Abstract
The incidence of investigator diagnosed myocardial infarction (MI) is greater in patients treated with haemoglobin-based oxygen carriers (HBOCs) than controls. Clinical trials and literature pertaining to possible HBOC toxicity mechanisms have been analyzed in order to identify possible reasons for this imbalance. MI diagnosis is hampered by potential interference of troponin assays by haemoglobin, haemolysis and bilirubin. Nevertheless, insofar as the reported incidence correlates with actual occurrence, there is a positive relationship between MI and HBOC dose and size. Preclinical and clinical data suggest that direct cardiac toxicity and coronary vasoconstriction are unlikely. More probable are detrimental intravascular interactions between HBOCs and components of the coagulation cascade, particularly dysfunctional endothelium. Elucidation of mechanisms is impeded by a lack of clinical data. Measurement of relevant biomarkers would be extremely useful in this regard and in improving patient selection criteria. Conduct of clinical trials in carefully selected patient populations after the development of improved protocols for MI diagnosis, along with concomitant biomarker data collection, is recommended.
Introduction
In later stage clinical trials, the incidence of investigator reported myocardial infarction is approximately three-fold higher in patients treated with haemoglobin-based oxygen carriers (HBOC) than corresponding control groups (), raising concern about the safety of this class of therapeutics [Citation1]. While several lines of experimental inquiry bear on the interpretation of these results, this literature is disparate and has not heretofore been reviewed holistically. This publication summarizes and evaluates pertinent clinical and preclinical observations.
Table 1. Incidence of myocardial infarction in phase II and III clinical trials of HBOCs and potential correlating parametersTable Footnotea.
Summary of clinical data
Results are compiled for five different HBOC formulations that have undergone extensive clinical testing in Europe, the US, and/or Canada (). References evaluated include all of those cited by Natanson [Citation1] and several subsequent publications [Citation2–6]. Four of the five formulations are derived from human haemoglobin, while the fifth is bovine sourced [Citation7]. One of the preparations (Hemospan®) is derivatized with activated polyethylene glycol (PEG), while the other four (HemAssist™, Hemolink™, Hemopure®, and Polyheme™) are crosslinked and/or polymerized with low molecular weight reagents [Citation7]. The average molecular weights (AMW) are taken from published values [Citation8–11] except for Polyheme. While the AMW for Polyheme was reported as 150 kD, with a molecular weight range up to 400 kD [Citation12], these values were taken prior to implementation of process changes to further reduce tetrameric haemoglobin components [Citation13]. Given that residual tetramer levels in Polyheme are considerably less than those in Hemopure (1% versus 3%) [Citation11,Citation13], the AMW of Polyheme formulations evaluated clinically was probably greater than that of Hempure (250 kD) but less than the upper range previously reported (400 kD). Therefore, the average molecular weight of Polyheme is taken to be 300 kD. Overall average doses are based on weighted averages of the doses given in multiple trials. When doses were not reported on a g/kg basis, an average patient weight of 70 kg was assumed. The average radius of gyration (Rg) was taken as that reported for HemAssist, Hemolink, and Hemospan [Citation8,Citation10]. Values for Hemopure and Polyheme were estimated using the following assumptions: (1) the average value would be that for a polymer consisting of the number of tetrameric haemoglobin subunits which most closely approximates the average molecular weight, (2) Rg would fall somewhere between the values calculated assuming that the polymer is a perfectly random coil or a rigid extended rod [Citation14], (3) the average value for Hemopure and Polyheme would fall between these two values in a similar ratio as that measured for Hemolink. Under these assumptions, the average polymer unit number was taken to be three for Hemolink, four for Hemopure, and five for Polyheme. The measured value for Rg for Hemolink (4.9 nm) falls approximately three-quarters of the way between the values calculated for the random coil (4.0 nm) and rigid rod (5.2 nm) assumptions; therefore, this same ratio was utilized in estimating Rg for Hemopure and Polyheme.
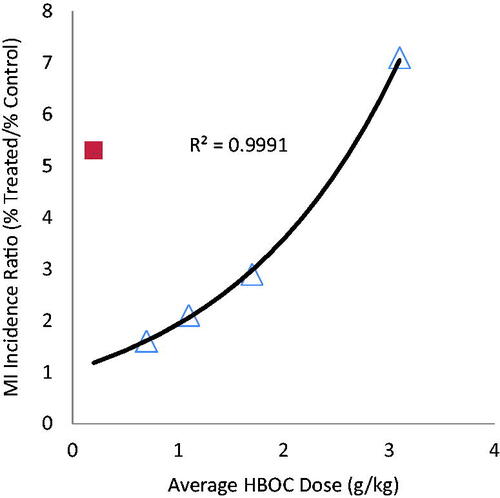
For the four HBOCs prepared by crosslinking and/or polymerization, there is a significant positive correlation (R2 = .9991 for a monoexponential function) between average dose and the ratio of incidence of myocardial infarction (MI) between treated versus control patients (). Hemospan does not exhibit the dose dependency exhibited by the other HBOCs, which is not surprising given the different physical-chemical characteristics conferred upon haemoglobins derivatized with activated polyethylene glycols [Citation15]. PEG modification increases the molecular hydrodynamic radius more than increasing the molecular weight to a comparable extent by polymerization [Citation8], and when used to modify uncrosslinked haemoglobins, PEG increases the extent of tetramer dissociation [Citation16]. Both of these differences could impact toxicity mechanisms.
Figure 1. Relative incidence of myocardial infarction as a function of average HBOC dose: crosslinked and/or polymerized HBOCs (Δ) and PEG modified HBOC (■).
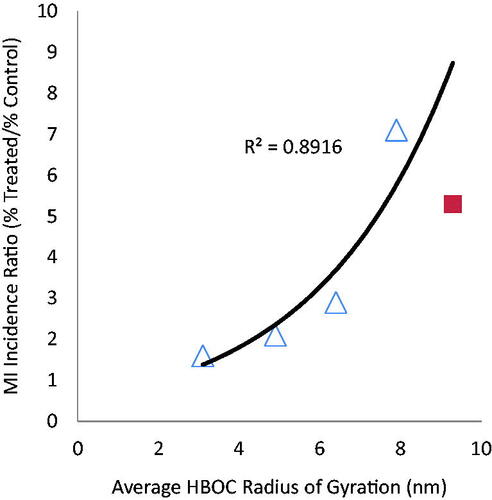
There is also a significant positive correlation (R2 = .8916 for the four crosslinked/polymerized HBOCs; R2 = .8436 including Hemospan) between molecular size as reflected in Rg and MI (). A higher incidence of MI after treatment with higher molecular weight HBOCs was previously noted [Citation17]. The fact that Hemospan falls much closer to the functional dependence between size and MI incidence for the crosslinked/polymerized HBOCs is interesting because it may be argued that the size dependency for the latter is only apparent due to the fact that the larger HBOCs were administered at higher doses. However, Hemospan, which was administered at lower doses than any of the other HBOCs, has the largest radius of gyration and the second highest relative incidence of MI, suggesting that molecular size may indeed be an independent risk factor. The positive correlations among MI, HBOC dose, and HBOC size suggest an intravascular aetiology, since a greater exposure of the endothelium and blood components to any physical or biochemical actions of HBOCs would be expected for increasing intravenous doses and increased intravascular persistence. The latter is positively correlated to HBOC size [Citation18].
MI diagnosis
The diagnosis of acute MI is based on the consideration of patient symptoms, electrocardiographic (ECG) examination and assessment of changes in biomarker proteins [Citation19]. Cardiac-specific troponins (cTnI and cTnT) are biomarkers of choice due to their high sensitivity and specificity for cardiac tissue injury [Citation20]. No mechanism is known whereby HBOCs might interfere with ECG determinations, but troponin assay interferences due to haemolysis, haemoglobin and the primary haemoglobin metabolite bilirubin are recognized [Citation21–23]. Positive, negative and neutral biases due to HBOCs have been reported, but possible concomitant effects of haemolysis and bilirubin have not been discussed [Citation24–26]. Furthermore, detecting haemolysis in the presence of HBOCs is problematic, and interference by haemolysis is not necessarily explicable solely by the presence of haemoglobin [Citation23]. Typical cutoff values for the exclusion of troponin results due to haemolysis (0.04 g/dL) are far below the plasma haemoglobin concentrations achieved after HBOC treatment [Citation21]. Accounting for possible bias due to bilirubin is also challenging, because bilirubin assay is subject to interference by haemoglobin to the extent that it is customarily not quantitated [Citation21,Citation22]. Even in the absence of haemoglobin interference, up to 20-fold variation in the absolute cTnI concentration was observed when comparing assays from different manufacturers [Citation27].
Further complicating the interpretation of plasma troponin elevations is the fact that these may be influenced by a myriad of cardiac conditions other than MI, including trauma, heart failure, and myocarditis, as well as conditions such as renal failure, sepsis, and even heavy exercise [Citation19,Citation20]. Thus, assignation of modest increases in troponin levels to myocardial infarction after HBOC infusion is not straightforward, particularly in the absence of corroborating indicators [Citation19,Citation20]. This has led to valid debate about the meaning of low-level increases in plasma troponin levels after HBOC administration [Citation28], but due to the aforementioned uncertainties, it is unclear whether the situation is better or worse than the reported troponin values suggest.
Potential mechanisms by which HBOCs may cause MI
In their analysis of mortality and MI after HBOC infusion, Natanson et al. speculated that HBOCs may cause vascular thrombosis of the heart due to “systemic vasoconstriction, decreased blood flow, increased release of proinflammatory mediators and potent vasoconstrictors, and a loss of platelet inactivation” [Citation1]. This argument conflates several toxicity hypotheses, with an emphasis on vasoconstriction and the generation of thrombosis. These hypotheses are discussed in the following.
Vasoconstriction
The ability of at least some HBOCs to increase systemic blood pressure and overall vascular resistance is well established and has been recently reviewed [Citation29]. The specific hypothesis that HBOCs cause coronary vasoconstriction originates from isolated rodent heart experiments demonstrating that haemoglobin perfusion increases coronary perfusion pressure [Citation30,Citation31]. This increase was diminished in more highly purified and/or modified preparations [Citation31,Citation32]. However, in these experiments, hearts are subjected to traumatic manipulation and disengaged from in vivo regulatory pathways.
In contrast, in vivo coronary blood flows are maintained or even increased in rats [Citation33], dogs [Citation34,Citation35], cats [Citation36] and swine [Citation37–39] after infusion of various HBOC preparations. Moreover, cardiac function was well supported after exchange transfusion [Citation34,Citation39], haemorrhagic shock resuscitation [Citation38], angioplasty [Citation37] and myocardial ischemia/reperfusion protocols [Citation35]. In two small studies conducted in human patients with heart disease, coronary blood flow and artery diameter were unaffected by peripheral or intracoronary perfusion of HBOC-201 [Citation40,Citation41]. Collectively, these experiments suggest that coronary vasoconstriction after HBOC infusion is uncommon. Results from human patients with cardiac disease are particularly encouraging; however, given that MI has been observed in only a few percent of HBOC treated patients, and cardiac blood flow after haemoglobin administration has been measured in only 42 patients, the results do not exclude the possibility that a minority population may exist who are susceptible to haemoglobin induced coronary vasoconstriction. In this regard, it is notable that Yu et al. showed that PolyHeme infusion caused systemic vasoconstriction in mice with endothelial dysfunction, but not in normal, healthy animals [Citation42]. Biro has argued forcefully that vascular responses to HBOCs in the presence of endothelial dysfunction may be very different than responses observed in the presence of normal endothelium [Citation43]. Thus, any general conclusions should be tempered with the realization that a minority of patients may respond differently. On the other hand, the lack of coronary vasoconstriction as a primary mechanism is consistent with the observed size dependency, since a higher incidence was observed with larger HBOCs which are less vasoactive [Citation44].
Procoagulant stimulus
A logical mechanism for any putative risk factor for MI is the promotion of thrombosis, which suggests consideration of the interactions of HBOCs with platelets, plasma coagulation factors, and endothelium. In this context, it must be recognized that the large doses of HBOCs utilized clinically results in significant dilution of coagulation factors. Ekseth et al. [Citation45] demonstrated that dilution of human blood samples with common intravenous solutions may enhance coagulation at low and medium doses, presumably because of differing effects of dilution on various pro- and anti-coagulant factors. At higher dilutions, coagulation returns to normal or is impaired. Thus, any other effects of HBOCs will be manifested against this complex dilutional background.
HBOC effects on platelets
In vitro experiments suggest that mixing human-based HBOCs with whole blood does not induce platelet aggregation or activation [Citation46,Citation47], but may enhance the response to certain agonists [Citation48]. In contrast, bovine-based HBOCs may actually impair clot formation [Citation49]. Ex vivo studies with blood after HBOC infusion into normal animals suggest that platelets are not activated and respond normally to agonists [Citation50], but in rodent models of endothelial injury or thrombosis, human-based HBOCs caused a modest increase in platelet deposition [Citation51] or more rapid thrombus occlusion [Citation48]. On the other hand, in a rabbit model of arterial thrombosis and bleeding, Hemopure infusion resulted in a reduced rate of thrombosis compared to saline-infused animals [Citation52]. Hemopure resuscitation also caused a mild impairment in clotting times in a swine model of haemorrhagic shock [Citation53].
In human clinical trials with HemAssist, Hemospan, or Hemlink, no differences in platelet count were observed between treated and control patients and/or normal values in normal volunteers [Citation26,Citation54,Citation55], haemorrhagic shock patients [Citation56], or surgical patients [Citation2,Citation3,Citation57]. However, while platelet counts were not changed in a Phase I study of Hemopure in normal volunteers [Citation58], a detailed analysis of a subset of surgical patients in a Phase III trial suggested those treated with this HBOC exhibited a slight increase in platelet clot closure times [Citation59]. Thus, platelet count data collected during human clinical trials of HBOCs do not suggest that this class of products is prothrombotic; however, localized effects would not necessarily be apparent, especially if they only occur in a small fraction of patients with other risk factors. Additional, and more nuanced, assessments will be needed to clarify the effect of HBOCs on platelet function in vivo.
HBOC interaction with plasma coagulation factors
Diaspirin cross-linked haemoglobin (DCLHb), the HBOC utilized in HemAssist, admixed in various proportions with human plasma did not alter the activated partial thromboplastin time (aPTT), prothrombin time (PT), or fibrinogen concentrations compared to buffer control solutions [Citation60]. Only a modest dilutional coagulopathy was observed in haemorrhaged swine after resuscitation with Hemopure [Citation53]. In Phase I normal human volunteer studies, coagulation factors remained within the normal range after the infusion of low doses of HemAssist [Citation54], Hemolink [Citation55], and Hemospan [Citation26]. In a Phase I study of Hemopure in which subjects were phlebotomized prior to test article infusion, there was a decrease in fibrinogen concentrations attributed to haemodilution, but no changes in PT or aPTT [Citation58]. In trials with patients in haemorrhagic shock, the incidence of coagulopathy, aberrant aPTT, or PT, was not significantly different between those receiving HemAssist and control patients [Citation56]. In surgical patients, aPTT and PT were not significantly different from pre-infusion values after HemAssist treatment [Citation57]. In patients haemodiluted with Hemopure or hydroxyethyl starch (HES) before liver resection, there were comparable decreases in fibrinogen levels and increases in aPTT, and these parameters returned to baseline levels within one week [Citation61]. These changes were ascribed to the haemodilution protocol and procedural blood loss. Finally, single unit infusions of Hemopure into surgical patients did not result in different fibrinogen levels, or aPTT or PT values compared to controls [Citation62]. Thus, data collected on the functionality of plasma coagulation factors as part of human clinical trials suggests that the predominant changes, if any, are due to haemodilution with no evidence of prothrombotic tendencies, insofar as these can be determined by standard hematologic ex vivo measurements.
Haemoglobin and endothelium
Most experiments investigating the effects of haemoglobins on endothelium have utilized cultured cells and shown that haemoglobins may cause differing degrees of endothelial cell activation and mortality. These effects vary as a function of haemoglobin modification, oxidation state, and the presence of other stressors. For example, Simoni et al. reported the enhanced expression of procoagulant adhesion molecules and oxidative and inflammatory stress in human endothelial cells after exposure to unmodified and glutaraldehyde-polymerized bovine haemoglobin preparations, but not haemoglobin modified with adenosine triphosphate and adenosine [Citation63]. Motterlini et al. found porcine aortic cell injury was increased after six hours of incubation with crosslinked human haemoglobin compared to unmodified human haemoglobin, a difference the authors attributed to the higher rate of oxidation of the former [Citation64]. In contrast, incubation of bovine aorta endothelial cells (BAECs) with unmodified, crosslinked or crosslinked and polymerized human haemoglobins for six hours did not cause significant cytotoxicity, nor did they enhance the cytotoxicity of low concentrations (≤0.2 mM) of hydrogen peroxide [Citation65]. At higher peroxide concentrations, 200 µM unmodified haemoglobin significantly inhibited cell death, but the two modified haemoglobins did not. Subsequent work demonstrated that the presence of crosslinked haemoglobin could exacerbate cytotoxicity due to hypoxia, oxidative stress, endotoxin, and combinations thereof [Citation66]. D’Agnillo showed that incubation of BAECs with lipopolysaccharide (LPS; endotoxin) alone or in combination with purified haemoglobin solutions did not induce apoptosis in the absence of serum; in the presence of serum, apoptosis was observed to a similar extent upon exposure to LPS with or without the haemoglobins [Citation67]. However, the addition of a peroxide flux resulted in enhanced apoptosis in the presence of haemoglobin. Belcher et al. reported that exposure to methaemoglobin or haeme resulted in rapid expression of P-selectin and von Willebrand factor (VWF) on cultured human endothelial cells [Citation68]. Furthermore, these authors demonstrated that haemoglobin and haeme infusion into sickle, but not normal, mice increased vaso-occlusion, an effect which was mediated by the uptake of haeme by TLR4 receptors. It was hypothesized that endothelial toxicity is due to haeme released from oxidized haemoglobins and subsequent uptake into cell membranes and low-density lipoproteins (LDL) exacerbating oxidant damage by enzymatically or leukocyte-derived reactive oxygen species (ROS) or cytotoxic LDL [Citation69]. Of particular relevance to the causation of MI is the demonstration that haemoglobin can engage in a synergistic oxidation with lipids from human atherosclerotic lesions which catalyzes red cell lysis and further mutual oxidation [Citation70]. In turn, these oxidized lipids are more cytotoxic to endothelial cell monolayers. Collectively, these results suggest that, in the presence of combined inflammatory and oxidative insults, certain haemoglobins may increase endothelial activation and cell mortality.
On the other hand, while haemoglobins may exacerbate the effects of LPS during simultaneous exposure, Cheng et al. found that lung microvascular endothelial cells preincubated with Polyheme and subsequently with LPS exhibited lower adhesion factor expression than cells preincubated with media alone, an effect attributed to the induction of haeme oxygenase (HO) [Citation71]. Transfection of rabbit coronary endothelial cells with an HO gene to increase HO activity resulted in greater cell survival after haeme or haemoglobin exposure [Citation72]. The first reported case of a human patient with HO deficiency presented with coagulation abnormalities which were ascribed to persistent endothelial damage [Citation73]. Therefore, an important question is the degree to which HO induction mitigates any adverse effects of HBOC exposure and how rapidly such mitigation begins after HBOC infusion.
Haemoglobin and VWF
Dysfunctional endothelium secretes large quantities of strongly prothrombotic ultralarge von Willebrand factor (UL-VWF) [Citation74] which is normally cleaved into smaller, less active forms by the metalloprotease, ADAMTS13 [Citation74]. This protease is inhibited by haemoglobin [Citation75]. Failure to cleave UL-VWF by missing or defective ADAMTS13 is a primary mechanism for thrombotic thrombocytopenic purpura, a condition associated with a high incidence of MI and stroke [Citation75]. Elevated plasma haemoglobin levels in patients with sickle cell anaemia correlate with higher levels of UL-VWF and an elevated sub-population of hyperactive VWF multimers in the circulation to which haemoglobin is bound [Citation76]. One significant unknown is the degree to which HBOCs can mimic the inhibitory action of unmodified haemoglobin on UL-VWF proteolysis and the degree that HBOCs may associate with VWF. Haemoglobin may also inhibit an alternative pathway for VWF breakdown that involves the reduction of the internal disulphide bonds linking the VWF subunits by creating a more oxidizing environment [Citation77]. Oxidative stress also increases the exocytosis of VWF from the Weibel–Palade bodies of cultured endothelial cells [Citation78]. Thus, there are several potential mechanisms by which exposure to HBOCs may increase the concentration of more thrombotic VWF species.
Direct myocyte toxicity
Only a modest number of studies have examined the direct effects of haemoglobins on myocyte viability. Walter and Chang reported no effects on cellular morphology in a spontaneously contracting rat ventricle cell culture after exposure to unmodified or polymerized bovine haemoglobin solutions [Citation79], but Feola et al. noted adverse effects on heart histopathology by haemoglobin solutions contaminated with stroma or endotoxin [Citation80]. During the preclinical testing of DCLHb, small areas of inflammation and necrosis were noted in the hearts of certain primates and swine, but not dogs, rats or sheep [Citation81]. These findings were limited in extent and resolved after several days, even after extremely high repetitive DCLHb doses. Cardiac function was not affected. Nevertheless, given the concern associated with any cardiac finding, extensive investigations were performed to characterize this phenomenon and explore potential mechanisms. Elimination of the blood pressure increase consistently observed after DCLHb infusion had no discernible effect on these findings, nor were they attributable to inflammation, coagulation system disturbances or oxidative stress. Furthermore, no thrombosis or infarction was detected after DCLHb administration. Lesion incidence and severity were reduced by polymerization [Citation81], consistent with reports that such findings have not been observed after the infusion of larger HBOCs [Citation82,Citation83]. In the context of MI, it is interesting to note that the correlation between molecular size and MI incidence in human clinical trials is opposite to that observed with the formation of these microscopic findings. Unless humans are substantially more sensitive to the formation of these microscopic lesions than the most sensitive preclinical species identified to date, it is unlikely that this pathway is a cause of MI.
In fact, HBOCs have been salutary when used to treat myocardial ischemia. In a swine angioplasty model, DCLHb infusion mitigated the deterioration of cardiac function resulting from balloon inflation [Citation37]. Similar observations were made after Hemopure infusion into dogs during coronary artery occlusion or stenosis [Citation35,Citation84], pigs during angioplasty balloon inflation [Citation85], and a small clinical study with humans [Citation41]. Several anecdotal clinical observations are also positive. Polyheme infusion supported good hemodynamic stability with no evidence of myocardial ischemia during a 60% blood volume replacement in an already anaemic patient [Citation86], and there was a rapid reversal of myocardial ischemia due to anaemia in a high-risk surgical patient treated with Hemopure [Citation87]. This same product reduced heart rate, normalized base excess and restored blood pH in a patient who refused blood transfusions despite suffering from severe ischemia [Citation88]. In addition, Hemopure administration reversed electrocardiogram abnormalities and decreased troponin levels in another patient who refused blood transfusions despite severe traumatic injuries and anaemia [Citation89]. Thus, HBOCs are effective at ameliorating cardiac ischemia in both animal models and human patients.
Discussion
Ascertaining the cause of a serious adverse event with a frequency of a few percent in studies which at most enrol only a few hundred patients is statistically and mechanistically challenging. Given the variability in dosing, patient populations, protocol design, and manner in which these parameters have been reported, the analyses presented in the foregoing should be considered as hypothesis generating rather than definitive. Another complicating factor is the diagnostic veracity of troponin is in question because of possible interference by HBOCs, HBOC metabolites, and haemolysis. Nevertheless, insofar as the published data correlate with the actual incidence of MI, several interesting trends are suggested.
The differential incidence of MI in patients treated with an HBOC relative to controls was statistically significant in only one individual study [Citation6]. To increase the statistical power of analysis, Natanson et al. performed a meta-analysis combining the results of different studies [Citation1]. The validity of this approach hinges on whether the different formulations are similar enough in key biochemical and physiological characteristics to justify the pooling of data. While this assumption has been vigorously contested [Citation90], there is enough commonality in protein composition and haeme chemistry to suggest these preparations may share one or more common mechanisms of toxicity. As Biro has noted in his thoughtful commentary on HBOC clinical trials, ‘It appears that with some variation the majority of adverse events reported are class effects, rather than individual and idiosyncratic effects specific to various products’ [Citation91]. It is also likely that HBOCs act by exacerbating the effects of other co-morbidities, such as inflammation, endothelial dysfunction, ischemia/reperfusion, depletion of antioxidant defences, and the presence of endotoxin. This hypothesis is consistent with the fact that MI has only been observed in a few percent of human patients and has not been observed in published preclinical HBOC studies, with the caveat that the latter have almost exclusively been performed in younger, healthy animals subjected to one or two acute insults. Indeed, a number of preclinical studies have demonstrated that HBOCs are quite effective at reducing tissue damage in models of MI and stroke. Thus, it may be the case that two somewhat seemingly conflicting realities are simultaneously true, namely, that HBOCs are quite effective in the support of cardiac function and the perfusion of ischemic tissue, while at the same time increasing the risk of thrombosis in certain at-risk patients.
Ironically, the most frequently proposed toxicity mechanism, coronary vasoconstriction, has the least evidentiary support. Multiple studies have demonstrated that HBOC formulations do not cause coronary artery constriction in vivo, even in human patients with coronary artery disease. This is consistent with the observation that the incidence of MI is higher in patients treated with higher molecular weight (and size) HBOCs, since the latter are generally less vasoactive than the smaller stabilized tetrameric formulations. While these observations do not eliminate the possibility that a small subset of patients is susceptible to such vasoconstriction, the preponderance of evidence suggests that, if HBOCs are indeed a risk factor for MI, the mechanism is more likely to derive from intravascular interactions. Such interactions are consistent with the higher incidence of MI observed in patients treated with higher molecular weight HBOCs, since these formulations persist in the circulation for a longer period of time and, therefore, increase the exposure of the endothelium, plasma coagulation factors and blood formed elements to the various chemistries catalyzed by haemoglobins. The correlation between molecular weight and MI incidence also argues against the type of direct cardiac toxicity observed with lower molecular weight HBOCs (e.g. HemAssist), since this finding is significantly diminished by polymerization.
Evaluation of putative toxicity mechanisms is hampered by a lack of human data. While a few observations have been obtained on the effect of HBOCs on coagulation factors and inflammatory mediators, more are needed, and there are essentially no published data on the effects of HBOC treatment on patient redox status. ROS are implicated in multiple aspects of tissue necrosis, reperfusion injury, and heart failure when antioxidant defences are overwhelmed [Citation92]. There are also complex effects exerted by nitric oxide (NO) on cardiac contractility and interactions between ROS and NO [Citation92]. Both ROS and reactive nitrogen species (RNS) derived from NO are important in the regulation of normal cardiac function, but in excess, both can be detrimental [Citation93]. It is also pertinent that haemoglobin can act as an antioxidant, exhibiting both peroxidase and superoxide scavenging activities [Citation65,Citation94]. Many other oxidant stress defences are present as well [Citation95]. Since HBOCs are involved in multiple interactions with oxygen, ROS and NO, both beneficial and detrimental [Citation96], it would not be surprising that the risk/benefit ratio of HBOC treatment with respect to cardiac function and viability may vary significantly depending on patients’-specific nitroso-redox balance and endothelial health. Obtaining additional information on patients’ inflammatory mediator, coagulation, and redox status should not only shed light on potential toxicity mechanisms but also enable the development of better inclusion/exclusion criteria to identify patients that can most benefit from HBOC treatment. Clinical results already suggest that certain patient subpopulations (e.g. elderly) are at greater relative risk of serious adverse events after HBOC treatment [Citation28], which probably reflects the higher incidence of inflammatory and redox stressors and dysfunctional endothelium in these populations. Obviously, identification of more specific biomarkers would enable better discrimination between those that should and should not be treated with HBOCs.
In summary, the reason for the observed imbalance in MI incidence between HBOC-treated and control patients is probably multifactorial. Clearly, further elucidation of the effects of HBOCs on troponin assays, troponin clearance, and mediators of coagulation, inflammation and oxidative stress are needed. However, on the basis of the preclinical and clinical experience to date, it is likely that in some patients the combination of HBOC infusions with concomitant risk factors will increase the chances of myocardial infarction. After all, few drugs and biologics are without side effects and HBOCs are unlikely to be the exception. On the other hand, extensive preclinical and clinical experience suggests that there are also patients who can benefit from HBOC treatment, and, if past learnings are heeded, future HBOC clinical trials could be designed and conducted at much lower risk to patients. Indeed, several authors have already proposed populations in which such trials would be appropriate [Citation97]. Most productive would be a dual approach wherein thoughtful clinical testing would be conducted in those populations for whom the risk of MI is likely to be low, while simultaneously collecting data that would clarify important side effect mechanisms.
Disclosure statement
The author is a consultant for Omniox, Inc., an early stage biopharmaceutical company developing new medicines for hypoxic diseases. No support was received from Omniox for the production of this manuscript.
References
- Natanson C, Kern SJ, Lurie P, et al. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;299:2303–2312.
- Olofsson C, Nygårds EB, Ponzer S, et al. A randomized, single-blind, increasing dose safety trial of an oxygen-carrying plasma expander (Hemospan®) administered to orthopedic surgery patients with spinal anaesthesia. Transfus Med. 2008;18:28–39.
- Olofsson CI, Górecki AZ, Dirksen R, et al. Evaluation of MP4OX for prevention of perioperative hypotension in patients undergoing primary hip arthroplasty with spinal anesthesia: a randomized, double-blind, multicenter study. Anesthesiology. 2011;114:1048–1063.
- Jahr JS, Mackenzie C, Pearce LB, et al. HBOC-201 as an alternative to blood transfusion: efficacy and safety evaluation in a multicenter phase III trial in elective orthopedic surgery. J Trauma. 2008;64:1484–1497.
- Hemelrijck JV, Levien LJ, Veeckman L, et al. A safety and efficacy evaluation of hemoglobin-based oxygen carrier HBOC-201 in a randomized, multicenter red blood cell controlled trial in noncardiac surgery patients. Anesth Analg. 2014;119:766–776.
- Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13.
- Silverman TA, Weiskopf RB. Hemoglobin-based oxygen carriers: current status and future directions. Transfusion. 2009;49:2495–2515.
- Vandegriff KD, McCarthy M, Rohlfs RJ, et al. Colloid osmotic properties of modified hemoglobins: chemically cross-linked versus polyethylene glycol surface-conjugated. Biophys Chem. 1997;69:23–30.
- Nelson DJ. Hemassist™: development and clinical profile. In: Rudolph AS, Rabinovici R, Feuerstein GZ, editors. Red blood cell substitutes basic principles and clinical applications. New York(NY): Marcel Dekker; 1998. p. 353–400.
- Vandegriff KD, Malavalli A, Wooldridge J, et al. MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion. 2003;43:509–516.
- Dubé GP, Vranckx P, Greenburg AG. HBOC-201: the multipurpose oxygen therapeutic. EuroIntervention. 2008;4:161–165.
- Gould SA, Sehgal LR, Sehgal HL, et al. The development of hemoglobin solutions as red cell substitutes: hemoglobin solutions. Transfus Sci. 1995;16:5–17.
- Gould SA, Moore EE, Hoyt DB, et al. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J Am Coll Surg. 1998;187:113–122.
- Flory PJ. Statistical mechanics of chain molecules. New York(NY): Wiley (Interscience); 1969.
- Talarico TL, Guise KJ, Stacey CJ. Chemical characterization of pyridoxalated hemoglobin polyoxyethylene conjugate. Biochim Biophys Acta. 2000;1476:53–65.
- Caccia D, Ronda L, Frassi R, et al. PEGylation promotes hemoglobin tetramer dissociation. Bioconjug Chem. 2009;20:1356–1366.
- Biopure. Scientific commentary on the Natanson et al. meta-analysis. [Online] 2008 [cited 2008 October 16]; Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1807-59322009000800016.
- Estep TN. Pharmacokinetics and mechanisms of plasma removal of hemoglobin-based oxygen carriers. Artif Cells Nanomed Biotechnol. 2015;43:203–215.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567.
- Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. Cmaj. 2005;173:1191–1202.
- Dasgupta A, Wells A, Biddle DA. Negative interference of bilirubin and hemoglobin in the MEIA troponin I assay but not in the MEIA ck-mb assay. J Clin Lab Anal. 2001;15:76–80.
- Wolthuis A, Peek D, Scholten R, et al. Effect of the hemoglobin-based oxygen carrier HBOC-201 on laboratory instrumentation: Cobas Integra, Chiron Blood Gas Analyzer 840, Sysmex SE-9000 and BCT. Clin Chem Lab Med. 1999;37:71–76.
- Sodi R, Darn SM, Davison AS, et al. Mechanism of interference by haemolysis in the cardiac troponin T immunoassay. Ann Clin Biochem. 2006;43:49–56.
- Ma Z, Monk TG, Goodnough LT, et al. Effect of hemoglobin- and perfluorbron-based oxygen carriers on common clinical laboratory tests. Clin Chem. 1997;43:1732–1737.
- Cameron SJ, Gerhardt G, Engelstad M, et al. Interference in clinical chemistry assays by the hemoglobin-based oxygen carrier, Hemospan. Clin Biochem. 2009;42:221–224.
- Björkholm M, Fagrell B, Przybelski R, et al. A phase I single blind clinical trial of a new oxygen transport agent (MP4), human hemoglobin modified with maleimide-activated polyethylene glycol. Haematologica. 2005;90:505–515.
- Wu AHB, Feng YJ, Moore R, et al. Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. Clin Chem. 1998;44:1198–1208.
- Freilich D, Pearce LB, Pitman A, et al. HBOC-201 vasoactivity in a phase III clinical trial in orthopedic surgery subjects-extrapolation of potential risk for acute trauma trials. J Trauma. 2009;66:365–376.
- Kim HW. Acellular hemoglobin-based oxygen carrier mediated blood pressure elevation and vasoconstriction: a review of proposed mechanisms and contributing factors. In: Kim HW, Greenburg AG, editors. Hemoglobin-based oxygen carriers as red cell substitutes and oxygen carriers. Heidelberg: Springer; 2013. p. 587–620.
- Vogel WM, Dennis RC, Cassidy G, et al. Coronary constrictor effect of stroma-free hemoglobin solutions. Am J Physiol. 1986;251:H413–H420.
- Macdonald VW, Winslow RM, Marini MA, et al. Coronary vasoconstrictor activity of purified and modified human hemoglobin. Biomater Artif Cells Artif Organs. 1990;18:263–282.
- Vogel MW, Hsia JC, Briggs LL, et al. Reduced coronary vasoconstrictor activity of hemoglobin solutions purified by ATP-agarose affinity chromatography. Life Sci. 1987;41:89–93.
- Sharma AC, Rebello S, Gulati A. Regional circulatory and systemic hemodynamic effects of diaspirin cross-linked hemoglobin in the rat. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:593–602.
- Kingma JG, Sandhu R, Hamelin ND, et al. The effects of hemodilution with Hemolink™ upon hemodynamics and blood flow distribution in anesthetized dogs. Artif Cells Blood Substit Immobil Biotechnol. 2002;30:137–154.
- Caswell JE, Strange MB, Rimmer DM, et al. A novel hemoglobin-based blood substitute protects against myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2005;288:H1796–HH801.
- Ulatowski JA, Nishikawa T, Matheson-Urbaitis B, et al. Regional blood flow alterations after bovine fumaryl beta beta-crosslinked hemoglobin transfusion and nitric oxide synthase inhibition. Crit Care Med. 1996;24:558–565.
- McKenzie JE, Cost EA, Scandling DM, et al. Effects of diaspirin crosslinked haemoglobin during coronary angioplasty in the swine. Cardiovasc Res. 1994;28:1188–1192.
- Van Iterson M, Siegemund M, Burhop K, et al. Hemoglobin-based oxygen carrier provides heterogeneous microvascular oxygenation in heart and gut after hemorrhage in pigs. J Trauma. 2003;55:1111–1124.
- Mongan PD, Moon-Massat PF, Rentko V, et al. Regional blood flow after serial normovolemic exchange transfusion with HBOC-201 (Hemopure) in anesthetized swine. J Trauma. 2009;67:51–60.
- Serruys PW, Vranckx P, Slagboom T, et al. Haemodynamic effects, safety, and tolerability of haemoglobin-based oxygen carrier-201 in patients undergoing PCI for CAD. EuroIntervention. 2008;3:600–609.
- Meliga E, Vranckx P, Regar E, et al. Proof-of-concept trial to evaluate haemoglobin based oxygen therapeutics in elective percutaneous coronary revascularization. Rationale, protocol design and haemodynamic results. EuroIntervention. 2008;4:99–107.
- Olson JS, Foley EW, Rogge C, et al. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–697.
- Yu B, Shahid M, Egorina EM, et al. Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier. Anesthesiology. 2010;112:586–594.
- Biro GP. Adverse HBOC-endothelial dysfunction synergism: a possible contributor to adverse clinical outcomes? Curr Drug Discov Technol. 2012;9:194–203.
- Ekseth K, Abildgaard L, Vegfors M, et al. The in vitro effects of crystalloids and colloids on coagulation. Anaesthesia. 2002;57:1102–1108.
- Toussaint M, Latger-Cannard V, Caron A, et al. Hemoglobin-based oxygen carriers do not alter platelet functions: study of three chemically modified hemoglobin solutions. Intensive Care Med. 2003;29:62–68.
- Leytin V, Mazer D, Mody M, et al. Hemolink™, an o-raffinose cross-linked haemoglobin-based oxygen carrier, does not affect activation and function of human platelets in whole blood in vitro. Br J Haematol. 2003;120:535–541.
- Lee DH, Bardossy L, Peterson N, et al. o-Raffinose cross-linked hemoglobin improves the hemostatic defect associated with anemia and thrombocytopenia in rabbits. Blood. 2000;96:3630–3636.
- Jahr JS, Weeks DL, Desai P, et al. Does Oxyvita, a new-generation hemoglobin-based oxygen carrier, or Oxyglobin acutely interfere with coagulation compared with normal saline or 6% hetastarch? An ex vivo thromboelastography study. J Cardiothorac Vasc Anesth. 2008;22:34–39.
- Burhop KE, Farrell L, Nigro C, et al. Effects of intravenous infusions of diaspirin cross-linked hemoglobin (DCLHb) on sheep. Biomater Artif Cells Immobilization Biotechnol. 1992;20:581–585.
- Olsen SB, Tang DB, Jackson MR, et al. Enhancement of platelet deposition by cross-linked hemoglobin in a rat carotid endarterectomy model. Circulation. 1996;93:327–332.
- Marret E, Bonnin P, Mazoyer E, et al. The effects of a polymerized bovine-derived hemoglobin solution in a rabbit model of arterial thrombosis and bleeding. Anesth Analg. 2004;98:604–610.
- Arnaud F, Hammett M, Asher L, et al. Effects of bovine polymerized hemoglobin on coagulation in controlled hemorrhagic shock in swine. Shock. 2005;24:145–152.
- Przybelski RJ, Daily EK, Kisicki JC, et al. Phase I study of the safety and pharmacologic effects of diaspirin cross-linked hemoglobin solution. Crit Care Med. 1996;24:1993–2000.
- Carmichael FJ, Ali AC, Campbell JA, et al. A phase I study of oxidized raffinose cross-linked human hemoglobin. Crit Care Med. 2000;28:2283–2292.
- Przybelski RJ, Daily EK, Micheels J, et al. A safety assessment of diaspirin cross-linked hemoglobin (DCLHb) in the treatment of hemorrhagic, hypovolemic shock. Prehosp Disaster Med. 1999;14:251–264.
- Schubert A, Przybelski RJ, Eidt JF, et al. Diaspirin-crosslinked hemoglobin reduces blood transfusion in noncardiac surgery: a multicenter, randomized, controlled, double-blinded trial. Anesth Analg. 2003;97:323–332.
- Hughes GS, Francom SF, Antal EJ, et al. Hematologic effects of a novel hemoglobin-based oxygen carrier in normal male and female subjects. J Lab Clin Med. 1995;126:444–451.
- Jahr JS, Liu H, Albert OK, et al. Does HBOC-201 (Hemopure) affect platelet function in orthopedic surgery: a single-site analysis from a multicenter study. Am J Ther. 2010;17:140–147.
- Burhop KE, Goldberg CM, Balma D, et al. Effects of diaspirin cross-linked hemoglobin (DCLHb) on hematologic variables [abstract]. Blood. 1992;80:221a.
- Standl T, Burmeister MA, Horn EP, et al. Bovine haemoglobin-based oxygen carrier for patients undergoing haemodilution before liver resection. Br J Anaesth. 1998;80:189–194.
- Sprung J, Kindscher JD, Wahr JA, et al. The use of bovine hemoglobin glutamer-250 (Hemopure®) in surgical patients: results of a multicenter, randomized, single-blinded trial. Anesth Analg. 2002;94:799–808.
- Simoni J, Simoni G, Martinez-Zaguilan R, et al. Improved blood substitute evaluation of its effects on human endothelial cells. Asaio J. 1998;44:M356–M367.
- Motterlini R, Foresti R, Vandegriff K, et al. Oxidative-stress response in vascular endothelial cells exposed to acellular hemoglobin solutions. Am J Physiol. 1995;269:H648–H655.
- Goldman DW, Breyer IIIRJ, Yeh D, et al. Acellular hemoglobin-mediated oxidative stress toward endothelium: a role for ferryl iron. Am J Physiol. 1998;275:H1046–H1053.
- D’Agnillo F. Redox activity of cell-free hemoglobin: implications for vascular oxidative stress and endothelial injury. In: Kim HW, Greenburg AG, editors. Hemoglobin-based oxygen carriers as red cell substitutes and oxygen carriers. Heidelberg: Springer; 2013. p. 665–682.
- D’Agnillo F. Redox active hemoglobin enhances lipopolysaccharide-induced injury to cultured bovine endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1875–H1882.
- Belcher JD, Chen C, Nguyen J, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123:377–390.
- Balla J, Vercellotti GM, Jeney V, et al. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 2007;9:2119–2137.
- Nagy E, Eaton JW, Jeney V, et al. Red cells, hemoglobin, heme, iron and atherogenesis. Atvb. 2010;30:1347–1353.
- Cheng AM, Moore EE, Johnson JL, et al. Polymerized hemoglobin induces heme oxygenase-1 protein expression and inhibits intercellular adhesion molecule-1 protein expression in human lung microvascular endothelial cells. J Am Coll Surg. 2005;201:579–584.
- Abraham NG, Lavrovsky Y, Schwartzman ML, et al. Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: protective effect against heme and hemoglobin toxicity. Proc Natl Acad Sci USA. 1995;92:6798–6802.
- Yachie A, Niida Y, Wada T, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135.
- Turne N, Molasco L, Moake J. Generation and breakdown of soluble ultralarge von Willebrand factor multimers. Semin Thromb Hemost. 2012;38:38–46.
- Studt JD, Hovinga JAK, Antoine G, et al. Fatal congenital thrombotic thrombocytopenic purpura with apparent ADAMTS-13 inhibitor: in vitro inhibition of ADAMTS-13 activity by hemoglobin. Blood. 2005;105:542–544.
- Zhou Z, Yee DL, Guchhait P. Molecular link between intravascular hemolysis and vascular occlusion in sickle cell disease. Cvp. 2012;10:756–761.
- Pimanda JE, Ganderton T, Maekawa A, et al. Role of thrombospondin-1 in control of von Willebrand factor multimer size in mice. J Biol Chem. 2004;279:21439–21448.
- Vischer UM, Jornot L, Wollheim CB, et al. Reactive oxygen intermediates induce regulated secretion of von Willebrand factor from cultured human vascular endothelial cells. Blood. 1995;85:3164–3172.
- Walter VS, Chang TMS. Chronotropic effects of in vitro perfusion with albumin, stroma-free hemoglobin, and polyhemoglobin solutions. Biomater Artif Cells Artif Organs. 1990;18:283–298.
- Feola M, Simoni J, Canizaro PC, et al. Toxicity of polymerized hemoglobin solutions. Surg Gynecol Obstet. 1988;166:211–222.
- Burhop K, Gordon D, Estep T. Review of Hemoglobin-induced myocardial lesions. Artif Cells Blood Substit Immobil Biotechnol. 2004;32:353–374.
- Muir WW, Ilangovan G, Zweier JL, et al. Vital organ tissue oxygenation after serial normovolemic exchange transfusion with HBOC-201 in anesthetized swine. Shock. 2011;35:597–603.
- Young MA, Malavalli A, Winslow N, et al. Toxicity and hemodynamic effects after single dose administration of MalPEG-hemoglobin (MP4) in rhesus monkeys. Transl Res. 2007;149:333–342.
- George I, Geng-Hua Y, Schulman AR, et al. A polymerized bovine hemoglobin oxygen carrier preserves regional myocardial function and reduces infarct size after acute myocardial ischemia. Am J Physiol Heart Circ Physiol. 2006;291:H1126–H1137.
- Hekkert MTL, Dubé GP, Regar E, et al. Preoxygenated hemoglobin-based oxygen carrier HBOC-201 annihilates myocardial ischemia during brief coronary artery occlusion in pigs. Am J Physiol Heart Circ Physiol. 2010;298:H1103–H1113.
- Norris EJ, Ness PM, Williams GM. Use of a human polymerized hemoglobin solution as an adjunct to acute normovolemic hemodilution during complex abdominal aortic reconstruction. J Clin Anesth. 2003;15:220–223.
- Niquille M, Touzet M, Leblanc I, et al. Reversal of intraoperative myocardial ischemia with a hemoglobin-based oxygen carrier. Anesthesiology. 2000;92:882–885.
- Mullon J, Giacoppe G, Clagett C, et al. Transfusion of polymerized bovine hemoglobin in a patient with severe autoimmune hemolytic anemia. N Engl J Med. 2000;342:1638–1643.
- Fitzgerald MC, Chan JY, Ross AW, et al. A synthetic haemoglobin-based oxygen carrier and the reversal of cardiac hypoxia secondary to severe anaemia following trauma. Med J Aust. 2011;194:471–473.
- Mackenzie CF. Key adverse events in recent HBOC phase III clinical trials and their causal relationship to test HBOC’s. In: Kim HW, Greenburg AG, editors. Hemoglobin-based oxygen carriers as red cell substitutes and oxygen carriers. Heidelberg: Springer; 2013. p. 527–542.
- Biro GP. Some critical comments on the major HBOC clinical trials. In: Kim HW, Greenburg AG, editors. Hemoglobin-based oxygen carriers as red cell substitutes and oxygen carriers. Heidelberg: Springer; 2013. p. 543–562.
- Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508.
- Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation. 2006;114:1531–1544.
- Gabbianelli R, Santroni AM, Fedeli D, et al. Antioxidant activities of different hemoglobin derivatives. Biochem Biophys Res Commun. 1998;242:560–564.
- Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and disease. Cia. 2018; 13:757–772.
- Estep TN. HBOCs and cardiac integrity. In: Kim HW, Greenburg AG, editors. Hemoglobin-based oxygen carriers as red cell substitutes and oxygen carriers. Heidelberg: Springer; 2013. p. 621–646.
- Weiskopf RB, Silverman TA. Balancing potential risks and benefits of hemoglobin-based oxygen carriers. Transfusion. 2013;53:2327–2333.