Abstract
Upon bioprinting, cells are mixed with a biomaterial to fabricate a living tissue, thus emphasizing the importance of biomaterials. The biomaterial used in this study was a bio-ink prepared using skin decellularized extracellular matrix (dECM). Skin dECM was extracted by treating the dermis with chemicals and enzymes; the basic structural and functional proteins of the ECM, including collagen, glycosaminoglycans (GAGs), bioreactive materials and growth factors, were preserved, whereas the resident cells that might cause immune rejection or inflammatory responses were removed. The bio-ink based on dECM powder, together with human dermal fibroblasts (HDFs), was loaded into the nozzle of the 3D bioprinter to create the 3D construct. This construct underwent gelation with changing temperature while its shape was maintained for 7 days. The cells showed over 90% viability and proliferation. By analysing the gene expression pattern in the cells of the construct, the skin regenerative mechanism of the bio-ink was verified. Microarray results confirmed that the gene expression related to skin morphology and development had been enhanced because the bioreactive molecules and growth factors, in addition to residual ECM in dECM, provided an optimal condition for the HDFs.
Introduction
In the field of tissue engineering and regenerative medicine, 3D bioprinting is a technique that allows biofabrication of 3D functional living tissues through the use of cells and a biomaterial. The biomaterial is a bio-ink that contains the cells to be incorporated into a 3D construct with a designed shape. Bioprinting allows the shape of the 3D construct to be maintained, while the construct provides a condition similar to the natural environment to the cells so that the living cells can maintain their unique activities and properties [Citation1,Citation2]. Many materials have been suggested to be used as bio-inks, among which decellularized extracellular matrix (dECM) has been shown to provide cells with an environment similar to the environment of living cells [Citation3–6].
The cell–cell interactions and interactions between the cell and ECM help in the construction of the tissue. The ECM is produced by the resident cells to support the 3D structure of the surrounding cells for maintaining the tissue and organ structures. The biophysical properties of ECM include its ability to regulate cell shape, proliferation and migration and the activity of signalling molecules. It also plays a pivotal role in tissue morphogenesis and organ development, thus providing the most suitable environment for the cells [Citation7–9]. Through physical, chemical and biochemical methods, dECM is prepared by preserving the basic structural and functional ECM proteins of tissue and removing the cellular components that might cause immune rejection or inflammatory responses [Citation10–12]. Although various studies have been focused on 3D constructs based on the use of dECM, there is a general lack of research on changes in gene expression within the 3D construct.
The tissue investigated in this study was the skin comprising the epidermis and dermis, the largest component of the human body. dECM was obtained by removing only the resident cells from porcine dermis; a printable bio-ink was developed using dECM, which was then mixed with human dermal fibroblasts (HDFs) to produce a human cell-laden construct. The residual ECM in the extracted dECM containing collagen and GAG as well as bioactive molecules and growth factors provided the cells with an environment identical to the one in the tissue, which helped with the viability and proliferation of HDFs in the construct. Evaluation of gene regulation proved the positive effects of the bio-ink on skin morphology and development.
Materials and methods
Process of decellularization
Porcine skin was purchased from a local supplier (Seoul, South Korea). The epidermis and hypodermis were removed from the skin, and after mincing the remaining dermis, it was treated with trypsin and triton X-100 to remove the porcine resident cells and preserve the ECM. The freeze-dried dECM was ground to powder using a freezer mill (SPEX®, USA). A solution of the bio-ink was prepared by 3 days of pepsin digestion. The bio-ink was freeze-dried to form a sponge (deCelluid), and after sterilization, 0.02 M acetic acid was applied to melt the bio-ink for subsequent use. All chemicals were purchased from Sigma (USA).
Analysis of physical properties and measurement of residual proteins
To confirm the removal of resident cells, DNA was isolated from 5 mg dECM powder by phenol-chloroform extraction, and residual DNA was separated on agarose gel. The amount of collagen, GAG, and elastin in the dECM was measured using a specific kit (Biocolor Ltd., UK) for each. Samples were prepared and sent to Biocon (Seoul, Korea) for LC/MS analysis. The analysis involved SDS electrophoresis followed by gel slicing. The Quantibody assay for analysis of growth factors was performed according to the manufacturer’s protocol (eBiogen Inc., Seoul, Korea).
Preparation of bio-ink
The prepared bio-ink was adjusted to pH 7.4 with 5 M NaOH, and while it was maintained at 37 °C for 30 min, a gross image of the gelation was obtained. Quantitative analysis was carried out by controlling the time and temperature using a rheometer (Malvern, UK).
Preparation of 3 D bioprinted cell-laden construct
To confirm the printability of bio-ink, different percentages (2% and 3%) of the bio-ink were prepared and loaded into the 3D printer for ejection through the 500-μm nozzle. HDFs (Lonza, Switzerland) were resuspended in the medium along with foetal bovine serum (FBS, Gibco, USA) and evenly mixed with the bio-ink; a colour change of the medium indicated the evenness of the mixture. The printer-loaded and discharged construct was maintained at 37 °C for inducing gelation, and after a certain period of time, the proportion of viable cells and cell proliferation were confirmed by the Live/Dead assay (Thermo Fisher, USA).
Microarray analysis
To monitor the changes in gene expression within the 3D printed cell-laden construct, collagen and bio-ink were each mixed with HDFs for printing. After 7 days of culture, the cells were lysed in TRIzol, and total RNA qualified by an Agilent 2100 Bioanalyzer (Agilent Technology, USA). Human Quant-Seq microarray was performed using the customized service provided by eBiogen Inc.
Statistical analysis
All results are expressed as means ± standard deviations (SD). The differences between the two groups were compared by the unpaired t-test. p values ≤ .05 were considered statistically significant.
Results
Decellularization of porcine dermal skin
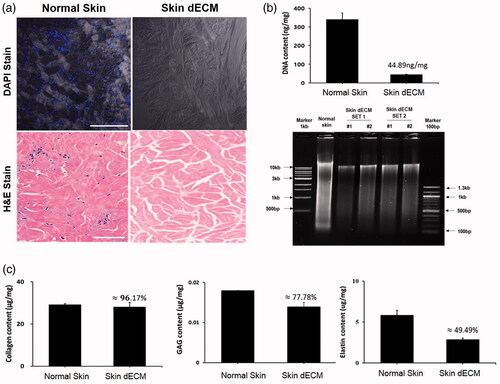
To create skin bio-ink, decellularization was performed to remove only the porcine resident cells from the dermal skin devoid of epidermis and hypodermis. To check the state of decellularization, DAPI staining with H&E was carried out. As shown in , the nuclei of the cells in skin dECM were not stained in comparison to those in cells of normal skin, while the ECM surrounding the cells had been maintained. To quantify this, DNA was extracted from normal skin and dECM, after which agarose gel electrophoresis and ELISA were carried out. shows the result. Residual DNA in skin dECM was 44.89 ng/mg, which was significantly different from that in normal skin from which the epidermis and adipose had been removed. Furthermore, the absence of DNA of 200 bp or fewer was confirmed via agarose gel electrophoresis. On the contrary, collagen, the representative component of ECM, was 96.17%, which was considerably higher than that in normal skin; 77.78% GAG and ∼50% elastin were also found in the cells. As a result, similar to the results of H&E staining, the proper extraction of dermal skin dECM was confirmed.
Figure 1. Molecular biology analysis of decellularized porcine dermis. (a) Histological and DAPI stains show the preserved ECM without residual cells. (b) Extracted porcine DNA was quantified and qualified. (c) The major components of ECM—collagen, GAG, and elastin—were preserved in the dECM.
In addition, to check residual proteins in dECM, SDS PAGE was carried out for LC/MS. Next, after gel slicing, an antibody microarray was used to study the growth factors. The result of LC/MS showed the presence of 51 proteins. Moreover, an identical set of 13 residual proteins in both the normal and skin dECM were found, all from the ECM. These proteins included COL1A1, COL1A2, COL3A1, COL5A2, COL6A2, COL6A3, asporin, dermatopontin, decorin and keratin. The Gene Ontology (GO) analysis of the analysed proteins revealed that the nucleus and cell membrane were detected in normal skin, whereas only the extracellular region and cytoplasm were found in dECM ().
Figure 2. Residual ECM and growth factors in skin dECM. (a) The LC/MS assay showed that ECM components were preserved and certain cellular components were removed by the decellularization process. (b) The remaining growth factors in skin dECM and collagen were analysed using the Quantibody array. Among the 40 growth factors, 24 growth factors were found at a higher degree than collagen. Schematic figure of Quantibody array presented by RayBiotech.
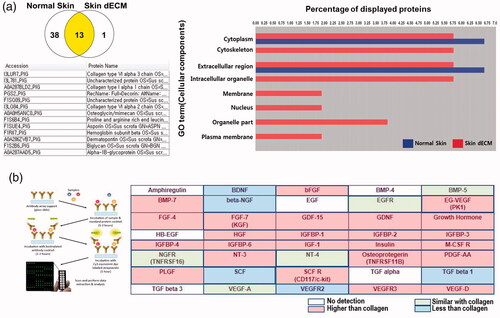
For the antibody microarray, a kit that can analyse 40 common growth factors was used to compare the content of collagen and the most representative and commercially available ECM. As shown in , among the 40 growth factors, 24 growth factors were found in greater abundance than collagen in dECM, and 5 growth factors were present in similar amounts. As a result, we confirmed that skin dECM was prepared with only the residual ECM and growth factors following the decellularization of dermal skin that removed the porcine resident cells.
Survival and proliferation of HDFs in the 3D construct
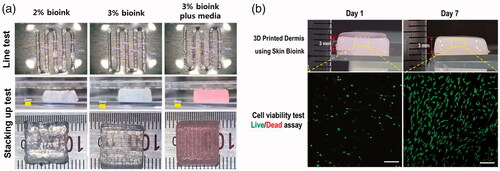
For 3D bioprinting, the bio-ink must be uniformly ejected through the nozzle to create a form, whose shape must be maintained through time. To confirm this, a bio-ink was prepared based on a previous report [Citation13]. Bio-ink (2% and 3%) was prepared, and after adjustment to a pH of 7.4, they were loaded into the 3D printer nozzle. The line width and layer thickness for bioprinting were set at 700 and 300 μm, respectively. The feed rate was fixed at 500, and a pressure of 20 and 43 kPa was applied to the 2% and 3% bio-inks, respectively. The shake of the printed sample is illustrated in . The surface produced by the 2% bio-ink had a tendency to sink as stacking increased, whereas the surface produced by the 3% bio-ink could maintain its shape. Nonetheless, in actual bioprinting, the bio-ink is made to include cells suspended in media. Thus, printing using 3% bio-ink to which media had been added was performed, and the result showed that a stable form was maintained after gelation without much difference from the printing using bio-ink only. This sample maintained the same shape even after storage at 37 °C for 7 days. The construct printed using the bio-ink mixed with cells also maintained its shape up to 7 days, and the cells that looked like dots on day 1 had assumed the 3D shape of the HDFs on day 7, with over 90% of the cells being viable and proliferating (). The result proved the suitability of the bio-ink in 3D bioprinting as a printable material that ensures cell viability.
Alteration of gene expression in 3D bioprintedcell-laden construct
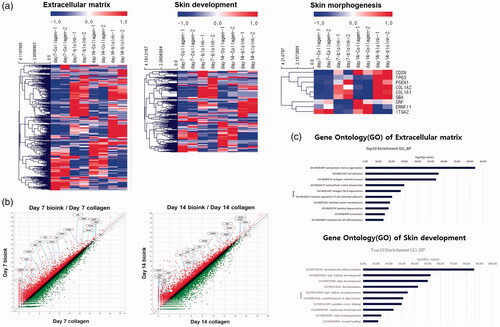
To investigate the influence of the bio-ink on HDFs, 1.5 × 106 HDFs were mixed with collagen and bio-ink for printing. After 7 and 14 days of culture, the construct was lysed using TRIzol for analysing the altered genes through microarray. To identify the changes in biological features due to bio-ink among the 25,737 human genes, GO was carried out. A heatmap was drawn based on GO involving skin morphogenesis, ECM, epidermis development, keratinocyte differentiation, keratinocyte proliferation, skin development and positive regulation of keratinocyte proliferation. The heatmap () showed that the genes encoding collagen, decorin, and laminin, proteins involved in HDF proliferation and dermis formation, increased in the bio-ink with time, and the genes related to epidermis formation had also increased (data not shown). This ultimately led to the expression of genes governing skin morphology and development.
Figure 4. Changes in gene expression in the 3D printed cell laden construct. (a) The heatmap shows that genes related to ECM, skin development, and skin morphology were increased in the 3D printed cell laden construct. (b) The scatter plots show that ECM and ECM-related genes in the skin bio-ink were upregulated. (c) Gene Ontology for ECM and skin development shows that skin development was enriched in the 3D printed cell laden construct.
Discussion
In this study, porcine dermal dECM, a material suitable for bio-ink development, was extracted and its composition was analyzed. An HDF laden 3D construct was constructed, and the increase in gene expression involved in skin morphology and development was confirmed for the cells in the bio-ink printed construct. The bio-ink was developed using dECM treated with detergent and enzymes to leave only the residual ECM in the porcine dermis, and the removal of resident cells was confirmed as shown in . The removal of DNA of 200 bp or fewer that might cause immune responses was also confirmed by quantifying the DNA via agarose gel electrophoresis [Citation14,Citation15]. The representative components in residual ECM, collagen and GAG, were detected. In particular, detection of decorin in dECM was important because decorin is abundant in skin dermal ECM, and by binding to the surface of type I collagen fibrils, it plays a vital role in collagen fibril assembly. Its deletion has been reported to cause abnormal collagen morphology that reduces the tensile strength of skin [Citation16,Citation17].
The ECM also contains cell signalling molecules and growth factors. Thus, we used an antibody array to analyze the skin-related growth factors, and as shows, 24 residual growth factors were found in contrast to purified collagen. Such factors are thought to play a positive role in the viability and proliferation of skin-related cells.
In this study, we systematically analyzed the residual components of bio-inks and evaluated the effect of bio-ink on HDF, while our previous study showed a heating system, a 3D printing method that can accumulate more than 5 mm using skin bio-ink [Citation13]. For this purpose, dECM was extracted using the same procedure, but biofabrication was performed through layer-by-layer rather than hollow-type squares. The upper layer was deposited in the lower layer at a 90° angle to form a lattice, resulting in a size 10 × 10 × 3 mm [Citation18,Citation19]. As shown in , maintaining the 3D construct at 37 °C resulted in gelation so that the shape of the construct was maintained even after 7 days, and encapsulated HDFs were also shown to have maintained their morphology and proliferation.
Through signal transduction, the ECM collagen influences cellular morphology, migration, differentiation, proliferation, and survival [Citation20–22]. The bio-ink contains GAG, bioactive molecules, and growth factors, in addition to collagen. Thus, HDFs were added to the collagen and to the bio-ink for generating respective 3D constructs. Then, the changes in gene expression were compared using microarray. The result showed that, for the HDFs in the bio-ink, the genes related to ECM such as collagen, laminin and decorin, as well as the genes governing skin morphology and development, exhibited enhanced expression in comparison to cells in collagen (). These results suggested that the bio-ink had positive roles on HDFs by providing an environment similar to the in vivo environment. Surprisingly, in a recent study, HEK was printed on 3D constructs, and it was reported that the attachment efficiency of HEK on dermis using skin dECM derived bio-ink was higher than that of collagen, resulting in increased proliferation and differentiation [Citation23]. Indeed, the dermis affects keratinocyte differentiation and epidermis development through interaction with the epidermis [Citation24,Citation25]. For this reason, we analyzed the altered expression of 25,737 human genes by bio-ink through microarray and evaluated epidermis development, keratinocyte differentiation, proliferation, and skin morphogenesis through GO, which provided insight into the skin related mechanism [Citation26].
Skin is the largest organ in the body and protects the body against external stimulus [Citation27]. However, for the cases where burns and other serious skin injuries pose limits to medical treatment, leaving skin transplantation as the only treatment option, many efforts have been made to use bioprinted skin. Based on the findings of this study, the bio-ink is expected to play a positive role in skin regeneration because dermal derived bio-ink can create a 3D environment in which the residual skin-related proteins induce survival and proliferation of human skin-derived cells in addition to skin development.ys
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ji S, Guvendiren M. Recent advances in bio-ink design for 3D bioprinting of tissues and organs. Front Bioeng Biotechnol. 2017;5:23.
- Zhu W, Ma X, Gou M, et al. 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol. 2016;40:103–112.
- Gungor-Ozkerim PS, Inci I, Zhang YS, et al. Bio-inks for 3D bioprinting: an overview. Biomater Sci. 2018;6:915–946.
- Pati F, Jang J, Ha DH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bio-ink. Nat Commun. 2014;5:3935.
- Yi S, Ding F, Gong L, et al. Extracellular matrix scaffolds for tissue engineering and regenerative medicine. Cscr. 2017;12:233–246.
- Skardal A, Devarasetty M, Kang HW, et al. A hydrogel bio-ink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015;25:24–34.
- Nakagawa S, Pawelek P, Grinnell F. Extracellular matrix organization modulates fibroblast growth and growth factor responsiveness. Exp Cell Res. 1989;182:572–582.
- Grinnell F. Fibroblast-collagen-matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 2000;10:362–365.
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677.
- Gao HW, Li SB, Sun WQ, et al. Quantification of alpha-gal antigen removal in the porcine dermal tissue by alpha-galactosidase. Tissue Eng Part C Methods. 2015;21:1197–1204.
- Yu D, Hanna KR, LeGallo RD, et al. Comparison of histological characteristics of acellular dermal matrix capsules to surrounding breast capsules in acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg. 2016;76:485–488.
- Shah RG, DeVore D, Silver FH. Biomechanical analysis of decellularized dermis and skin: initial in vivo observations using optical cohesion tomography and vibrational analysis. J Biomed Mater Res A. 2018;106:1421–1427.
- Ahn G, Min KH, Kim C, et al. Precise stacking of decellularized extracellular matrix based 3D cell-laden constructs by a 3D cell printing system equipped with heating modules. Sci Rep. 2017;7:8624.
- Keane TJ, Londono R, Turner NJ, et al. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771–1781.
- Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34.
- Li Y, Liu Y, Xia W, et al. Age-dependent alterations of decorin glycosaminoglycans in human skin. Sci Rep. 2013;3:2422.
- Danielson KG, Baribault H, Holmes DF, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743.
- Das S, Pati F, Choi YJ, et al. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015;11:233–246.
- Datar A, Joshi P, Lee MY. Biocompatible hydrogels for microarray cell printing and encapsulation. Biosensors (Basel). 2015;5:647–663.
- Koohestani F, Braundmeier AG, Mahdian A, et al. Extracellular matrix collagen alters cell proliferation and cell cycle progression of human uterine leiomyoma smooth muscle cells. PLoS One. 2013;8:e75844.
- Hoganson DM, O’Doherty EM, Owens GE, et al. The retention of extracellular matrix proteins and angiogenic and mitogenic cytokines in a decellularized porcine dermis. Biomaterials. 2010;31:6730–6737.
- Holzl K, Lin S, Tytgat L, et al. Bio-ink properties before, during and after 3D bioprinting. Biofabrication. 2016;8:032002.
- Kim BS, Kwon YW, Kong JS, et al. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bio-ink: a step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53.
- Medalie DA, Eming SA, Collins ME, et al. Differences in dermal analogs influence subsequent pigmentation, epidermal differentiation, basement membrane, and rete ridge formation of transplanted composite skin grafts. Transplantation. 1997;64:454–465.
- Segal N, Andriani F, Pfeiffer L, et al. The basement membrane microenvironment directs the normalization and survival of bioengineered human skin equivalents. Matrix Biol. 2008;27:163–170.
- Bachelor M, Binder RL, Cambron RT, et al. Transcriptional profiling of epidermal barrier formation in vitro. J Dermatol Sci. 2014;73:187–197.
- Rimann M, Bono E, Annaheim H, et al. Standardized 3D bioprinting of soft tissue models with human primary cells. J Lab Autom. 2016;21:496–509.