?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Hydroxyapatite (HAP) is a significant bone mineral that establishes bone strength. HAP composites in combination with biodegradable and bioactive polymer poly xylitol sebacic adipate (PXSA) would result in a constant release at target sites. Numerous studies have shown that vitamin K (VK) might possess a vital function in bone metabolism. The purpose of the present study was to inspect the synthesized composite HAP/PXSA/VK in developing polymeric biomaterials composite for the application of bone tissue regeneration. FTIR, X-ray diffraction, SEM and TEM techniques were applied to characterize the prepared composites. The release of VK from the HAP/PXSA/VK composite was evidenced through UV–Vis spectroscopy. In vitro studies proved that the HAP/PXSA/VK composite is appropriate for mesenchymal stem cell culture. Compared to pure HAP prepared following the same method, HAP/PXSA/VK composite provided favourable microstructures and good biodegradation distinctiveness for the application of tissue engineering, as well as tissue in-growth characteristics and improved scaffold cell penetration. This work reveals that the HAP/PXSA/VK composites have the potential for applications in bone tissue engineering.
Introduction
Tissue engineering offers a process to renovate and or substitute wounded tissue [Citation1–3]. Huge bone imperfections need reconstructive surgeries as the improvement process is further than the potential of the human’s self-repair mechanism [Citation4,Citation5]. Autografts are currently the golden standard for bone imperfection treatment [Citation6], although the method has limitations such as pain hemorrhage and infection at the contributor site. Due to the shortcoming linked with allografts and autografts, rising fresh approaches for bone rejuvenation is stimulated by pain connected with bone defects.
In spite of the healthy regenerative character of bone, loss of regenerative strength in certain disruptive circumstances is inevitable [Citation7]. One developing approach is the relevance of nano-biomaterials as directed models in facilitating tissue rejuvenation [Citation8,Citation9]. To overcome these problems, research on natural regeneration of bone via tissue engineering approaches has emerged in recent years [Citation10–13].
Hydroxyapatite (HAP) Ca10(PO4)6(OH)2, has fascinated immense attention as orthopedic biomaterial owing to its chemical and structural similarity to bone mineral. HAP has wonderful biocompatibility, fine cell adhesiveness high porosity, more osteoconductivity in the process of bone rejuvenation and admirable biodegradability [Citation14–16]. Hydroxyapatite is one of the mainly admired biomaterials worn in any biomaterial relevance. The attractive mechanism of HAP was accounted that extracellular PO43− could encourage osteogenic differentiation in stem cells through promoting metabolic signaling of ATP [Citation17]. These nanoparticles are intended as release medium causative to the proscribed discharge of bioactive factors and also as the environment on condition that an appropriate microenvironment is created for the cells [Citation18]. In the imminent years, it is apparent that new invention of bone implant materials will be developed based on the natural rejuvenation and characteristic possessions of any multicellular organism [Citation19]. Therefore, innovative and elegant biomaterials that dynamically contribute to the formation of useful tissues are required.
Ecological polymers comprise of a well-liked group of biomaterials that are extensively deliberated for use in various resorbable sutures, implants and other features like tissue scaffolds, breakage fascination devices and drug/gene delivery [Citation20]. Few studies have presented that a topographic substrate is able to mimic an in vivo microenvironment self-possessed of channels, pores, and ridges to support physically prompted cells at a nanoscale level [Citation21,Citation22]. Polyesters are generally favoured among eco-friendly polymers for both drug delivery and rejuvenative drugs due to their countless compensation, in exacting, their hydrolytic cleavage in the hydrophilic environment in vivo [Citation23–25]. The dicarboxylic acids worn in this exertion were sebacic acid, adipic acids that have been exposed to be cytocompatible [Citation26,Citation27].
Numerous reports have demonstrated that the roughness of scaffold surfaces and topographical composition can regulate osteogenesis through cell adhesion on the outer cytoskeletal signaling cascade [Citation28–32]. Particularly, the surface roughness mimics the extracellular matrix properties of tissue at nanoscale level and supports both mesenchymal and osteoblast stem cell attachment and proliferation. Consequently, enhancing the roughness of the scaffold surface is an effective approach towards improving its bioactivity [Citation33].
Vitamin K (VK) is an expression used to describe chemically analogous liposoluble compounds, which are different in their purpose and source. VK is recognized as significantly intended for the standard implementation of blood coagulation, likewise, it is also considered to have a critical function in bone metabolism through regulating the commencement of bone matrix proteins [Citation34]. VK is known to retard bone loss and improve bone strength [Citation35]. VK acts on osteocalcin which is a non-collagenous protein in bone, and also on γ-carboxyglutamic acid. Osteocalcin is assumed to have a connection in bone mineralization, owing to its unambiguous interaction by means of hydroxyapatite vitamin [Citation36–40].
Based on the standard of nanomaterials, we attempted to produce a type of nanocomposite to increase the roughness of the scaffold surface. Among the nanomaterials we used in rejuvenation therapy, nanohydroxyapatite (HAP)/poly xylitol sebacic adibate (PXSA)/VK is the nanocomposite that possesses the highest similarity with the mineral components of natural bone. HAP/PXSA/VK nanocomposite has been used to the extent of bone graft replacement and hence, has the aptitude for tissue engineering applications.
Materials and methods
Materials
Calcium chloride dehydrate (CaCl2.2H2O), adipic acid, ammonium hydroxide (NH4OH), ethyl alcohol (C2H5OH), diammonium hydrogen phosphate ((NH4)2HPO4), sebacic acid, VK, phosphate-buffered saline (PBS) were purchased from Sigma-Aldrich, St. Louis, MO. Analytical grade chemicals and deionized water were used for all experiments.
Preparation
Synthesis of hydroxyapatite (HAP)
HAP was synthesized by dissolving 0.05 M of CaCl2.2H2O in distilled water added with 0.03 M (NH4)2HPO4 in the solution and mixed under magnetic stirring. The pH was maintained at 10.0 using aqueous ammonia. The solution was stirred for 30 min and then transferred to 50 ml Teflon-lined sealed stainless steel autoclave tubes and heated in a muffle furnace for 2 h at 180 °C. The solution was later allowed to cool at room temperature (27 °C) and the precipitate formed was collected, rinsed with ethanol and deionized water through centrifugation at 5000 rpm for 10 min. Finally, the part of the purified product was disseminated in deionized water to form aqueous dispersion and freeze dried. The synthesized HAP was sintered in muffle furnace for 6 h at 600 °C to obtain pure HAP nanoparticles.
Synthesis of HAP/PXSA nanocomposite
Poly-condensation reaction was applied to synthesize PXSA. A prepolymer was formed by stirring xylitol, sebacic acid and adipic acid in a round bottom flask for 1 h at 140 °C under nitrogen gas blanket. The mixture was heated further at 150 °C and condensation of h was continued for another 5 h to obtain the PXSA polymer. The formed product gradually showed increasing viscosity with increased reaction time. The cross-linked elastomer was applied for further characterization. Ester bond formed between monomers of sebacic acid, xylitol and adipic acid was verified through FTIR analysis. Upon confirmation, 10 wt% of PXSA polymer were mixed with pure HAP and heated for ∼10 min in microwave oven (2.5 GHz, LG, India) at 720 W to form HAP/PXSA composite.
Loading of VK on HAP/PXSA composites
VK was dissolved in ethanol solution (5 mg/mL) to load into HAP/PXSA composite. The dissolved VK was added to HAP/PXSA composite, stirred for 30 min using magnetic stirrer and then sonicated for 30 min. The solution was freeze-dried to recover the HAP/PXSA/VK composite.
Physiochemical characterizations
Fourier transform infrared spectroscopy (FTIR) analysis
All the synthesized nanoparticles and composites (HAP, PXSA, HAP/PXSA, HAP/PXSA/VK) were characterized using FTIR spectrometer (Bruker Tensor 27 Series, Bruker Inc., Billerica, MA) in the region of 400–4000 cm−1 with 2 cm−1 resolution and 16 scans. Preparation of test samples was done by grinding 0.2 g of sample powder with 1 g KBr and pressed onto a transparent disc.
X-ray diffraction (XRD) analysis
To investigate the crystallinity and phase composition of the synthesized nanoparticles and composites, XRD analysis was done in a Bruker D8 Advance Diffractometer with monochromatic Cu Kα source operated at 30 mA and 40 kV. A current of 15 mA and an acceleration voltage of 30 kV were used. The analysis was carried out over 2θ range of 10–60° in step scan mode with a size of 0.02° and a scan rate of 0.02°/min.
Scanning electron microscope (SEM) analysis
The microstructure and morphology of synthesized nanoparticles and composites were studied under SEM (VEGA3 TESCAN) equipped with energy dispersive X-ray analysis (EDAX) operating at an accelerating voltage of 10 kV.
High-resolution transmission electron microscope (HRTEM) analysis
The samples for HRTEM analysis were prepared by disseminating the nanoparticles and composites in ethanol and sonicated for 5 min. Consequently, a droplet of the suspension was smeared onto 200 mesh copper-deposited carbon grid and subjected to analysis.
Biodegradability analysis
Scaffold biodegradability was evaluated by placing them in PBS for 28 d at pH 7.4 by maintaining the solid ratio of 0.5 mg/mL at 37 °C with shaking speed of 100 rpm. The buffer solution was refreshed at every 3 d interval till 28th day. The scaffold was dried for 24 h at 60 °C in an oven till constant weight was reached. Degradation percentage was calculated following the equation:
(1)
(1)
Encapsulation efficiency analysis
The efficiency of VK-encapsulation was determined by separating HAP/PXSA through ultracentrifugation for 30 min at 5000 rpm at room temperature from the aqueous medium containing free drug. The concentration of VK was determined through spectrophotometric analysis. The efficiency of encapsulation was calculated following the formula:
(2)
(2)
In vitro release
Dialysis method was applied to estimate the in-vitro release characteristics of HAP/PXSA/VK composites at different time intervals in PBS at pH 7.4. The supernatant of the release medium was replaced with an equal amount of PBS solution and the concentration VK in supernatant solution was determined at the λmax value of 292–295 nm through UV–Vis spectrophotometric analysis.
Culture of MSCs
Human Wharton’s jelly-derived MSCs were cultured in medium containing DMEM with F-12 nutrient mixture (Gibco, Carlsbad, CA) supplemented with 1% penicillin/streptomycin and 10% FBS. The cells were seeded at a density of 2000 cells/cm2 in a 25 cm2 flask. The flask was monitored daily after incubation in a humidified atmosphere containing 5% CO2 at 37 °C. Fresh MSC culture medium was replaced every 3 d. The adherent cells were subjected to differentiation upon reaching confluence, using cells of third to fifth passages.
Preparation of differentiation media
Osteogenic medium OsteoMAX-XF Differentiation Medium (Merck, Kenilworth, NJ) was prepared following the manufacturer’s instructions. Biomaterial HAP, SHC and HS-6 were incorporated to the differentiation medium and diluted with DMSO to 12.5 µg/mL.
In vitro differentiation of MSCs into osteocyte cells
Osteogenic differentiation of cultured adherent cells was carried out in 24-well tissue culture plates. Cells after trypsinization were seeded at a seeding density of 2 × 104 cells/cm2 in 500 µL of DMEM/F12 medium supplemented with 1% penicillin/streptomycin and 10% FBS and cells were cultured in a5% CO2 incubator at 37 °C. Once the cells achieved 90% confluency, osteogenic differentiation of MSCs was performed by differentiation media prepared. MSCs differentiated by OsteoMAX-XF differentiation medium only act as control for the experiment. In brief, MSC culture medium was aspirated from the confluent adherent cells and the wells were rinsed twice with 1× PBS (Gibco, Carlsbad, CA) pH 7.2. 1 ml of pre-warmed differentiation media was added to each of the well before incubation of the well plate at 37 °C in a humidified atmosphere containing 5% CO2. Fresh differentiation media was replaced every 3 d for 15 d. The morphological changes in cell cultures were observed using an inverted light microscope.
Human bone alkaline phosphatase ELISA
The deposition of mineralized extracellular matrix by the osteocytes was assessed by ELISA quantitative determination of bone-specific alkaline phosphatase concentrations. The supernatants were collected at days 5, 10, and 15 following induction of osteogenic differentiation. The supernatants were subjected to ELISA by Human BALP ELISA Kit (Elabscience, Houston, TX) following manufacturer’s protocol. In brief, a serial dilution producing eight concentrations of ELISA reference standards were prepared prior to ELISA assay. The standards and samples were added to each well and incubated for 90 min at 37 °C. After sample binding to the ELISA well plate pre-coated with an antibody, the liquid was discarded. The biotinylated detection antibody was added to each well and the ELISA plate was incubated for 1 h at 37 °C. The liquid was then removed and the plate was washed thrice with wash buffer before avidin-horseradish peroxidase (HRP) conjugate was added to each well and incubated for 30 min at 37 °C. The washing process was repeated five times to remove possible residual. Substrate reagent was added to the wells and the plate was incubated for 15 min at 37 °C. After that, stop solution was added and the optical density of each well was determined using a microplate reader (Bioscience, Franklin Lakes, NJ) set to 450 nm.
The cell viability of MSCs after osteogenic differentiation
The cell viability of MSCs at days 5, 10 and 15 of osteogenic differentiation was determined with trypan blue exclusion test. The wells were washed twice with 1 × PBS prior to trypsinization and centrifugation at 300 g for 5 min. The pellet was then subjected to trypan blue exclusion test to determine the cell viability. In parallel, the growth curve of MSCs under OsteoMAX-XF differentiation medium only was determined and served as a comparison.
Staining with Alizarin Red S dye
At days 5, 10 and 15 of osteogenic differentiation, the cells were fixed and stained with Alizarin Red S dye (Sigma-Aldrich, St. Louis, MO) for mineralization. Stained cells were then observed under an inverted light microscope.
Statistical analysis
Mean data were examined from obtained results with one-way analysis of variance (ANOVA) and the consequences are presented as mean ± standard deviation (SD). All experiments were performed in triplicate. The consequences with p < .05 were considered statistically significant.
Results and discussion
FTIR
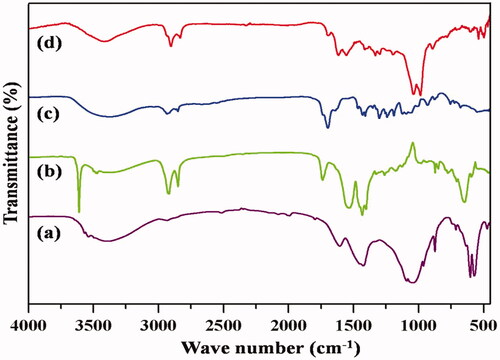
Characteristic bands of PO43− groups were shown in the spectra at 1096, 1047, 962, 601, 563 and 472 cm−1. Absorbed water might be influencing the bands observed at approximately 3440 and 1637 cm−1 (). Hydroxyl (OH) absorption bands corresponding to vibration and stretching were observed for all the samples at 3572 and 633 cm−1. Additional peaks were also observed for PXSA ( due to asymmetric CH stretching at 2963 cm−1 and CH3 symmetric deformation at 1258 cm−1. Symmetrical and asymmetrical stretching modes of O–H were depicted in the synthesized PXSA polymer, which showed variations in the transmittance intensity at 3500–3000 cm−1, which changes upon reduction. shows the CH3 asymmetric stretching mode at 2958 cm−1, CH stretching mode at 2856 cm−1 and CH bending at 1447 cm−1, indicating the manifestation of PXSA. The ester bond formation to the polymer was confirmed from the IR spectra at 1730 cm−1 where C=O stretching was noticed and free hydroxyl groups were also present in the PXSA. The band at 1640 cm−1 confirmed the composite formation between PXSA and hydroxyapatite COO– and Ca2+ as shown in . New peaks are observed in at 1660, 1618, 1595,714 and 1333 cm−1 corresponding to C=O, C=C, aromatic ring vibration, and CH out of plane deformation vibration for the four adjacent ring hydrogen and C=O with aromatic C=C interactions, respectively, present in the HAP/PXSA/VK composite.
XRD
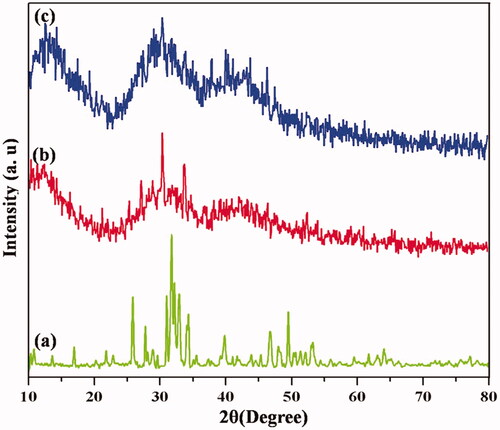
represents the XRD spectra of calcined HAP and HAP/PXSA nanocrystals. Diffraction peaks of hexagonal Ca10(PO4)6(OH)2 were observed for both nanocrystals, which could be indexed in accordance to standard data (JCPDS no. 09-0432). shows the characteristic peaks of HAP 26, 32, 33 and 40 at 2θ regions attributing to (002), (211), (300), and (310) planes, respectively, indicating the formed hydroxyapatite’s crystalline nature similar to previous reports. However, it is noted that three intense peaks corresponding to (211), (112) and (300) became less intense in synthesized HAP/PXSA composite compared to HAP, indicating the low crystallinity of PXSA as shown in . This influences the nanoparticles as that of polymers present in HAP/PXSA composites as shown in . corresponds to the VK-loaded HAP/PXSA/VK composite.
Morphological analysis
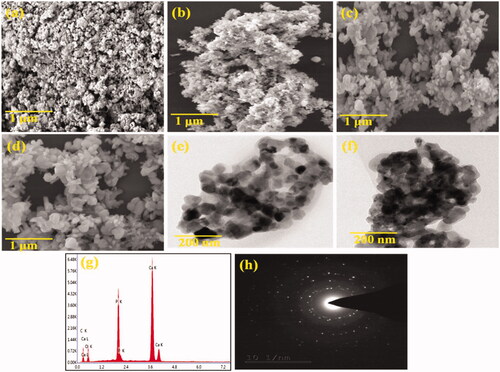
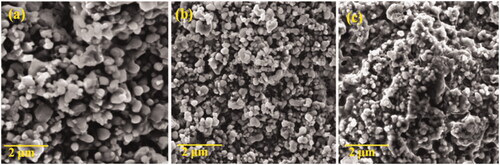
SEM images of HAP nanoparticles are shown in . Morphology of nanoparticles in sizes smaller than 200 nm was characterized. represents the spherical shaped HAP nanoparticles similar to previous reports. The SEM images of PXSA are shown in . PXSA possesses the disc-like morphology. After the HAP and 10 wt% of PXSA composite formation, the disc and rod-like morphology were observed. In HAP/PXSA composite formation by hydrothermal method, the morphology of HAP and PXSA was changed as presented in . The formed morphology will favourable for the application in tissue engineering. VK-loaded HAP/PXSA composite morphology is shown in . The TEM images of HAP/PXSA and HAP/PXSA/VK are shown in . represents the EDAX spectra and SAED image of HAP/PXSA/VK.
Biodegradation
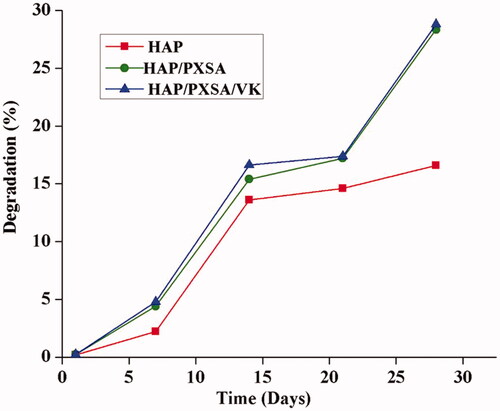
Results of scaffold biodegradability were increased after the introduction of PBS. The incorporation of PXSA to HAP nanoparticles yields acceptable results. The requirement for a specific tissue engineering application can be satisfied by adding the PXSA to hydroxyapatite. No differences were observed in the HAP/PXSA and HAP/PXSA/VK composites. It is noted that VK cannot affect the biodegradation of the material ().
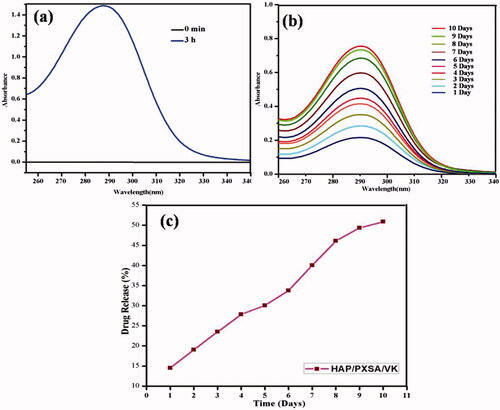
In vitro drug release studies
As shown in , the drug encapsulation efficiency for HAP/PXSA reached approximately 80.3%, which is satisfactory for a drug delivery system. In addition, the amount of VK released from the composite was significantly higher. The days increase, the release was increased and VK concentration in the medium changed after 10 d. The results revealed that 50.87% of VK was released from the VK-loaded HAP/PXSA composite after 10 d. The percentage drug release profile is presented in and the UV spectra of released VK and release profile is presented in . The release of VK will hold back bone loss and improve bone strength.
In vitro SBF analysis
SBF analysis was carried out to determine in-vitro bone regeneration properties of scaffolds. Scaffold guided bone regeneration and bioactivity can be assessed by incubating scaffolds in SBF solution with sufficient ion concentration to simulate human blood plasma. This method is applicable in estimating the quantitative and qualitative assessment of scaffold’s in vitro bone activity. ) shows the SEM images of calcium phosphate mineral formations on the surface of composites HAP/PXSA/VK composite prior and after incubation in SBF solution for 1, 3 and 7 d. The freshly formed calcium crystals with growth and nucleation can be observed on the surfaces of HAP/PXSA/VK composites are shown in . They were found wrapped around surfaces of individual molecules after 7 d of incubation in SBF solution.
Human bone alkaline phosphatase ELISA assessment of osteogenic potential of the biomaterials
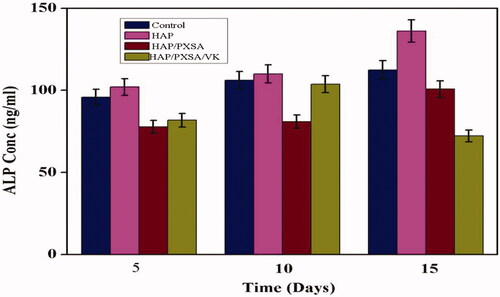
The osteogenic differentiation potential of various biomaterials to induce MSCs differentiation into bone was examined by the concentration of ALP produced by the MSCs. Cell adherence and differentiation are vital characteristics to be analyzed on a preliminary scale for biomaterials and is essential for stem-cell mediated bone regeneration. The results show that the biomaterials were able to induce ALP activity up to day 15 except for HAP/PXSA/VK composites, which showed a marked decrease in ALP activity after day 10. At day 15, MSCs differentiated by HAP achieved the highest concentration of ALP (136.21 ng/mL) as shown in . The results indicate the osteoprogenitor cells would able to differentiate on the mineralized scaffolds due to an increase in the ALP activities. The drop in activity on day 15 for HAP/PXSA/VK composites was most likely due to an increase in mineralization ().
Cell viability of MSCs after osteogenic differentiation
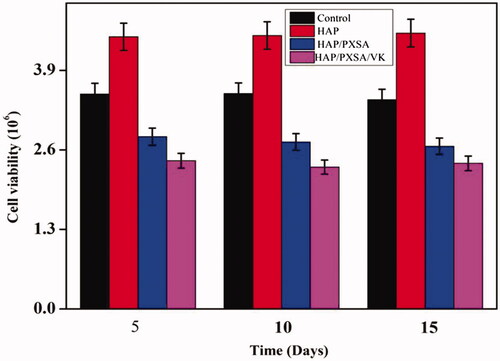
Cell viability of MSCs after osteogenic differentiation by biomaterials was determined using trypan blue exclusion test at days 5, 10, and 15. The results show that MSCs differentiated by HAP achieved the highest cell viability and maintained to be the highest until day 15. There were no significant differences (p > .05) within each biomaterial treatment for 15 d, which shows that there is no time-dependent effects on the nanoparticles suspension on MSCs cell viability. Cell viability is commonly influenced in a positive way at a longer time at the presence of larger suspension load. The results indicate that the nanocomposites have high cytocompatibility with MSCs.
Alizarin Red S staining of human Wharton’s jelly MSCs (hWJ-MSCs) differentiated by biomaterials
Alizarin Red S stains were performed to visualize the scaffold mineralization at days 10 and 15. The results are displayed in and . The results show that calcifications started to develop at day 10 as imaged with red stains. More mineralization appeared to be formed by the induction of HAP. The results at day 15 show that more calcifications were developed which can be seen with red stains. MSCs differentiated by HAP appeared to have more mineralization formed. The results indicate that osteogenic cells differentiated with the biomaterials were able to mineralize with an increase in time. Therefore, the biomaterial differentiated osteogenic cells will promote rapid bone regeneration.
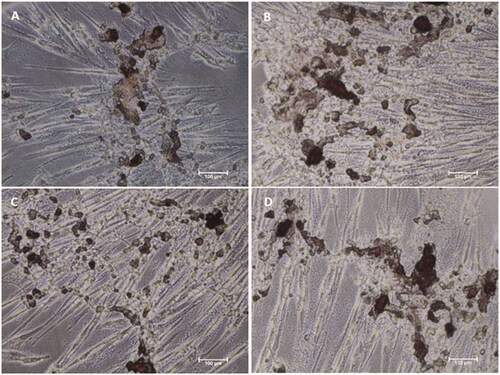
Figure 9. Alizarin Red S staining of MSCs differentiated by biomaterials at day 10. Microscopic images of MSCs after differentiation by (A) Osteo MAX-XF differentiation medium only, (B) Osteo MAX-XF differentiation medium and HAP, (C) OsteoMAX-XF differentiation medium and HAP/PXSA and (D) Osteo MAX-XF differentiation medium and HAP/PXSA/VK.
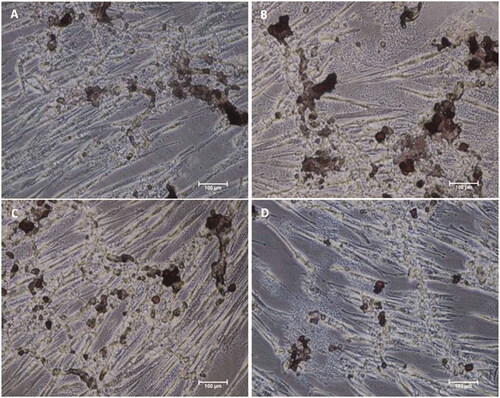
Figure 10. Alizarin Red S staining of MSCs differentiated by biomaterials at day 15. Microscopy images of MSCs after differentiation by (A) Osteo MAX-XF differentiation medium only; (B) Osteo MAX-XF differentiation medium and HAP. The image showed high numbers of attached MSCs on the well, indicating high cell viability; (C) Osteo MAX-XF differentiation medium and HAP/PXSA; (D) Osteo MAX-XF differentiation medium and HAP/PXSA/VK.
Conclusion
We developed a bioactive PXSA-based composite scaffold for the application of bone tissue engineering. Our study established that the as-prepared HAP/PXSA/VK composite exhibited improved biocompatibility. The HAP/PXSA/VK nanocomposite could encourage hMSCs spreading, propagation, and adhesion of MG63 osteoblasts like cells. However, the in vitro study would be the finest validation of the efficiency of VK in bone formation. Convincing data of the biological plausibility of the significance of VK for bone health have been studied. In conclusion, the present study revealed more favourable changes in bone regeneration for the VK-loaded HAP/PXSA composite. These whole results emphasize the guided bone-tissue regeneration possessions of the nanocomposite with viability properties (on demand), which would discover their function as an engineered osteogenic composite for bone regeneration applications.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926.
- Yang S, Leong KF, Du Z, et al. The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Eng. 2002;8:1–11.
- Stoop R. Smart biomaterials for tissue engineering of cartilage. Tissue Eng. 2001;7:679–689.
- Petite H, Viateau V, Bensaid W, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–963.
- Wang Q, Xu J, Jin H, et al. Artificial periosteum in bone defect repair – a review. Chin Chem Lett. 2017;28:1801–1807.
- Dimitriou R, Jones E, McGonagle D, et al. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66–76.
- Qazi TH, Mooney DJ, Pumberger M, et al. Biomaterials based strategies for skeletal muscle tissue engineering: existing technologies and future trends. Biomaterials. 2015;53:502–521.
- Stevens MM. Biomaterials for bone tissue engineering. Mater Today. 2008;11:18–25.
- Ajellal N, Thomas CM, Aubry T, et al. Encapsulation and controlled release of L-leuprolide from poly(β-hydroxyalkanoate)s: impact of microstructure and chemical functionalities. New J Chem. 2011;35:876–880.
- Wang D, Xuan L, Zhong H, et al. Incorporation of well-dispersed calcium phosphate nanoparticles into PLGA electrospunnano fibers to enhance the osteogenic induction potential. RSC Adv. 2017;7:23982–23993.
- Sun F, Shi T, Zhou T, et al. 3D poly(lactic-co-glycolic acid) scaffolds for treating spinal cord injury. J Biomed Nanotechnol. 2017;13:290–302.
- Fu S, Wang P, Chu B, et al. In vivo biocompatibility and osteogenesis of electrospun poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone)/nano-hydroxyapatite composite scaffold. Biomaterials. 2012;33:8363–8371.
- Ling Q, Wang T, Yu X, et al. UC-VEGF-SMC three dimensional (3D) nano scaffolds exhibits good repair function in bladder damage. J Biomed Nanotechnol. 2017;13:313–323.
- Shakir M, Jolly R, Khan MS, et al. Nano-hydroxyapatite/β-CD/chitosan nanocomposite for potential applications in bone tissue engineering. Int J Biol Macromol. 2016;93:276–289.
- Cunniffe GM, Curtin CM, Thompson EM, et al. Content-dependent osteogenic response of nanohydroxyapatite: an in vitro and in vivo assessment within collagen-based scaffolds. ACS Appl Mater Interfaces. 2016;8:23477–23488.
- Fujisaki K, Tadano S. Relationship between bone tissue strain and lattice strain of HAp crystals in bovine cortical bone under tensile loading. J Biomech. 2007;40:1832–1838.
- Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363–408.
- Place ES, George JH, Williams CK, et al. Synthetic polymer scaffolds for tissue engineering. Chem Soc Rev. 2009;38:1139–1151.
- Shearer MJ. Vitamin K. Lancet. 1995;345:229–234.
- Katti DS, Robinson KW, Ko FK, et al. Bioresorbablenano fiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res. 2004;70:286–296.
- Das RK, Zouani OF, Labrugere C, et al. Influence of nanohelical shape and periodicity on stem cell fate. ACS Nano. 2013;7:3351–3361.
- Jeon O, Alsberg E. Regulation of stem cell fate in a three-dimensional micropatterned dual-crosslinked hydrogel system. Adv Funct Mater. 2013;23:4765–4775.
- Vilela C, Sousa AF, Fonseca AC, et al. The quest for sustainable polyesters – insights into the future. Polym Chem. 2014;5:3119–3141.
- Narendran K, Nanthini R. In vitro biocompatibility evaluation of biscoumarin based random copolyesters. New J Chem. 2015;39:4948–4956.
- Killi N, Paul VL, Gundloori RVN. Antibacterial non-woven nanofibers of curcumin acrylate oligomers. New J Chem. 2015;39:4464–4470.
- Heller J. Ocular delivery using poly(ortho esters). Adv Drug Deliv Rev. 2005;57:2053–2062.
- Migneco F, Huang Y-C, Birla RK, et al. Poly(glycerol-dodecanoate), a biodegradable polyester for medical devices and tissue engineering scaffolds. Biomaterials. 2009;30:6479–6484.
- Krishna L, Dhamodaran K, Jayadev C, et al. Nanostructured scaffold as a determinant of stem cell fate. Stem Cell Res Ther. 2016;7:188.
- Galli C, Piemontese M, Lumetti S, et al. Actin cytoskeleton controls activation of Wnt/β-catenin signaling in mesenchymal cells on implant surfaces with different topographies. Acta Biomater. 2012;8:2963–2968.
- Ozdemir T, Xu LC, Siedlecki C, et al. Substrate curvature sensing through Myosin IIaupregulates early osteogenesis. Integr Biol. 2013;5:1407–1416.
- Shi M, Chen Z, Farnaghi S, et al. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomaterial. 2016;30:334–344.
- Cai X, Xie J, Yao Y, et al. Angiogenesis in a 3D model containing adipose tissue stem cells and endothelial cells is mediated by canonical Wnt signaling. Bone Res. 2017;5:17048.
- Ohji T, Fukushima M. Macro-porous ceramics: processing and properties. Int Mater Rev. 2012;57:115–131.
- Nelsestuen GL, Zytkovicz TH, Howard JB. The mode of action of vitamin K. Identification of gamma-carboxyglutamic acid as a component of prothrombin. J Biol Chem. 1974;249:6347–6350.
- Shimizu T, Takahata M, Kameda Y, et al. Vitamin K-dependent carboxylation of osteocalcin affects the efficacy of teriparatide (PTH(1-34)) for skeletal repair. Bone. 2014;64:95–101.
- Price PA, Williamson MK, Lothringer JW. Origin of the vitamin K-dependent bone protein found in plasma and its clearance by kidney and bone. J Biol Chem. 1981;256:12760–12766.
- Hoang QQ, Sicheri F, Howard AJ, et al. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003;425:977–980.
- Stenflo J, Fernlund P, Egan W, et al. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc Natl Acad Sci USA. 1974;71:2730–2733.
- Hauschka PV, Lian JB, Gallop PM. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci USA. 1975;72:3925–3939.
- Abrahams MJ, Price J, Whitlock FA, et al. The Brisbane floods, January 1974: their impact on health. Med J Aust. 1976;2:936–939.