups based on their cut-off values of PNI and γ-GT/ALT. HCC patients between high and low PNI groups showed significant differences in serum AFP level (p = .007), AST (p = .010), albumin (p < .001), ALP (p = .037), MVTT (p = .021), NLR (p < .001), PLR (p < .001) and APRI (p < .001) (). An elevated γ-GT/ALT was associated with high level of AST (p = .004), ALP (p < .001) and γ-GT (p < .001), larger tumour size (p < .001), advanced TNM stage (p = .001) and greater NLR (p = .002), PLR (p = .002), APRI (p = .004) (). There were no significant differences in the other variables among the groups with different PNI and γ-GT/ALT.
A preoperative inflammation-based prognostic scoring model
We established a preoperative inflammation-based prognostic scoring model combining the systemic inflammation marker (PNI) and liver inflammation marker (γ-GT/ALT), which was found to be significant by multivariate analysis. Each factor was given a score of 1, and then, patients were divided into three categories (score 0, score 1 and score 2). Significant survival differences were shown in according to the stratified pre-operative prognostic score. The median overall survival for patients with score 0, 1, 2 was 111.8, 86.6 and 47.0 months, respectively (p < .001). We compared the prognostic significance among PNI, γ-GT/ALT and the inflammation-based prognostic scoring model. The results summarized in demonstrated that the predictive sensitivity and specificity of the scoring model for five-year survival of HCC were 66.9% and 67.5% respectively, and the accuracy was 67.3%. The diagnostic performance of the inflammation-based prognostic scoring model was better than PNI and γ-GT/ALT. The combination of PNI and γ-GT/ALT could improve the prognostic values of the parameters.
Table 4. Prognostic values of the inflammation-based prognostic scoring model and TNM stages for 5-year overall survival of the patients.
In addition, ROC curve analysis of the pre-operative inflammation-based prognostic score versus that of 7th TNM stage was shown in . The pre-operative inflammation-based prognostic score had a higher AUC value at predicting the five-year survival of patients with HBV-associated HCC after curative resection (0.659) in comparison with 7th TNM stage (0.600) though no statistically significant difference was observed (p = .1036).
Discussion
Currently, the BCLC system is one of the most commonly used classification systems for HCC, which can be applied for prognosis evaluation and guidance treatment [Citation17]. Compared with other classification systems, the most prominent advantage of the BCLC system is that it can provide therapeutic strategies for patients at different stages. However, some study has reported that the patients with the same stage of BCLC may exhibit obviously different clinical symptoms, thus resulting in distinct clinical outcomes [Citation18]. Thus, more prognostic models are required to improve the diagnostic accuracy of the BCLC system. The BCLC staging system refers to multiple parameters, such as tumour burden, liver functional reserve and performance status, but not inflammatory factors [Citation16]. Given the pivotal roles of inflammatory response in the initiation and development of HBV-associated HCC, we speculated that the prognostic models constructed based on inflammatory biomarkers might provide super prognostic value for HCC patients.
The initial evidence linking inflammation with cancer was reported in the 19th century by Rudolf Virchow who noted inflammation was one of the predisposing conditions for a tumour process. With increasing knowledge the connection between chronic inflammation and cancer has been recognized for a long time. Epidemiological studies have shown that chronic inflammation increases the risk of numerous cancers. HCC is the most typical one that usually develops in a context of liver inflammation caused by hepatotropic viruses, aflatoxin, metabolic liver disease or autoimmunity [Citation19]. More than 70–80% of HCC cases occur in the area of high HBV infections, and chronic HBV infections are responsible for 50% of HCC cases worldwide [Citation20]. Patients with HBV infection experience chronic inflammation, increasing the risk of liver cancer. Inflammation is now recognized to play a key role in HCC initiation, development and progression especially in the background of chronic HBV infection [Citation21]. In the last decade or so, it has become clear that disease progression in HCC is dependent on a complex interaction of the characteristics of both the liver and systemic inflammatory responses [Citation22]. The predictive role of systemic inflammation and hepatic inflammation markers in the prognosis of patients with HCC is of increasing interest.
The presence of a systemic inflammatory reaction which is sustained by the aberrant release of pro-inflammatory mediators, such as tumour necrosis factor-α, interleukins, and interferon-γ, seems to be a cancer-associated phenomenon or a part of the host’s innate immune response towards tumour [Citation23]. Clinically, there is no the most accurate method of quantifying the degree of the systemic inflammation reaction [Citation24,Citation25]. The most common reported biochemical or blood markers of the systemic inflammatory responses in cancer patients included elevated CRP levels, hypoalbuminaemia or increased neutrophils and platelet counts. Combinations of such factors have been used to derive simple inflammation-based prognostic scores [Citation9,Citation26] such as GPS, NLR, PLR, PNI, and inflammation-based index (IBI) to predict survival. It has been found that liver inflammation can be evaluated by means of APRI [Citation14,Citation27] and γ-GT/ALT [Citation15]. To our knowledge, the present study is the first report to investigate the combined value of preoperative NLR, PLR, PNI, APRI and γ-GT/ALT as predictive markers for HBV-associated HCC after curative resection. We used ROC analysis to determine preoperative cut-off values of 1.90, 83.80, 48.50, 0.39 and 1.52 for NLR, PLR, PNI, APRI and γ-GT/ALT. The univariate analysis in this study demonstrated that the NLR, PLR, PNI, APRI and γ-GT/ALT were significantly associated with overall survival. However, the multivariate analysis showed that only decreasing PNI and evaluating γ-GT/ALT are independent predictive factors and superior in this aspect to other markers. An established novel pre-operative inflammation-based prognostic scoring model led to a considerable improvement in prognostic values compared with 7th TNM stage by the simple combination of them. However, in our study, 350 (87.3%) patients were divided into BCLC stages 0-A, while stages B-C included 51 (12.7%) patients. Due to the uneven distribution, BCLC stages had no obvious association with prognosis of the patients. Thus, the direct comparison between BCLC staging and the novel prognostic model was not performed in the current study. Further investigations will be required to compare the prognostic significance of the inflammatory model with the BCLC system.
Although the relationship between NLR and prognosis of HCC patients has been investigated by many studies, the results are not consistent. Several studies have shown that high NLR is an independent risk factor for overall survival in patients with HCC after surgical resection [Citation28], liver transplantation [Citation29], transcatheter arterial chemoembolization (TACE) [Citation30], and radiofrequency ablation (RFA) [Citation31]. However, the cut-off values of NLR in these studies were different. The current study evaluated the cut-off values of NLR at 3, 4, 5, and 1.90, and revealed that the NLR was not an independent factor for survival of HCC patients after curative resection at any of the cut-off levels (part of data not shown). Similarly, we also used 150, 300 and 83.80 as the cut-off value of PLR and showed that elevated PLR was not significantly affected patients’ survival independently (part of data not shown). These findings agreed with the results of A Kinoshita et al [Citation9]. This study compared the NLR, PLR, PNI, Glasgow Prognostic Score (GPS), modified GPS (mGPS) and prognostic index (PI), and demonstrated that GPS was an independent predictor of poor prognosis in patients with HCC and outperformed other inflammation markers in terms of the ability to prognosticate. But it is worth mentioning that the enrolled patients were not limited to only HBV infected; undergo curative resection and child-Pugh A. So it is very natural that we confirm that significant value of PNI in predicting the prognosis of HCC patients. But Kinoshita et al didn’t have the same conclusion. And it is our weakness that we did not compare the prognostic scores based on the value of CRP such as GPS and PI because CRP level was not the routine test in HCC patients on the day of admission within one week before surgery.
In accordance with Pinato et al.’s study [Citation8], they found that the PNI significantly affected patient survival independently in patients with HCC. The PNI is calculated using only two values: the serum albumin concentration and total lymphocyte count in the peripheral blood, which are the clinical routine examination indexes and easily obtained. It was initially designed to evaluate the immunonutritional status of patients who underwent operation of the gastrointestinal tract [Citation32], and pancreatic cancer [Citation33] because that albumin is one of the most commonly used indicators to reflect nutritional status, and circulating lymphocytes are known to play an important immunological role in various carcinomas. However, there is increasing evidence that a sustained presence of systemic inflammation is strongly associated with weight loss, poor performance status, nutritional decline, which further lead to shorter survival, and increased mortality in patients with cancer [Citation34]. This is of special attention in HCC patients due to the possible impaired nutritional status secondary to liver cirrhosis [Citation8,Citation35]. It can be interpreted that liver is the main organ for the synthesis of albumin. When liver gets exposured to a sustained pro-inflammatory response stimuli, hepatic albumin biosynthesis is down-regulated [Citation36]. Impaired synthetic function interacts with end-stage liver disease including liver cirrhosis. The combined effects result in the reduced level of serum albumin. So, the correlation between systemic inflammation and malnutrition can be reflected by hypoalbuminaemia.
Moreover, some investigators found that significant relationship between peripheral lymphocytopenia and the poor nutritional and weaken immune response in patients with liver disease [Citation37]. Recently, clinical research come to emphasize that the critical role of peritransplant low absolute lymphocyte count in predicting the short-term prognosis of HCC patients [Citation38]. It is consistent with the value of lymphocytes infiltrating in tumour. There is evidence demonstrating that a low number of tumour-infiltrating lymphocytes in HCC specimens are associated with poorer survival rates after operation [Citation39]. Therefore, it is feasible that the PNI could be a novel indicator of systemic inflammation. The present study adds further weight to the important role of PNI in predicting the prognosis of survival in HCC patients as a marker of systemic inflammation.
It is well known that HCC recurrence and metastasis after operation often occurred in the postoperative residual liver [Citation40]. The inflammatory state of residual liver after hepatic resection for HCC is negatively related to its ability to regenerate. The inflammatory reaction is heavier; the regeneration ability is poorer, easier to form tumour recurrent lesions. So, the background liver inflammation and fibrosis have an important impact on the development and survival of HCC patients. The clinical index of liver inflammation such as ALT, AST and γ-GT has been shown to positively related with the recurrence and survival of liver cancer [Citation41–43]. APRI is the simple and feasible tests for assessing the stage of fibrosis and cirrhosis in patients with HBV infection [Citation44], which is based on two routine tests, AST and PLT. And we speculate that APRI not only is a surrogate marker in assessing the degree of liver fibrosis, but also a powerful prognostic factor for HCC patients as a marker of liver inflammation. For now, few studies have confirmed the impact of APRI on HCC recurrence and prognosis [Citation14,Citation28,Citation45] and had consistent conclusion that elevated APRI based inflammatory induces was significantly associated with poor prognosis in HCC. In our study, there is no obvious association between high APRI and prognosis in HCC patients despite in the univariate analysis APRI was associated with overall survival. Different enrolling criteria, sample size and follow-up time may explain the variance in the results of our study as compared with other studies. The ratio of γ-GT/ALT is another parameter reflecting liver inflammation that can independently predict HCC outcomes. Some studies suggested that pre-treatment γ-GT/ALT was suitable for monitoring the activity of viral hepatitis and to assess the effect of antiviral treatments [Citation46,Citation47]. For now, only Min-Jie Ju et al [Citation15] verified the value of preoperative serum γ-GT/ALT ratio contributing in the prediction of tumour recurrence and survival of patients with HCC. It was the first evidence that the γ-GT/ALT ratio is a powerful prognostic for the recurrence and survival of Child-Pugh a HCC patients after hepatic resection. This is highly agreed with our findings.
We therefore have constructed a novel inflammation-based prognostic score model based on PNI and γ-GT/ALT, which were found to be significant in the multivariate Cox model. The median overall survival for patients with score 0, 1, 2 was 111.8, 86.6 and 47.0 months, respectively (p < .001). The inflammation-based preoperative prognostic score model is superior to the 7th TNM stage with an AUC of 0.659 compared with 7th TNM stage of 0.600 though no statistically significant difference was observed (p = .1036). TNM stage is formed based on the pathological analysis of operation samples. But there is no consensus on its prognostic value worldwide in part due to the complexity of the potential prognostic value [Citation48]. For an effective staging system, it must be simple and consider the biological characteristics of HCC to apply for prognostication before treatment. In this respect, the novel inflammation-based prognostic score model established comprising several biochemical markers that are routinely tested on the day of admission before treatment is superior to 7th TNM stage. In addition, some prognostic evaluation systems were developed to predict the outcome of HCC patients based on inflammation markers [Citation49,Citation50]. But these inflammation-related prognostic systems only consider systemic inflammation factors and neglect the impact of liver inflammation. It is our novelty that the systemic inflammation and hepatic inflammation are both taken into account.
It is beyond doubt that our investigation has some limitations. Firstly, this study is a single institution study of a fairly homogenous population. All patients enrolled had a history of HBV infection which may bias our study because Hepatitis C virus (HCV) infection and alcoholism are the predominant etiological factors for HCC in Western countries. Whether our findings can be applied to patients with HCC in Western countries is also our concern. Secondly, the major HCC patients in our study were diagnosed with TNM stages I (84%) and BCLC 0-A stages (86%). Due to the uneven distribution and relatively small sample size, the prognostic values of the inflammation-based prognostic scoring model for the patients with different stages were not estimated in our study. An unexpected result in our should be noted. The BCLC stages had no obvious influences on overall survival of the patients. The abnormal results might be also attributed to the relatively small sample size and the uneven tumour stage distribution. Thirdly, the AUC value of the constructed model for five-year prognosis of the included patients was only 0.659, with the sensitivity of 66.9%, specificity of 67.5% and accuracy of 67.3%. Although the predictive performance was super better than the factors used alone, but the unsatisfactory performance might limit its clinical application. Relatively small sample size, uneven tumour stage distribution, as well as the inevitable experimental error might influence the prognostic value of the constructed model. Prognostic value of the inflammation-based prognostic score in different stages was not analysed in our study. Thus, it was necessary to improve the inflammation-based prognostic scoring model with more factors. Additionally, the present study was a single institution study that was performed in a retrospective way. Hence, further large-scale, prospective and multicentre study is needed to confirm the results.
In conclusion, the results of the present study highlight the importance of systemic inflammation and hepatic inflammation in the clinical outcomes of patients with HCC. The novel inflammation-based prognostic score model combing the systemic and hepatic inflammation marker is suitable for the postoperative prognosis evaluation in patients with HBV-associated HCC.
| Abbreviation list | ||
| HCC | = | Hepatocellular carcinoma |
| HBV | = | Hepatitis B virus |
| CRP | = | C-reactive protein |
| NLR | = | Neutrophil-to-lymphocyte ratio |
| PLR | = | Platelet-to-lymphocyte ratio |
| AFP | = | α-fetoprotein |
| γ-GT | = | γ-glutamyl transferase |
| ALT | = | Alanine aminotransferase |
| APRI | = | Aspartate aminotransferase to platelet ratio index |
| AST | = | Aspartate aminotransferase |
| CT | = | Computed tomography |
| MRI | = | Magnetic resonance imaging |
| HBeAg | = | Hepatitis Be antigen |
| ALP | = | Alkaline phosphatase |
| TB | = | Total bilirubin |
| MVTT | = | Micro-vascular tumour thrombus |
| TNM | = | Tumour node metastasis |
| DFS | = | Disease-free survival |
| OS | = | Overall survival |
| ROC | = | Receiver operating characteristic |
| AUC | = | Area under the curve |
| AJCC | = | American Joint Committee on Cancer |
| BCLC | = | Barcelona Clinic Liver Cancer |
| IBI | = | Inflammation-based index |
| PNI | = | Prognostic nutritional Index |
| TACE | = | Transcatheter arterial chemoembolization |
| RFA | = | Radiofrequency ablation |
| GPS | = | Glasgow Prognostic Score |
| mGPS | = | Modified Glasgow Prognostic Score |
| PI | = | Prognostic index |
| HCV | = | Hepatitis C virus |
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- El–Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576.
- Ferenci P, Fried M, Labrecque D, et al. Hepatocellular carcinoma (HCC): a global perspective. J Clin Gastroenterol. 2010;44:239–245.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
- Hashimoto K, Ikeda Y, Korenaga D, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103:1856–1864.
- Xiao W-K, Chen D, Li S-Q, et al. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:117.
- Feng J-F, Huang Y, Chen Q-X. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Onc. 2014;12:58.
- Pinato D, North B, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer. 2012;106:1439–1445.
- Kinoshita A, Onoda H, Imai N, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. 2012;107:988–993.
- Yao Q, Bao X, Xue R, et al. Prognostic value of immunoscore to identify mortality outcomes in adults with HBV-related primary hepatocellular carcinoma. Medicine (Baltimore). 2017;96:e6735.
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526.
- Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–736.
- Cheng J, Zhao P, Liu J, et al. Preoperative aspartate aminotransferase-to-platelet ratio index (APRI) is a predictor on postoperative outcomes of hepatocellular carcinoma. Medicine (Baltimore). 2016;95:e5486.
- Hung H-H, Su C-W, Lai C-R, et al. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. 2010;4:691–699.
- Ju M-J, Qiu S-J, Fan J, et al. Preoperative serum gamma-glutamyl transferase to alanine aminotransferase ratio is a convenient prognostic marker for Child-Pugh A hepatocellular carcinoma after operation. J Gastroenterol. 2009;44:635–642.
- EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338.
- Yoo JJ, Chung GE, Lee JH, et al. Advance-stage hepatocellular carcinoma: a cohort study including 612 patients treated with sorafenib. Cancer Res Treat. 2018;50:366-373.
- Berasain C, Castillo J, Perugorria M, et al. Inflammation and liver cancer. Ann New York Acad Sci. 2009;1155:206–221.
- Yang F, Zhang L, Huo X, et al. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54:1679–1689.
- Pan H, Fu X, Huang W. Molecular mechanisms of liver cancer. ACAMC. 2011;11:493–499.
- Alison MR, Nicholson LJ, Lin WR. Chronic inflammation and hepatocellular carcinoma. Inflammation and Gastrointestinal Cancers. Springer; 2011. pp. 135–148.
- Esper DH, Harb WA. The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutr Clin Pract. 2005;20:369–376.
- Clarke S, Chua W, Moore M, et al. Use of inflammatory markers to guide cancer treatment. Clin Pharmacol Ther. 2011;90:475–478.
- Pinato DJ, Sharma R. An inflammation-based prognostic index predicts survival advantage after transarterial chemoembolization in hepatocellular carcinoma. Transl Res. 2012;160:146–152.
- Pinato DJ, Stebbing J, Ishizuka M, et al. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI). J Hepatol. 2012;57:1013–1020.
- Shen S-L, Fu S-J, Chen B, et al. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. 2014;21:3802–3809.
- Gomez D, Farid S, Malik H, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–1762.
- Bertuzzo VR, Cescon M, Ravaioli M, et al. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers. Transplantation. 2011;91:1279–1285.
- Huang Z-L, Luo J, Chen M-S, et al. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interventional Radiol. 2011;22:702–709.
- Chen TM, Lin CC, Huang PT, et al. Neutrophil‐to‐lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012;27:553–561.
- Nozoe T, Ninomiya M, Maeda T, et al. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40:440–443.
- Kanda M, Fujii T, Kodera Y, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–274.
- Argilés JM, Moore-Carrasco R, Fuster G, et al. Cancer cachexia: the molecular mechanisms. Int J Biochem Cell Biol. 2003;35:405–409.
- Meng Q-H, Yu H-W, Li J, et al. Inadequate nutritional intake and protein-energy malnutrition involved in acute and chronic viral hepatitis Chinese patients especially in cirrhosis patients. Hepato-gastroenterol. 2009;57:845–851.
- Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunology Today. 1994;15:81–88.
- O'Keefe S, Carraher T, E1-Zayadi A, et al. Malnutrition and immuno-incompetence in patients with liver disease. Lancet. 1980;316:615–617.
- Nagai S, Abouljoud MS, Kazimi M, et al. Peritransplant lymphopenia is a novel prognostic factor in recurrence of hepatocellular carcinoma after liver transplantation. Transplantation. 2014;97:694–701.
- Unitt E, Marshall A, Gelson W, et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006;45:246–253.
- Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890–897.
- Cheung YS, Chan HL, Wong J, et al. Elevated perioperative transaminase level predicts intrahepatic recurrence in hepatitis B-related hepatocellular carcinoma after curative hepatectomy. Asian J Surg. 2008;31:41–49.
- Tarao K, Rino Y, Takemiya S, et al. Serum alanine aminotransferase levels and survival after hepatectomy in patients with hepatocellular carcinoma and hepatitis C virus‐associated liver cirrhosis. Cancer Sci. 2003;94:1083–1090.
- Silva IS, Ferraz MLC, Perez RM, et al. Role of γ‐glutamyl transferase activity in patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2004;19:314–318.
- Shin W, Park S, Jang M, et al. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Digestive Liver Dis. 2008;40:267–274.
- Kao WY, Chiou YY, Hung HH, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: the clinical implication of aspartate aminotransferase–platelet ratio index. Eur J Gastroenterol Hepatol. 2011;23:528–536.
- Mihm S, Monazahian M, Grethe S, et al. Ratio of serum γ-GT/ALT rather than ISDR variability is predictive for initial virological response to IFN‐α in chronic HCV infection. J Med Virol. 1999;58:227–234.
- Tarantino G, Sorrentino P, Conca P, et al. Low daily dosage of interferon for 1 year after HCV-related end-therapy response. A randomized-controlled study. Liver Int. 2003;23:413–419.
- Henderson J, Sherman M, Tavill A, et al. AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. HPB. 2003;5:243–250.
- Huang J, Xu L, Luo Y, et al. The inflammation-based scores to predict prognosis of patients with hepatocellular carcinoma after hepatectomy. Med Oncol. 2014;31:1–8.
- Wang G-Y, Yang Y, Li H, et al. A scoring model based on neutrophil to lymphocyte ratio predicts recurrence of HBV-associated hepatocellular carcinoma after liver transplantation. PLoS One. 2011;6:e25295.