Abstract
The present study was aimed to develop an effective nanoliposomal vaccine delivery system with P435 HER2/neu-derived peptide conjugated to Maleimide-PEG2000-DSPE. The nanoliposome formulation composed of DSPC/DSPG/Chol/DOPE and monophosphoryl lipid A was used as an adjuvant. Liposomal formulations were prepared and their physical properties were characterized. Anti-tumoral efficacy of formulations was evaluated by immunization of tumor-bearing BALB/c mice and the generated immune response was studied by using ELISpot and flow cytometry analysis. The results of the study demonstrated Lip + DOPE + P535 formulation caused the lowest tumor size and the longest survival time in TUBO mice model and could make it a promising candidate in developing effective vaccines against HER2-positive breast cancers.
Introduction
Cancer is still a major cause of death worldwide, despite many researches. Chemotherapy, radiotherapy and surgery are the major cancer treatments, but chemotherapy destroys normal cells and ionizing radiotherapy or surgery cannot prevent metastases. The tumor relapse and drug resistance mechanisms in tumor cells are important problems, so additional therapeutic approaches must be established [Citation1]. Due to this disadvantage of current treatments, tumor immunotherapy has been paid attention during past decades [Citation2]. Immunotherapy is the treatment of disease with enhancing or suppressing the immune response [Citation3]. In cancer immunotherapy strategies, the immune system is activated against proteins expressed by cancerous cells and shows the effective potential to selectively inhibit cancer growth [Citation4]. Humoral immunity has a low potential to kill solid tumors, but induction of an effective cell-mediated immunity by CTLs activation, specifically CD8+ T cells, is an important goal in cancer immunotherapy [Citation5,Citation6]. Synthetic peptides derived from tumor-associated antigens (TAA) can elicit potent cytotoxic T lymphocytes (CTL) response. To activate an efficient CTL response, TAA-derived peptide should be taken up by antigen-presenting cells (APCs), delivered into the cytosol, bound to major histocompatibility complex (MHC) class I molecules in the endoplasmic reticulum, and finally presented to CD8+ CTLs [Citation7,Citation8]. Therefore, different strategies including design the efficient antigenic peptides, using effective delivery systems and strong adjuvants, have been developed to increase the efficacy of peptide-based cancer immunotherapy [Citation9–12].
Liposomes are safe, well-tolerated and biodegradable carriers that can be formulated with different cationic or anionic lipid constituents and present all types of peptide antigens and adjuvants to cell-mediated immunity system [Citation13–15]. Adjuvants can enhance and prolong immune responses [Citation16]. Monophosphoryl lipid A (MPL) has been frequently used as an efficient adjuvant in liposomal vaccines and has shown adjuvant activity in both cellular and humoral immunity [Citation17]. It is a FDA approved and non-toxic derivative from lipopolysaccharides or endotoxin that stimulates Toll-like receptor 4 (TLR4) which activates the innate immune system [Citation18–21]. HER2/neu (human epidermal growth factor receptor 2) is a 185 kDa transmembrane glycoprotein and one of member of the epidermal growth factor receptor family that is over expressed in 20–40% of primary breast cancers. HER2/neu-derived epitopes have potential of developing breast cancer vaccines [Citation22,Citation23]. Increasing cellular and humoral response against HER2/neu has been associated with decreased tumor development and improved prognosis [Citation24]. In our previous study, several HER2/neu epitopes were formulated in different nanoliposomes, which could induce effective immune responses against HER2-positive murine tumor model [Citation25–28]. Our findings demonstrated that P435, as a short HER2 epitope, was an effective peptide which inducing CTL responses and improving prognosis of cancer in TUBO mice model of breast cancer [Citation29]. Localization of antigens in lymphatic organs such as lymph nodes and interaction of APCs which presents specific antigens to T cells is critical in induction of immune responses [Citation30,Citation31]. In this regard, PEGylation is one way that can be useful. It has been shown that PEGylation reduces the accumulation of particles in the site of injection and increases the migration of particles to the lymph nodes [Citation32–35] and also effectively influence the uptake of conjugated peptides to dendritic cells [Citation36]. An important point in induction of cell-mediated immunity is the delivering antigens through the cytosol to MHC class I molecules [Citation37]. One strategy for this is the use of pH-sensitive phospholipids such as DOPE in composition of liposomes vesicles which cause the endosomal escape of antigens and effective delivery to MHC molecules [Citation38,Citation39]. According to our findings about the effective role of MPL adjuvant, liposomes containing DOPE and also conjugation of peptide to liposomal formulation in mediating cellular immunity [Citation25], in this study besides conjugation peptide to liposomes and MPL, fusogenic liposomes are used to efficiently introduce P435 peptide to cytosol of APCs and enhancing CTL response.
Materials and methods
Materials
P435 is a peptide with 21 amino acids, whose the sequence is as follow (IRGRILHDGAYSLTLQGLGIHGGGC) [Citation29,Citation40]. The peptide with a purity >95% was synthesized by China Peptides Co. (Shanghai, China). 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-distearoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DSPG), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), and Maleimide-PEG2000-DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanolamine) were purchased from Avanti Polar Lipid (Alabaster, AL, USA). MPL from Salmonella enterica was purchased from Sigma-Aldrich (Steinheim, Germany). Cytofix/Cytoperm Plus, phorbol myristate acetate (PMA)/ionomycin cocktail, anti-CD8a-PE-cy5, anti-CD4-PE-cy5, anti-IFN-γ-FITC and anti-IL-4-PE antibodies were provided from BD Biosciences (San Diego, CA, USA). All other solvents and reagents were used as a high purified chemical grade.
Mice
Female BALB/c mice, 4–6 weeks old, were purchased from Pasteur Institute (Tehran, Iran). The mice were kept under controlled condition and all the experiments were carried out under the approval of Ethical Committee and Research Advisory Committee of Mashhad University of Medical Sciences in accordance with animal welfare standards.
Cell lines
BALB/c mammary cancer cell, TUBO, that overexpress rat HER2/neu were generously gifted by Dr. Pier-Luigi Lollini (Department of Clinical and Biological Sciences, University of Turin, Orbassano, Italy) and was maintained in Dulbecco’s modified Eagle’s medium) – containing 20% foetal bovine serum (FBS). The CT26, a murine colon carcinoma cell line, was purchased from Pasteur Institute (Tehran, Iran) and cultured in RPMI (Roswell Park Memorial Institute) contained – 10% FBS.
Conjugation of the P435 peptide to Maleimide-PEG 2000 – DSPE
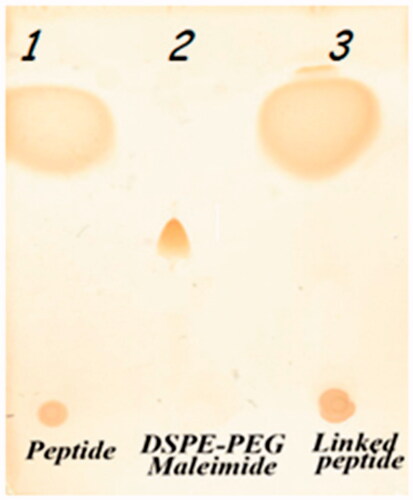
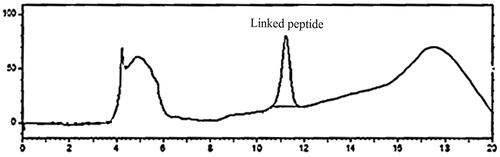
The P435 peptide was conjugated to Maleimide-PEG2000-DSPE with the thioether covalent binding between thiol group of a terminal cysteine of peptide and pyrrole group of maleimide. DSPE-PEG-Maleimide(2000) ammonium salt and P435 peptide were dissolved in chloroform:DMSO solution (1:1) at lipid/peptide molar ratio of 1:1.2. Coupling reaction took place for 48 h at room temperature in an argon atmosphere. The synthesized product was identified by thin layer chromatography (TLC) method which was performed in the mobile phase containing chloroform, methanol and water at 90:18:2 (v/v) and stained with iodine vapor. After verifying the conjugation, the DMSO/chloroform solution was dried by rotary evaporator (Heidolph, Germany) and overnight freeze-drying (VD-800f, Taitech, Japan). The dried product was hydrated with sterile deionized water (pH 7.2) at 30 °C to construct DSPE-PEG-peptide micelles. The prepared micelles were detected via SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) method. The SDS-PAGE consisted of running gel (16% (w/v) acrylamid/6 M urea), stacking gel (4% (w/v) acrylamide) and spacer gel (10% (w/v) acrylamide). The gel thickness was 0.7 mm. The anode buffer was 0.1 M Tris, pH 8.9 and cathode buffer was 0.1 M Tris, 0.1 M Tricine, 0.1% SDS, PH 8.25. Electrophoresis was carried out with an initial voltage of 30 V, which was increased to 300 V at end of the run then stained with silver method [Citation41]. The true value of the conjugated micelles was measured by calculating efficiency of conjugation, determined by high performance liquid chromatography (HPLC) analysis, and content of total lipid, evaluated by the Bartlett phosphateassay method [Citation42]. The conjugated peptide in the prepared micelles was indirectly quantified by measuring fraction of non-conjugated peptide by using HPLC analysis. The conjugation of the peptide with PEG 2000-DSPE was also quantified indirectly by determining unconjugated peptide via HPLC. KNAUER smart line HPLC (Berlin, Germany) with a Nucleosil C18, 5 μm, 150 × 4.6 mm, 100A° column (KENAUER) and a UV detector (KENAUER S2600) set at 220 nm. The mobile phases were A (water + 0.1% TFA) and B (acetonitrile + 0.1% TFA). Eluent gradient started with 100% A and increasing to 30% B in 2 min, 60% B in 10 min and 90% B in 2 min. The flow rate was set at 1 ml/min.
Preparation of nanoliposomal formulations
Liposomes (40 µM Lip/DOPE) composed of DSPC/DSPG/Chol/DOPE at a molar ratio of 15:2:3:5 were prepared using lipid film hydration method, dried to a thin film under reduced pressure by rotary evaporation (Heidolph, Germany) freeze-dried (VD-800F, Taitech, Japan) overnight in order to remove the solvents completely. Then, the lipid films were hydrated in HEPES buffer (10 mM, pH 7.2) containing 5% dextrose, vortexed and bath-sonicated to disperse completely into the buffer. These multilamellar vesicles (MLVs) were extruded through 400 nm, 200 nm, and 100 nm polycarbonate membrane using a mini-extruder (Avestin, Canada). To formulate Lip + DOPE-containing MPL (Lip + DOPE + MPL), through liposome preparation MPL (25 µg/mice) was mixed with lipids. To construct Lip + DOPE + MPL + P435 vaccine, P435-PEG-DSPE micelle was post-inserted into the liposome nanoparticles through incubation at TM threshold of the liposome formulation (45 °C) shaking with 250 rpm (Innova 4080 Incubator shaker) for 4 h.
Characterization of nanoliposomal formulations
The conjugation of P435-PEG-DSPE micelle and liposomal nanoparticles was quantified by HPLC analysis as mentioned above. Total lipid phosphorus was determined by the method of Bartlett [Citation42]. In order to disrupt liposomes, formulations were added to 1.5% (v/v) C12E10 detergent and then MPL content was assayed by the limulus amebocyte lysate (LAL) chromogenic endpoint assay (QCL-1000, Lonza, Walkersville, MD) [Citation43]. Liposome nanoparticles size, polydispersity and zeta potential were characterized by dynamic light scattering (Malvern Instruments, Malvern, UK). Liposomes were stored at 4 °C under argon [Citation42,Citation43].
Immunization schedule
Seven groups of BALB/c mice were subcutaneously injected three times at 2-week intervals with different nanoliposomal formulations. The dose of 10 μM/mouse of liposomes, 10 μg/mouse of free P435 peptide and HEPES-dextrose buffer were used for each injection of liposomal, free peptide and control groups, respectively. Fourteen days after the last inoculation, the mice (three per group) were killed and their splenocytes collected in an aseptic situation to evaluate immune responses.
Enzyme-linked immunospot (ELISpot) assay
ELISpot assays were done by mouse ELISpot kits from U-CyTech (Utrecht, The Netherlands) according to the manufacturer’s instructions to detect cytotoxic T-cell activation via measuring the number of cytokines-producing cells. One day before mice killing, ELISpot plates were coated with 15 μg/ml anti-IFN-γ and/or anti-IL-4 murine antibodies and incubated overnight at 4 °C. Splenocytes were cultured triplicately in wells in a final volume of 200 μl with medium containing P435 peptide (10 μg/ml) in pre-coated plates. Subsequently, the isolated splenocytes were added at 3 × 105 cells per well and re-stimulated either with 10 μg/ml P435 peptide, 10 μg/ml PHA (phytohemagglutinin) (Gibco) or RPMI medium (Gibco) supplemented with 10% foetal calf serum (FCS) (Gibco) and incubated for 24 h at 37 °C. Afterwards, the biotinylated anti-mouse IFN-γ and/or IL-4 detection antibodies were added and incubated for 1 h at 37 °C. Gamma-aminobutyric acid (GABA) solution containing anti-biotin antibody was then added and incubated for 1 h at 37 °C. Eventually, spot-forming cells (SFC) were detected according to the manufacturer’s guidelines, and the amount of INF-γ- and/or IL-4-producing cells were determined by counting the number of spots per well using Kodak 1D image analysis software (Version 3.5, Eastman Kodak, Rochester, NY). Results were expressed as the number of anti-INF-γ and/or anti-IL-4 SFC per 106 cells. For each group, triplicate measurements were done.
Flow cytometric analysis of intracellular cytokines
Evaluation of intra-cellular INFγ and IL-4 cytokines was performed as explained [Citation44]. Briefly, 106 splenocytes/ml in medium containing GolgiPlug (1 μl/ml) was stimulated with PMA/ionomycin cocktail (2 μl/ml) for 4 h at 37 °C. Then, in order to stain the cell extra- and intracellularly, 105 splenocytes of each groups were transferred into flow cytometry tubes. For extracellular staining, splenocytes were stained with 1 μl of each anti-CD4-PE-cy5 and anti-CD8a-PE-cy5 antibodies in different tubes for 30 min at 4 °C. The cells were washed with stain buffer and fixed by Cytofix/Cytoperm solution. Washing was done two times with Perm/Wash buffer and then for intracellular cytokines stained with 1 μl anti-IFN-γ-FITC and IL-4 PE antibodies for 30 min at 4 °C for CD8 and CD4 T cells and just CD4 T cells, respectively. Then washed with Perm/Wash buffer and suspended in 300 μl stain buffer for flow cytometric analysis (BD FACSCalibur, BD Biosciences, San Jose, CA, USA).
In vitro CTL assay
Two weeks after the last inoculation, splenocytes were isolated from each group (three mice per groups) and then re-stimulated with P435 peptide (10 μg/ml). Targeted cells, TUBO cell line, were incubated with 12.5 μM Calceine AM at 37 °C for 1 h in the dark [Citation45]. TUBO cells (2 × 104) were incubated with different ratio of stimulated splenocytes, as effector cells, in round-bottom plates in triplicate wells for 4 h in the dark at 37 °C. To determine the minimum and maximum release by TUBO cells, culture medium only and medium containing 2% Triton X-100 were added to the wells, respectively. Triton X-100 can tear up all the cells and release the dye. Fluorescence in supernatants was read by fluorimeter (FLx800, BioTek Instruments Inc., USA) with excitation at 485 nm and emission at 538 nm. The specific lysis was calculated by this formula: percentage of specific lysis = (release by CTLs − minimum release by TUBO cells)/(maximum release by TUBO cells − minimum release by TUBO cells). Like TUBO cells, CT26 cells were also labelled and used as negative control.
In vivo tumor prophylaxis assay
Two weeks after the last inoculation, the immunized mice (six per group) were subcutaneously challenged in the right flank with 5 × 105 TUBO cells in PBS buffer. The tumor volume of each mouse ([length × width × height] × 0.52) was measured regularly [Citation46,Citation47]. For ethical consideration, mice were killed if the tumor volume was greater than 1000 mm3, body weight decreases over 15% of initial weight or the mice became lethargic or sick and unable to feed.
Statistical analysis
The results were analyzed using one-way ANOVA and in case of significance, Tukey test used to evaluate the differences among formulations. Mouse survival was analyzed by log-rank test (GraphPad Prism, version 5, San Diego, CA). The results with p < .05 were considered as statistically significant.
Results
Preparation of P435-PEG-DSPE micelle
As depicted in , TLC analysis verified the binding of P435 to Maleimide-PEG2000-DSPE and formation of P435-PEG-DSPE. As found by HPLC analysis, the efficiency of reaction between maleimide group in PEG2000-DSPE and thiol in P435 peptide was up to 100% ().
Characterization of nanoliposome formulations
The physical properties, including size, polydispersity index (PDI) and zeta potential of prepared formulations, were determined as shown in . All liposomal formulations showed a size ranged from 159.1 nm to 183.1 nm in diameter, in which PDI was <0.2, showing homogeneity of particles. Zeta potential analysis also revealed negative charge on the surface of liposome nanoparticles. Since the amounts of peptide antigen and adjuvant can significantly influence the efficacy of immunization [Citation48], contents of P435 peptide, phospholipids, and MPL were determined in different liposomal formulations properly (). According to the lipid dose (4 µm), MPL dose and peptide dose per mouse were determined as 25 µg and 10 µg, respectively, for each formulation.
Table 1. Physical properties of liposomal formulations.
Table 2. Content of P435 peptide and MPL in liposomal formulations.
SDS-PAGE analysis
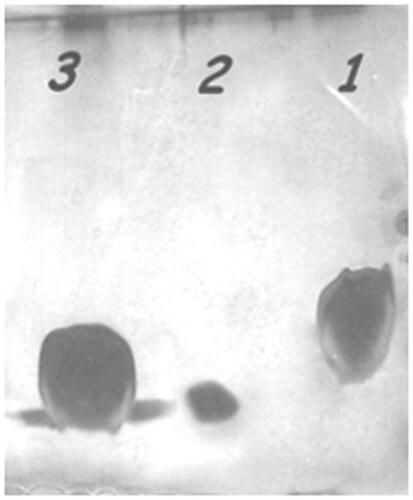
The presence of P435-PEG-DSPE micelles in liposome nanoparticles was further confirmed by SDS-PAGE analysis ().
Determination of cytokine-producing splenocytes
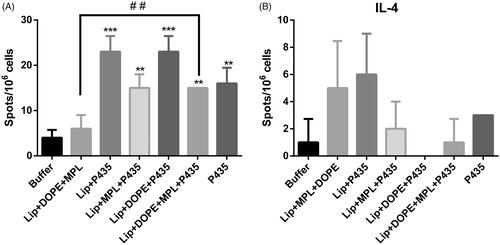
The levels of splenocytes producing IFN-γ and IL-4 cytokines in the isolated spleens were measured using ELIspot assay (). The results showed that splenocytes of the mice immunized with nanoliposomes composed of DSPC/DSPG/Chol/DOPE containing P435 peptide (Lip + DOPE + P435) produced significantly highest amount of IFN-γ and the lowest amount of IL-4 than the other liposomal formulations, P435 peptide alone, and HEPES buffer. Among different groups, Lip + P435 group showed the highest level of IL-4-producing cells in the mice’s spleen.
Figure 4. Extracellular determination of IFN-γ, IL-4 by ELIspot. The efficacy of different liposomal formulations in inducing IFN-γ (A) and IL-4 (B) production. BALB/c mice were immunized three times at two-week intervals with different liposomal formulations. On 14 days’ post-last booster, four mice from each group were killed and their splenocytes were re-stimulated with peptide. IFN-γ and IL-4 release from splenocytes induced by different liposomal formulations was determined using ELISpot assay. The data indicate the mean ± SEM (n = 3).
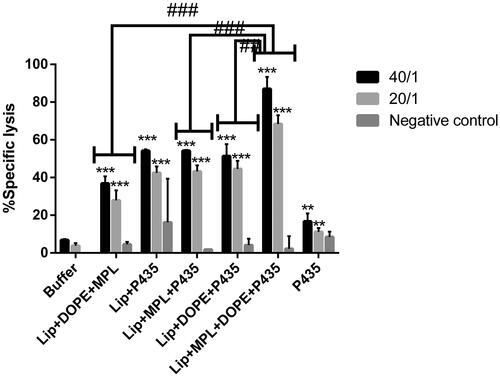
In vitro antigen-specific cytotoxicity
Exposing the TUBO cells to different ratios of splenocytes (40/1, 20/1 and 10/1 ratio of effector cells to target cells) isolated from different groups revealed that splenocytes from all immunized groups generated a significantly higher cytotoxicity against TUBO tumor cell than those from buffer group. However, splenocytes from Lip + DOPE + MPL + P435 group showed the most cytotoxicity effect with two different ratios (20/1 and 40/1) of effector/target cells ().
Figure 5. Antigen-specific CTL response induced by various formulations at two different ratios of (20/1 and 40/1) effector to target cells (E/T) was assessed using an in vitro CTL activity assay. Splenocytes isolated from mice (three in each group) were incubated with Calcein AM-loaded rHER2/neu-expressing TUBO tumor cells and rHER2/neu-expressing negative CT26 cells. The data indicate the mean ± SEM (n = 3).
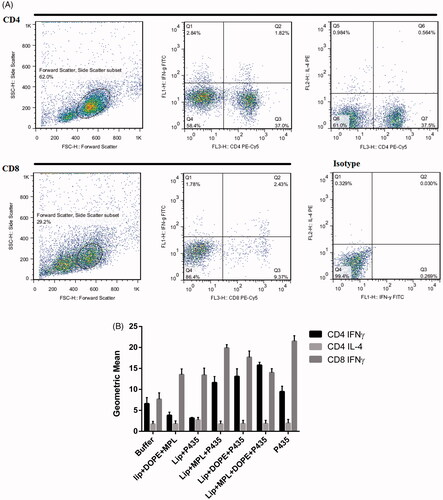
Intracellular cytokine assay
Intracellular cytokine assay was evaluated to analyse the effect of different formulations on induction of CD4+ and CD8+ T cells. shows the dot plot graphs of both activated Th1 and CTL responses. As shown in , Lip + MPL + P435 and Lip + MPL + DOPE + P435 induced the highest level of CD8+ and CD4+ cells secreting IFN-γ compared to other liposomal groups.
Figure 6. Flow cytometric dot plot analysis of extracellular and intracellular cytokines (A) and the geometric mean fluorescence intensity (MFI) level for IFN-γ and IL-4 in gated CD8 and CD4 and CD4 T-lymphocyte populations (B), respectively. Isolated splenocytes of immunized mice were re-stimulated in vitro with PMA/ionomycin and stained with CD4, CD8, IFN-γ and IL-4 markers. The data indicate the mean ± SEM (n = 3).
In vivo anti-tumor effects of vaccination in BALB/c
The protection effect of CTLs responses of different formulations were evaluated in HER2-overexpressing tumor-bearing mice. Lip + DOPE + MPL + P435 formulation showed the most tumor growth inhibition in mice-bearing TUBO tumor cells (). The survival time was also significantly longer in the Lip + DOPE + MPL + P435 group compared to the other formulations (; ). Median survival time (MST), time to reach end point (TTE) and tumor growth delay % (TGD %) data for each group are illustrated in .
Figure 7. Protective effects of vaccination with different formulations in BALB/c mice against a TUBO tumor model. Immunized mice (seven in each group) were challenged 14 days’ post-last booster with 5 × 105 TUBO cells. Tumor size was calculated based on three dimensions. The values are means of tumor size ± SEM (n = 7).
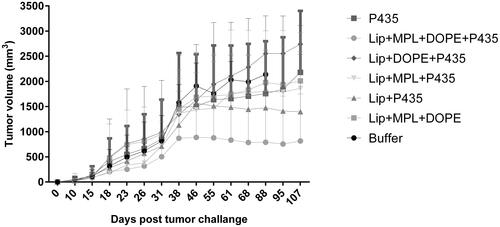
Figure 8. Survival test. Effects of immunization on survival time were monitored for a period of 107 days among BALB/c mice (n = 7) the Lip + DOPE + MPL + P435 formulation showed the most tumor growth inhibition.
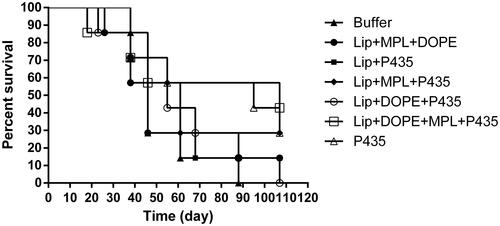
Table 3. Effects of immunization on survival time were monitored for a period of 107 days among BALB/c mice (n = 7).
Table 4. Therapeutic efficacy data from different liposomal vaccine formulations in mice-bearing TUBO tumor.
Discussion
According to our previous study, P435 peptide, both in free or liposomal form, was sufficiently potent to induce CTL responses in tumor-bearing mice [Citation29]. Moreover, fusion of this peptide to another HER2/neu peptide, P5 epitope, resulted in expansion of Th1 and CTL lymphocytes, decreasing tumor growth, and prolonged survival of the vaccinated mice [Citation49]. In the present study, we enhanced the immunogenicity of P435 peptide, a synthetic multi-epitope peptide from rHER2/neu protein, by conjugation with different nanoliposomal formulations. It has been showed although P435 peptide in conjugation with liposomes (Lip + P435) can induce immune responses [Citation50,Citation51], but this formulation has no potential to conduct antigens into the cytosol. Adding cholesterol to this formulation increases the in vivo stability of the liposomes, with more inflexible structure which promotes stimulation of CD8+ T-cell responses. Cholesterol can also enhance the release of antigens from cytoplasm and avoid the vesicle lysosomal degradation [Citation52,Citation53]. DOPE a pH-sensitive lipid in the liposome structure has been demonstrated as an efficient tool to introduce antigens in to cytosol and MHC class I pathway. It induces the fusion of liposomes with the endosomal membrane and subsequently liposomal antigens are released into the cytosol, leading to stimulation of cellular immune response that is very important in cancer treatment [Citation47,Citation54–59]. Further, MPL is an excellent adjuvant that can co-stimulate CTL response and enhance the peptide immunogenicity. In addition, MPL can induce intracellular signaling pathways through TLR4 stimulation leading to production of co-stimulatory molecules [Citation60]. It has been also shown that the size of liposome can influence lymphatic uptake and lymph node localization in subcutaneous administration [Citation28]. In this investigation, P435-linked liposome formulations were prepared with particle sizes ranged from 159 to 183 nm that are suitable for efficient delivery to lymph nodes where CD8+ lymphoid DCs are present [Citation61,Citation62]. As detected by ELIspot assay, results showed that Lip + DOPE + P435 can stimulate significantly higher amount of IFN-γ, and Lip + P435 formulation induced the highest amount of IL-4 in mice. Lip + DOPE + MPL + P435 formulation showed the most cytotoxicity effect with two different ratios of effector/target cells. Lip + MPL + P435 trigged the most amount of IFN-γ production by CD8+ cells among liposomal formulations, but the P435 peptide provoked the most amount of IL-4 production by CD8+ cells. Lip + DOPE + MPL + P435 formulation showed the most tumor growth inhibition in the TUBO tumor mice model, and the survival time was also significantly longer in the mice immunized by Lip + DOPE + MPL + P435 formulation compared to the other administrated formulations.
In conclusion, Lip + DOPE + MPL + P435 can make a potential candidate to develop liposomal vaccines in terms of antitumor prophylaxis of HER2-positive breast cancers and merits further investigations.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Borghaei H, Smith MR, Campbell KS. Immunotherapy of cancer. Eur J Pharmacol. 2009;625:41–54.
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433.
- Wei Y. The influence of calcium infiltration on the sintering behavior and dissolution of beta-tricalcium phosphate [Master thesis]. University of Pittsburgh; 2015.
- Jaffee EM. Immunotherapy of cancer. Ann N Y Acad Sci. 1999;886:67–72.
- Hanson HL, Donermeyer DL, Ikeda H, et al. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–276.
- Matsui K, O’Mara LA, Allen PM. Successful elimination of large established tumors and avoidance of antigen‐loss variants by aggressive adoptive T cell immunotherapy. Int Immunol. 2003;15:797–805.
- Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357:1777–1789.
- Burgdorf S, Kurts C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr Opin Immunol. 2008;20:89–95.
- Babai I, Barenholz Y, Zakay-Rones Z, et al. A novel liposomal influenza vaccine (INFLUSOME-VAC) containing hemagglutinin–neuraminidase and IL-2 or GM-CSF induces protective anti-neuraminidase antibodies cross-reacting with a wide spectrum of influenza A viral strains. Vaccine. 2001;20:505–515.
- Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta. 2002;1589:1–13.
- Krieg A, Davis H. Enhancing vaccines with immune stimulatory CpG DNA. Curr Opin Mol Therap. 2001;3:15–24.
- Amir Jalali S, Parmiani G. Pre-clinical and clinical aspects of peptide-based vaccine against human solid tumors. Recent Pat Biotechnol. 2011;5:108–117.
- Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30:2256–2272.
- Alving CR. Liposomes as carriers of antigens and adjuvants. J Immunol Methods. 1991;140:1–13.
- Zamani P, Momtazi-Borojeni AA, Nik ME, et al. Nanoliposomes as the adjuvant delivery systems in cancer immunotherapy. J Cell Physiol. 2018;233:5189–5199.
- Nordly P, Madsen HB, Nielsen HM, et al. Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immunostimulators. Exp Opin Drug Deliv. 2009;6:657–672.
- Ulrich JT, Myers KR. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharm Biotechnol. 1995;6:495–524.
- Alving CR. Lipopolysaccharide, lipid A, and liposomes containing lipid A as immunologic adjuvants. Immunobiology. 1993;187:430–446.
- Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231.
- Vacchelli E, Galluzzi L, Eggermont A, et al. Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology. 2012;1:894–907.
- Brubaker SW, Bonham KS, Zanoni I, et al. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–290.
- Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61:1–13.
- Baxevanis CN, Sotiriadou NN, Gritzapis AD, et al. Immunogenic HER-2/neu peptides as tumor vaccines. Cancer Immunol Immunother. 2006;55:85–95.
- Disis ML, Calenoff E, McLaughlin G, et al. Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res. 1994;54:16–20.
- Shariat S, Badiee A, Jalali SA, et al. P5 HER2/neu-derived peptide conjugated to liposomes containing MPL adjuvant as an effective prophylactic vaccine formulation for breast cancer. Cancer Lett. 2014;355:54–60.
- Mansourian M, Badiee A, Jalali SA, et al. Effective induction of anti-tumor immunity using p5 HER-2/neu derived peptide encapsulated in fusogenic DOTAP cationic liposomes co-administrated with CpG-ODN. Immunol Lett. 2014;162:87–93.
- Yazdani M, Amir Jalali S, Badiee A, et al. Stimulation of tumor-specific immunity by p5 HER-2/neu generated peptide encapsulated in nano-liposomes with high phase transition temperature phospholipids. Curr Drug Deliv. 2017;14:492–502.
- Talesh GA, Ebrahimi Z, Badiee A, et al. Poly (I:C)-DOTAP cationic nanoliposome containing multi-epitope HER2-derived peptide promotes vaccine-elicited anti-tumor immunity in a murine model. Immunol Lett. 2016;176:57–64.
- Jalali SA, Sankian M, Tavakkol-Afshari J, et al. Induction of tumor-specific immunity by multi-epitope rat HER2/neu-derived peptides encapsulated in LPD Nanoparticles. Nanomed Nanotechnol Biol Med. 2012;8:692–701.
- Banchereau JSRM. Dendritic cells and the control of immunity. Nature. 1998;395:245–252.
- Banchereau JBF, Caux C, Davoust J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811.
- Moghimi SMHAE, Christy NM, Gray T, et al. Surface engineered nanospheres with enhanced drainage into lymphatics and uptake by macrophages of the regional lymph nodes. FEBS Lett. 1994;344:25–30.
- Hawley AE, Illum L, Davis SS. Preparation of biodegradable, surface engineered PLGA nanospheres with enhanced lymphatic drainage and lymph node uptake. Pharm Res. 1997;14:657–661.
- Illum LCAE, Butterworth MD, Arien A, et al. Development of systems for targeting the regional lymph nodes for diagnostic imaging: in vivo behaviour of colloidal PEG-coated magnetite nanospheres in the rat following interstitial administration. Pharm Res. 2001;18:640–645.
- MS M. The effect of methoxy-PEG chain length and molecular architecture on lymph node targeting of immuno-PEG liposomes. Biomaterials. 2006;27:136–144.
- Zhan X, Tran KK, Shen H. Effect of the poly(ethylene glycol) (PEG) density on the access and uptake of particles by antigen-presenting cells (APCs) after subcutaneous administration. Mol Pharm. 2012;9:3442–3451.
- Eiji Y. Design of pH-sensitive polymer-modified liposomes for antigen delivery and their application in cancer immunotherapy. Polym J. 2016;48:761–771.
- Bungener LSK, Bijl L, Leserman L, et al. Virosome-mediated delivery of protein antigens to dendritic cells. Vaccine. 2002;20:2287–2295.
- Bungener LHA, de Mare A, de Vries-Idema J, et al. Virosome-mediated delivery of protein antigens in vivo: efficient induction of class I MHC-restricted cytotoxic T lymphocyte activity. Vaccine. 2005;23:1232–1241.
- Shariat S, Badiee A, Jaafari MR, et al. Optimization of a method to prepare liposomes containing HER2/Neu-derived peptide as a vaccine delivery system for breast cancer. Iran J Pharm Res. 2014;13:15–25.
- Schagger H. Tricine-SDS-PAGE. Nat Protoc. 2006;1(1):16–22.
- Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468.
- Harmon P, Cabral-Lilly D, Reed RA, et al. The release and detection of endotoxin from liposomes. Anal Biochem. 1997;250:139–146.
- Nikpoor AR, Tavakkol-Afshari J, Sadri K, et al. Improved tumor accumulation and therapeutic efficacy of CTLA-4-blocking antibody using liposome-encapsulated antibody: in vitro and in vivo studies. Nanomed Nanotechnol Biol Med. 2017;13:2671–2682.
- Lichtenfels R, Biddison WE, Schulz H, et al. CARE-LASS (calcein-release-assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J Immunol Methods. 1994;172:227–239.
- Steinman RM, Bonifaz L, Fujii SI, et al. The innate functions of dendritic cells in peripheral lymphoid tissues. Adv Exp Med Biol. 2005;560:83–97.
- Reddy R, Zhou F, Huang L, et al. pH sensitive liposomes provide an efficient means of sensitizing target cells to class I restricted CTL recognition of a soluble protein. J Immunol Methods. 1991;141:157–163.
- Matyas GR, Mayorov AV, Rice KC, et al. Liposomes containing monophosphoryl lipid A: a potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine. 2013;31:2804–2810.
- Ghaffari-Nazari H, Tavakkol-Afshari J, Jaafari MR, et al. Improving multi-epitope long peptide vaccine potency by using a strategy that enhances CD4+ T help in BALB/c mice. PLoS One. 2015;10:e0142563.
- Hale AH. H-2 antigens incorporated into phospholipid vesicles elicit specific allogeneic cytotoxic T lymphocytes. Cell Immunol. 1980;55:328–341.
- Raphael L, Tom B. Liposome facilitated xenogeneic approach for studying human colon cancer immunity: carrier and adjuvant effect of liposomes. Clin Exp Immunol. 1984;55:1.
- Firouzmand H, Badiee A, Khamesipour A, et al. Induction of protection against leishmaniasis in susceptible BALB/c mice using simple DOTAP cationic nanoliposomes containing soluble Leishmania antigen (SLA). Acta Tropica. 2013;128:528–535.
- Barati N, Nikpoor AR, Razazan A, et al. Nanoliposomes carrying HER2/neu-derived peptide AE36 with CpG-ODN exhibit therapeutic and prophylactic activities in a mice TUBO model of breast cancer. Immunol Lett. 2017;190:108–117.
- Nair S, Zhou F, Reddy R, et al. Soluble proteins delivered to dendritic cells via pH-sensitive liposomes induce primary cytotoxic T lymphocyte responses in vitro. J Exp Med. 1992;175:609–612.
- Chang J-S, Choi M-J, Cheong H-S, et al. Development of Th1-mediated CD8+ effector T cells by vaccination with epitope peptides encapsulated in pH-sensitive liposomes. Vaccine. 2001;19:3608–3614.
- Luo L, Li Y, Chang J-S, et al. Induction of V3-specific cytotoxic T lymphocyte responses by HIV gag particles carrying multiple immunodominant V3 epitopes of gp120. Virology. 1998;240:316–325.
- Siegel D, Epand R. The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: implications for membrane fusion mechanisms. Biophys J. 1997;73:3089–3111.
- Connor J, Yatvin MB, Huang L. pH-sensitive liposomes: acid-induced liposome fusion. Proc Natl Acad Sci. 1984;81:1715–1718.
- Düzgüneş N, Straubinger RM, Baldwin PA, et al. Proton-induced fusion of oleic acid-phosphatidylethanolamine liposomes. Biochemistry. 1985;24:3091–3098.
- Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov Today. 2003;8:934–943.
- Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787.
- Badiee A, Khamesipour A, Samiei A, et al. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: rgp63 as a model antigen. Exp Parasitol. 2012;132:403–409.