Abstract
Labour is considered to be one of the most painful procedures in human experience. The most effective technique for pain relief during labour is neuraxial labour analgesia which provides analgesia without maternal or fetal sedation. Genetic predisposition may be of importance for pain perception and women experience varying degrees of pain in labour. Genetic variations in opioid receptor (OPR) genes may influence the response to epidural opioid analgesia during labour. The single-nucleotide polymorphism, A118G of the mu opioid receptor gene (oprm1), has been associated with altered pain perception. Targeted drug delivery reduces toxic side effects. Liposomes, nano-particles, nanofibres hydrogel, have been suggested to deliver anaesthetic drugs.
Introduction
Labour is recognized as being desired experiences in life [Citation1]. An important feature of childbirth is pain [Citation2]. It is well known that labour is one of the most painful processes. Psychological mechanisms, duration of labour and the weight of fetus are main factors that affect the perception of pain during labour [Citation3]. Women have different degree of pain in the first and second stage of labour [Citation4]. Genetic factors are very important in the perception of pain. Various approaches have been identified that polymorphisms at several gene loci are related to the perception of pain [Citation5]. It is called functional pain genomics [Citation6]. Good obstetric care has proper pain relief during labour. Psychological techniques are time-consuming, with unpredictable, inconsistent and incomplete results [Citation7]. James Young in 1847 administered ether during labour. He also administered chloroform later that year [Citation8].
Labour analgesia
Neuraxial anaesthesia
The most effective technique for relieving labour pain is neuraxial labour analgesia, which provides analgesia without maternal or fetal sedation [Citation9]. A number of diverse neuraxial techniques are also available; e.g. continuous epidural anaesthesia (EDA) and combined spinal epidural (CSE) analgesia. The use of epidural analgesia is associated with better pain relief than are systemic opioids [Citation10]. Epidural analgesia has better pain relief.
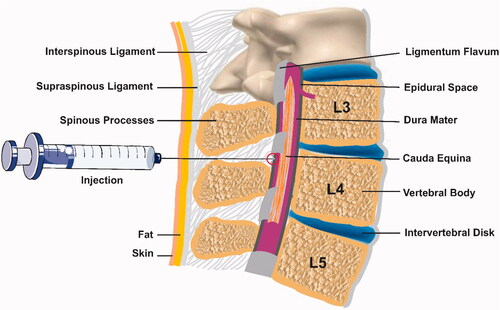
Epidural analgesia has been used to labour for more than 40 years and it is the most effective method of pain relief [Citation11]. Labour analgesia has become extensively recognized in clinical practice. It is applied for women when the cervix is dilated 2 cm or more and catheterization is performed at the L2-L3 interspace [Citation12]. The anaesthetic solution is injected only in the epidural space and not in the cerebrospinal fluid (CSF) during labour [Citation13] ().
Rapid onset and greater spread of sacral analgesia have been produced by combined spinal epidural (CSE) analgesia.
A new technique, DPE (Dural Puncture Epidural), involves the performance of a CSE but without administration of an intrathecal medication dose. In contrast with standard epidural analgesia, DPE produces greater analgesia [Citation14].
Labour pain and drug
Pain is distressing sensation which is connected to potential or actual tissue injury which acts as preventer for further damage. Pain-sensation is based on precise receptors embedded in primary afferent fibres. Pain sensation performs according to a sequential pathway initiate with pain sense, peripheral nervous system signal transmission to dorsal horn of spinal cord, and finally signal transmission to brain through central nervous system. Pain is classified in three main sub-groups including nociceptive, neuropathic and inflammatory pains [Citation15]. Labour as a set of regular and painful contractions in uterus whit gradual increases in intensity and frequency is belonging to nociceptive pains. Labor-pain comprises two main phases including visceral- and somatic-pain, visceral-pain in uterus is conducted by tiny unmyelinated C-fibres accompanied with sympathetic fibres which pass through uterus, cervical and hypogastric nerve plexuses to reach sympathetic chain that is connected with T10, T11, T12 and L1 in spinal-cord. Early pain of labour is referred to T10-T12 which is not completely responsive to opioids. Late-labor-somatic-pain is conducted by A-fibres into S2-S4 nerve roots, also Somatic fibres access L1 and L2 in spinal-cord. Literature suggest relief in labour-pain is depended on personal aspiration, cultural features and peer group influences [Citation16–18]. Pharmacological-based analgesia for relieving labour-pain beside alternative and complementary therapies are common, which fall in systemic (intravenous, intramuscular and inhalation) or locoregional (epidurals, spinals and combined spinal-epidurals) groups. Systemic analgesics represent less-invasive approach in contrast to locoregional techniques. Opioids like morphine, fentanyl, and meperidine beside mixed opioid agonists-antagonists like nalbuphine and butorphanol affect receptors in maternal brain to reduce pain and also reduce fetal heart rate as side-effect [Citation19,Citation20]. Opioids-receptors mostly are located in brain, they are classified as μ, δ and κ receptors which all mediate spinal analgesia, exceptionally μ-receptor-subtype-1 also contributes to supraspinal analgesia. Opioid-receptors are constructed include 7-transmembrane spanning proteins which pair with G-proteins. Opioid-receptors are tightly interconnected with inhibitory G proteins, Opioids suppress neurotransmitter release by neurons through enhanced conduction of potassium and hyperpolarization of cells to reduce responsivity to depolarization pulses then inhibit calcium influx and prevent postsynaptic impulse. Numerous interactions of neurons are needed for opioids analgesic effect, at the level of supraspinal they bound μ-receptor in the GABAergic (gamma-aminobutyric acid-ergic) neurons which result in activation of descending inhibitory neurons of brainstem but at the level of spinal, inhibition of pain-mediators (nitric oxide, glutamate and substance P) of nociceptive-afferent-neurons indicates analgesic outcome [Citation21,Citation22]. Opioids members may indicate complete- or partial- agonistic effect on receptors, dihydrocodeine, fentanyl, heroin, codeine and Morphine completely act as agonist on μ-receptors but weakly against δ- and κ-receptors; also, Buprenorphine is known as agonist and antagonist of μ-receptor which result in μ depended analgesia. Meptazinol as an analgesic-opioid-agonist on μ-receptor has also agonist function on muscarinic acetylcholine-receptors. Methadone and tramadol as agonists of μ-receptor increase analgesic function by reuptake prevention of serotonin and norepinephrine, Methadone also reduce pain as antagonist through glutamatergic N-methyl-D-aspartate receptor. Pain relief with opioids is due to rising of pain threshold [Citation21,Citation23,Citation24]. Fentanyl as synthetic opioid beside related congeners (sufentanil, alfentanil and remifentanil) are newer opioid with shorter duration apply for labour-pain relief. Fentanyl binding to μ-receptor of sensory neurons activates G-proteins which inhibits adenylate cyclase enzyme to decrease intracellular c-AMP beside inactivation of calcium channels leads to neuron to hyperpolarization and reduces neurotransmitter release with blocking of perception of the pain signals [Citation24–26]. Nonopioids like promethazine, phenothiazine, hydroxyzine and anti-histamine are used in combination with opioids to reduce common side effects like nausea and vomiting [Citation27].
Nano-carriers for pain therapy
Gold, silver and magnetic nanoparticles are nanotheranostic agents and can be conjugated to drugs, ligands, antibodies and nanoliposomes to enhance delivery rate and efficiency of therapy are applied in different types [Citation28]. Drug-loaded nanoparticles have higher therapeutic efficacy and reduce drug toxicity [Citation29]. More recent evidence suggests that bupivacaine used in liposomal formulations represent the most cited local anaesthetic, whereas liposomes loaded with benzocain or tetracain are already used in commercial topic formulations such as Optisome™ [Citation30]. Much work on the potentional of lidocaine-loaded nanofibers has been carried out and there is a prolonged release of lidocaine for more than 2 weeks [Citation31].
As mentioned by Tseng et al., lidocaine-loaded nanofibres confirmed can provide a sustained release in rats [Citation32]. Nanoparticle technology can provide a simple and useful method for delivering of fentanyle [Citation33] and producing a greater analgesic effect [Citation34].
Genetic correlation of pain and analgesia
Genetic is responsible for pain perception and degree of pain in labour are associated with polymorphism at several gen loci [Citation5]. Number of genes are reported to play role in analgesia which the most well-known ones are listed in Human Pain Genetics Database (www.humanpaingenetics.org). Opioids are considered as the first line medication to relief moderate or severe pain in patients. Regarding heterogeneous genetic background of patient’s various drug metabolizing enzymes and transporters (pharmacokinetics) beside receptor and signal transduction (pharmacodynamics) pathways are considered. ATP Binding Cassette Subfamily B Member 1 (ABCB1) is an opioid transporter which interfering drug response through single nucleotide polymorphisms [Citation35]. Genetic variants of delta opioid receptor genes (OPRD1) and catechol-O-methyl transferase gene (COMT) are correlated with analgesia. Variation of OPRD1 as drug receptor can highly change drug response in patients. COMT is known as an intracellular enzyme placed in the postsynaptic neurons to metabolizes dopamine, noradrenaline and adrenaline which are common catecholamine neurotransmitters. COMT variations are reported to modulate pain in patens, especially in cancer patients. rs4680 is reported to influence morphine in cancer patients [Citation36,Citation37]. Potassium Voltage-Gated Channel Subfamily J Member 6 (KCNJ6) is associated with potassium channel function which is reported to alter pain and analgesia. Various SNPs and haplotypes are reported for KCNJ6 which are integrated into Mild Pain and Severe Pain classes and can present pain phenotype in patients, also risk alleles alternation cause reduction in pain tolerance [Citation38]. UDP-Glucuronosyltransferase-2B7 (UGT2B7) and Cytochrome P450 3A4 (CYP3A4) are involved in transdermal buprenorphine metabolic pathway which can modulate pain and analgesia in patients. UGT2B7 is recognized as a phase II metabolism iso-enzyme located in different organs including brain, kidneys, liver and lower gastrointestinal epithelial cells to couple with drugs to abolish them. Variation cause higher activity of UGT2B7 predisposes patients to suffer severe pain regarding rapid drug elimination. CYP3A4 is a liver enzyme controls the metabolism of drugs including analgesia drugs, it is reported that variations of CYP3A4highly affect analgesia response in post-operative periods through altered drug metabolism [Citation39].
Opioid receptors (OPRs)
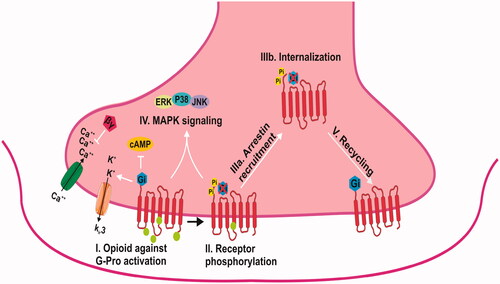
Opioid systems widely considered to be the most important role in pain control. Genes of opioid systems have been characterized at cellular, molecular and pharmacological levels [Citation40]. OPR is interacting with heterotrimeric G proteins (). They are part of the Rhodopsin family of G-protein coupled receptors (GPCRs) and activate downstream signalling. The μ-opioid receptor (MOR), δ-opioid receptor (DOR) and κ-opioid receptor (KOR) are the three most common types which encoded by the OPRM1, OPRD1 and OPRK1 genes [Citation41]. The µ-opioid receptor (MOR), encoded by the OPRM1gene [Citation42]. Optimal methadone dose was associated with single nucleotide polymorphism (SNPs) including A118G [Citation43].
OPRM1
OPRM1 gene encodes µ-opioid receptor. It is mediating the analgesic and euphoric effects of opioid drugs [Citation44]. The role of OPRM1 in obstetric and labour pain is important [Citation45,Citation46]. OPRM1 (MIM# 600018; GenBank NM_000914) is interacting with multiple endogenous opioid peptides including beta-endorphin and endomorphins [Citation47]. It is a member of the G-protein. Epidural opioids, including morphine, meperidine, sufentanil and fentanyl, have many applications in the field of labour analgesia [Citation48]. OPRM1 is located on chromosome 6q24-q25, spanning 1.9 kb, and it contains two exons [Citation49]. There is a vast amount of polymorphism in the OPRM1 gene [Citation50]. Recent developments in labour analgesia have indicated that the OPRM1 A118G polymorphism has a main role in the response to epidural analgesia with fentanyl [Citation51] ().
Table 1. A118G Variant of OPRM1 in labour analgesia.
A118G variant in exon 1 is the most common SNP of the OPRM1. This polymorphism exchanges asparagine to aspartic acid at N-glycosylation site [Citation52]. Aspartic acid may increase the binding affinity of b-endorphin [Citation53] and influences the response to epidural opioid analgesia during labour [Citation54]. Experiments on the effect of A118G variant of OPRM1 carried out by Landau et al. on Swiss nulliparous women [Citation55]. Higher dose of fentanyl is required for A118 homozygotes. In the classical approach, the proper dose of drugs is required to improve clinical outcomes [Citation56]. Wong et al. reported the correlation of the duration of intrathecal fentanyl analgesia and A118G variant of OPRM1 in the first stage of labour [Citation57]. Camorcia et al. evaluated the response to epidural sufentanil and showed that A118 homozygotes women required higher doses [Citation58]. Researchers did not confirm any significant correlation between A118G variant of OPRM1 and cervical dilation on arrival at the delivery unit or use of any type of analgesia (epidural or second-line analgesia) during labour [Citation54]. Therefore, it is biologically plausible that genetic variations of OPRM1 A118G polymorphism may modulate an individual’s response to epidural analgesia with fentanyl during labour [Citation58].
Guanosine triphosphate cyclohydrolase (GCH1)
Further studies which take pain-protective SNP combination of GCH1 in labouring women into account will need to be undertaken [Citation59]. As mentioned by Tegeder and colleagues, the correlation between specific SNPs in the guanosine triphosphate cyclohydrolase (GCH1) gene and reduced pain sensitivity in humans is interesting [Citation60].
GCH1 transcription is motivated by the cytokines interferon gamma (IFNγ) and tumour necrosis alpha (TNFα) and at a lesser extent interleukin 1β lipopolysaccharide nerve growth factor (NGF). GCH1 expression is also less than the control of estrogens possibly through a NO-mediated establishment of CREB [Citation61,Citation62]. Dabo et al. in their study confirmed that SNP combination of GCH1 has an important role during the first stage of labour and the use of second-line labour analgesia in homozygous carriers [Citation59].
Enzyme catechol-O-methyl transferase (COMT)
COMT (enzyme catechol-O-methyl transferase) is a Protein-Coding gene [Citation63]. It is identified in the 1950s and located on chromosome 22q11. A single-nucleotide polymorphism (SNP) (COMTval 158 met, rs4680) occurs in coding region in which a guanine to adenine substitution and exchanges valine-to-methionine (Val/Met) in codons 108 and 158 [Citation64]. This polymorphism reduces COMT activity [Citation65]. The importance of COMT gene in metabolism and inactivation of dopamine, norepinephrine, epinephrine, caffeine, estrogen and other catechol compounds is identified [Citation66]. Met homozygotes exhibit reduced tolerance to pain during labour that may affect the response to analgesia with IV fentanyl [Citation67] and they require a higher dose of remifentanil [Citation68]. In contrast with A allele, carriers of the G allele of COMT have more sensitivity [Citation67].
Beta-adrenergic receptors (ADRB1)
Beta-adrenergic receptors (ADRB1) are known to facilitate catecholamine-induced activation of adenylate cyclase through G proteins, ADRB1 indicates similar affinity to epinephrine and norepinephrine hormones. Ras activation through G(s)-alpha- and cAMP is mediated by ADRB1 [Citation68]. ADRB1 is comprised of single exon with a short un-translated region. rs1801252 and rs1801253 are embedded single nucleotide polymorphisms (SNPs) in ADRB1 gene with clinical significance in hypertension, stroke, heart disease, pain and analgesic sensitivity. In Chinese population, different response according to variety of rs1801252 and rs1801253 SNPs to pain is reported, also Japanese population response to Fentanyl is affected by these SNPs [Citation69–71].
Conclusion
Evaluating labour pain and the response to labour analgesia are challenging. Finding relevant genetic associations is particularly complicated. The use of drug-loaded nano-particles would be promising for several acute and chronic pain condition.
Acknowledgements
We would like to thank authorities of Treatment Affair of Tabriz University of Medical Sciences.
Disclosure statement
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Landau R, Ortner CM, Vuilleumier PH. The impact of genetics and other factors on intra-and post-partum pain. Curr Anesthesiol Rep. 2013;3:264–274.
- Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445.
- George RB, Allen TK, Habib AS. Intermittent epidural bolus compared with continuous epidural infusions for labor analgesia: a systematic review and meta-analysis. Anesth Analg. 2013;116:133–144.
- Capogna G, Camorcia M, Stirparo S, et al. Multidimensional evaluation of pain during early and late labor: a comparison of nulliparous and multiparous women. Int J Obstet Anesth. 2010;19:167–170.
- Max MB, Stewart WF. The molecular epidemiology of pain: a new discipline for drug discovery. Nat Rev Drug Discov. 2008;7:647.
- Diatchenko L, Nackley AG, Tchivileva IE, et al. Genetic architecture of human pain perception. TRENDS Genet. 2007;23:605–613.
- Hiltunen P, Raudaskoski T, Ebeling H, et al. Does pain relief during delivery decrease the risk of postnatal depression? Acta Obstet Gynecol Scand. 2004;83:257–261.
- Hingson RA, Edwards WB. Continuous caudal analgesia: an analysis of the first ten thousand confinements thus managed with the report of the authors' first thousand cases. JAMA. 1943;123:538–546.
- Bucklin BA, Hawkins JL, Anderson JR, et al. Obstetric anesthesia workforce survey twenty-year update. Anesthesiology. 2005;103:645–653.
- Lucas MJ, Sharma SK, McIntire DD, et al. A randomized trial of labor analgesia in women with pregnancy-induced hypertension. Am J Obstet Gynecol. 2001;185:970–975.
- Gambling D, Berkowitz J, Farrell TR, et al. A randomized controlled comparison of epidural analgesia and combined spinal-epidural analgesia in a private practice setting: pain scores during first and second stages of labor and at delivery. Anesth Analg. 2013;116:636–643.
- Salman C, Kayacan N, Ertuğrul F, et al. Combined spinal-epidural anesthesia with epidural volume extension causes a higher level of block than single-shot spinal anesthesia. Braz J Anesthesiol (English Ed). 2013;63:267–272.
- Loubert C, Hinova A, Fernando R. Update on modern neuraxial analgesia in labour: a review of the literature of the last 5 years. Anaesthesia. 2011;66:191–212.
- Meng M-L, Smiley R. Modern neuraxial anesthesia for labor and delivery. F1000Res. 2017;6:1211.
- Yam M, Loh Y, Tan C, et al. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int J Mol Sci. 2018;19:2164.
- Green JM, Coupland VA, Kitzinger JV. Expectations, experiences, and psychological outcomes of childbirth: a prospective study of 825 women. Birth. 1990;17:15–24.
- Labor S, Maguire S. The pain of labour. Rev Pain. 2008;2:15–19.
- Brownridge P. The nature and consequences of childbirth pain. Eur J Obstet Gynecol Reprod Biol. 1995;59:S9–S15.
- Petrie R, Yeh S, Murata Y, et al. The effect of drugs on fetal heart rate variability. Am J Obstet Gynecol. 1978;130:294–299.
- Mattingly JE, D'Alessio J, Ramanathan J. Effects of obstetric analgesics and anesthetics on the neonate: a review. Paediatr Drugs. 2003;5:615–627.
- Ghelardini C, Mannelli LDC, Bianchi E. The pharmacological basis of opioids. Clin Cases Miner Bone Metab. 2015;12:219.
- Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. J Am Soc Anesthesiol. 2011;115:1363–1381.
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–1953.
- Barbani F, Angeli E, De Gaudio AR. Intravenous sedatives and analgesics. In: De Gaudio AR, Romagnoli S, editors. Critical care sedation. Cham: Springer; 2018. p. 69–101.
- Prommer E. The role of fentanyl in cancer-related pain. J Palliat Med. 2009;12:947–954.
- Evron S, Ezri T. Options for systemic labor analgesia. Curr Opin Anaesthesiol. 2007;20:181–185.
- Hensley JG, Collins MR, Leezer CL. Pain management in obstetrics. Crit Care Nurs Clin North Am. 2017;29:471–485.
- Kafshdooz L, Pourfathi H, Akbarzadeh A, et al. The role of microRNAs and nanoparticles in ovarian cancer: a review. Artif Cells Nanomed Biotechnol. 2018;46:1–7.
- Vahabi S, Eatemadi A. Nanoliposome encapsulated anesthetics for local anesthesia application. Biomed Pharmacother. 2017;86:1–7.
- de Paula E, Cereda C, Tofoli GR, et al. Drug delivery systems for local anesthetics. Ddf. 2010;4:23–34.
- Tseng Y-Y, Liu S-J. Nanofibers used for the delivery of analgesics. Nanomedicine (Lond). 2015;10:1785–1800.
- Tseng Y-Y, Liao J-Y, Chen W-A, et al. Biodegradable poly ([D, L]-lactide-co-glycolide) nanofibers for the sustainable delivery of lidocaine into the epidural space after laminectomy. Nanomedicine. 2014;9:77–87.
- Csaba N, Garcia-Fuentes M, Alonso MJ. The performance of nanocarriers for transmucosal drug delivery. Expert Opin Drug Deliv. 2006;3:463–478.
- Christie JM, Simmonds M, Patt R, et al. Dose-titration, multicenter study of oral transmucosal fentanyl citrate for the treatment of breakthrough pain in cancer patients using transdermal fentanyl for persistent pain. JCO. 1998;16:3238–3245.
- Dzambazovska-Trajkovska V, Nojkov J, Kartalov A, et al. Association of single-nucleotide polymorhism C3435T in the ABCB1 gene with opioid sensitivity in treatment of postoperative pain. Prilozi. 2016;37:73–80.
- Olesen AE, Sato H, Nielsen LM, et al. The genetic influences on oxycodone response characteristics in human experimental pain. Fundam Clin Pharmacol. 2015;29:417–425.
- Nielsen LM, Christrup LL, Sato H, et al. Genetic influences of OPRM 1, OPRD 1 and COMT on morphine analgesia in a multi‐modal, multi‐tissue human experimental pain model. Basic Clin Pharmacol Toxicol. 2017;121:6–12.
- Langford DJ, Paul SM, West CM, et al. Variations in potassium channel genes are associated with distinct trajectories of persistent breast pain after breast cancer surgery. Pain. 2015;156:371–380.
- Blanco F, Muriel C, Labrador J, et al. Influence of UGT 2B7, CYP 3A4, and OPRM 1 gene polymorphisms on transdermal buprenorphine pain control in patients with critical lower limb ischemia awaiting revascularization. Pain Pract. 2016;16:842–849.
- Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229.
- Stockton SD Jr, Devi LA. Functional relevance of μ–δ opioid receptor heteromerization: a role in novel signaling and implications for the treatment of addiction disorders: From a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121:167–172.
- Flores CM, Mogil JS. The pharmacogenetics of analgesia: toward a genetically-based approach to pain management. Pharmacogenomics. 2001;2:177–194.
- Hung C-C, Chiou M-H, Huang B-H, et al. Impact of genetic polymorphisms in ABCB1, CYP2B6, OPRM1, ANKK1 and DRD2 genes on methadone therapy in Han Chinese patients. Pharmacogenomics. 2011;12:1525–1533.
- Deb I, Chakraborty J, Gangopadhyay PK, et al. Single‐nucleotide polymorphism (A118G) in exon 1 of OPRM1 gene causes alteration in downstream signaling by mu‐opioid receptor and may contribute to the genetic risk for addiction. J Neurochem. 2010;112:486–496.
- Baber M, Bapat P, Nichol G, et al. The pharmacogenetics of opioid therapy in the management of postpartum pain: a systematic review. Pharmacogenomics. 2016;17:75–93.
- Landau R. Genetic contributions to labor pain and progress. Clin Perinatol. 2013;40:575–587.
- Shi J, Hui L, Xu Y, et al. Sequence variations in the mu‐opioid receptor gene (OPRM1) associated with human addiction to heroin. Hum Mutat. 2002;19:459–460.
- Hawkins JL. Epidural analgesia for labor and delivery. N Engl J Med. 2010;362:1503–1510.
- Kleinjan M, Poelen EA, Engels RC, et al. Dual growth of adolescent smoking and drinking: evidence for an interaction between the mu‐opioid receptor (OPRM1) A118G polymorphism and sex. Addict Biol. 2013;18:1003–1012.
- Song Z, Du B, Wang K, et al. Effects of OPRM1 A118G polymorphism on epidural analgesia with fentanyl during labor: a meta-analysis. Genet Test Mol Biomarkers. 2013;17:743–749.
- Zhang S, Li S, Tan X. Human µ-opioid receptor A118G polymorphism affects epidural patient-controlled analgesia with fentanyl. Nan Fang yi ke da Xue Xue Bao = J Southern Med Univ. 2013;33:309–311.
- Bond C, LaForge KS, Tian M, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci. 1998;95:9608–9613.
- Rhodin A, Grönbladh A, Ginya H, et al. Combined analysis of circulating β-endorphin with gene polymorphisms in OPRM1, CACNAD2 and ABCB1 reveals correlation with pain, opioid sensitivity and opioid-related side effects. Mol Brain. 2013;6:8.
- Pettersson FD, Grönbladh A, Nyberg F, et al. The A118G single-nucleotide polymorphism of human µ-opioid receptor gene and use of labor analgesia. Reprod Sci. 2012;19:962–967.
- Landau R, Kern C, Columb MO, et al. Genetic variability of the mu-opioid receptor influences intrathecal fentanyl analgesia requirements in laboring women. Pain. 2008;139:5–14.
- Crowley JJ, Oslin DW, Patkar AA, et al. A genetic association study of the mu opioid receptor and severe opioid dependence. Psychiatr Genet. 2003;13:169–173.
- Wong C, McCarthy R, Blouin J, et al. Observational study of the effect of μ-opioid receptor genetic polymorphism on intrathecal opioid labor analgesia and post-cesarean delivery analgesia. Int J Obstet Anesth. 2010;19:246–253.
- Camorcia M, Capogna G, Stirparo S, et al. Effect of μ-opioid receptor A118G polymorphism on the ED50 of epidural sufentanil for labor analgesia. Int J Obstetr Anesth. 2012;21:40–44.
- Dabo F, Grönbladh A, Nyberg F, et al. Different SNP combinations in the GCH1 gene and use of labor analgesia. Mol Pain. 2010;6:41.
- Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269.
- Kaneko YS, Ikemoto K, Mori K, et al. Expression of GTP cyclohydrolase I in murine locus ceruleus is enhanced by peripheral administration of lipopolysaccharide. Brain Res. 2001;890:203–210.
- Shimizu S, Shiota K, Yamamoto S, et al. Hydrogen peroxide stimulates tetrahydrobiopterin synthesis through the induction of GTP-cyclohydrolase I and increases nitric oxide synthase activity in vascular endothelial cells. Free Radic Biol Med. 2003;34:1343–1352.
- Lee S-G, Joo Y, Kim B, et al. Association of Ala72Ser polymorphism with COMT enzyme activity and the risk of schizophrenia in Koreans. Hum Genet. 2005;116:319–328.
- Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250.
- Boecker-Schlier R, Holz NE, Buchmann AF, et al. Interaction between COMT Val158Met polymorphism and childhood adversity affects reward processing in adulthood. Neuroimage. 2016;132:556–570.
- Lundstr K, Salminen M, Jalanko A, et al. Cloning and characterization of human placental catechol–methyltransferase cDNA. DNA Cell Biol. 1991;10:181–189.
- Zubieta J-K, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects µ-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243.
- Pak Y, Pham N, Rotin D. Direct binding of the β1 adrenergic receptor to the cyclic AMP-dependent guanine nucleotide exchange factor CNrasGEF leads to Ras activation. Mol Cell Biol. 2002;22:7942–7952.
- Moriyama A, Nishizawa D, Kasai S, et al. Association between genetic polymorphisms of the β1-adrenergic receptor and sensitivity to pain and fentanyl in patients undergoing painful cosmetic surgery. J Pharmacol Sci. 2013;121:48–57.
- Feng S, Li N, Xu S, et al. Association of ADRB1 gene polymorphisms with pain sensitivity in a Chinese population. Int J Clin Exp Med. 2015;8:11514.
- Wei W, Tian Y, Zhao C, et al. Correlation of ADRB1 rs1801253 polymorphism with analgesic effect of fentanyl after cancer surgeries. Med Sci Monit. 2015;21:4000.