Abstract
Inflammatory bowel disease (IBD) is an intestinal chronic inflammatory disease, and is related to imbalance of CD4+T subsets. However, the current treatments of chronic colitis are not ideal and have potential side effects. Therefore, more effective and safer biologically active substances which are extracted from natural plants have been widely concerned. In this study, it was found that Inonotus obliquus polysaccharides (IOP), the main bioactive constituent of Inonotus obliquus, can alleviate dextran sodium sulfate-induced chronic murine intestinal inflammation. Oral administration of IOP (100, 200, 300 mg/kg) can significantly reduce the disease active index and alleviate the pathological changes in colitis mice, where the tight junction proteins Occludin and ZO-1 losses in colon tissues were reduced. It can also regulate imbalanced Th1/Th2 and Th17/Treg in colon tissues, mesenteric lymph nodes and spleen using Reverse Transcription-Polymerase Chain Reaction detection and flow cytometry. Immunohistochemistry and western blot assays further revealed the modulatory effect of IOP on the p-STAT1, p-STAT6, p-STAT3 expression, which promoted the balance of Th1/Th2, Th17/Treg in the colon of chronic colitis mice. In short, these results indicated that IOP was potentially effective therapeutic agent for IBD.
Introduction
Inflammatory bowel disease (IBD), including indeterminate colitis, ulcerative colitis (UC) and Crohn's disease (CD), have been regarded as abnormal chronic IBD [Citation1]. So far, 396 per hundred thousand Americans were affected by IBD and 8–12 per hundred thousand individuals worldwide suffer from UC [Citation2–4]. The long-term irreversible damage to the gastrointestinal structure and function in IBD patients contributed to an increased risk of colon cancer. Current treatments include aminosalicylic acid (ASA), corticosteroids, immunomodulatory drugs and anti-TNF-α drugs, but, the efficacy cannot be expected [Citation5–7]. Therefore, more effective and safer drugs with fewer side effects are expected for IBD.
So far, the pathogenesis of IBD is not completely clear, it may be affected by environmental, genetic, infection and immune factors. The immune response by intestinal injury and the excessive expression of inflammatory cytokines may play an important part in IBD [Citation8,Citation9]. CD4+ T cells are also known as T helper (Th) cells, and inflammatory responses mediated by Th1, Th2 and Th17 are associated with the development and progression of IBD [Citation10–12]. Imbalances in Th1/Th2 and Th17/Treg are considered to be an important cause of IBD [Citation13–15]. A large number of studies have shown that phytochemical agents can alleviate experimental IBD by modulating the JAK-STAT pathway to regulate Th subsets. For example, Li et al. [Citation16] confirmed that Berberine can alleviate DSS-induced chronic inflammatory effects by inhibiting the STAT3-Th17 pathway. Tao et al. [Citation17] demonstrated that natural flavonoid glucoside icariin could suppress the activation of STAT1 and STAT3 to inhibit Th1/Th17 responses, hence alleviating the experimental colitis in mice.
Medicinal fungi have been reported to possess medicinal values. Fungal polysaccharides are the most active ingredients, which show such effects as anti-hyperlipidaemia, delay of decrepitude, anti-tumour, anti-virus, detoxification, and promoting biosynthesis of protein and nucleic acid [Citation18] and there is no toxicity on normal cells. Inonotus obliquus, the most promising medicinal fungus, grow on the birch trunks at a low latitude (ca. 45–50°N) in Europe and North America; this fungus belongs to basidiomycotina’s hymenochaetaxeae family and is a white-rot fungus [Citation19]. Inonotus obliquus polysaccharides (IOPs) may potentially be employed for the prevention and treatment of cancer, asthma, heart disease, diabetes, AIDs and hyperlipoidaemia [Citation20–22].
Dextran sulphate sodium (DSS), a chemical substance widely used for colitis induction, can be utilized to construct DSS models with plenty of symptoms comparable with those of UC in human body [Citation16]. Furthermore, repeated treatment of mice with DSS can simulate the clinical features of recurrent human chronic UC. Therefore, in this study, DSS-induced experimental models of chronic IBD were used to confirm that IOP can alleviate experimental colitis by inhibiting the JAK-STAT signalling pathway to regulate CD4+ T cells, and that IOP may be a potential therapeutic agent for IBD.
Chemicals and assays
Chemicals
IOP was prepared by Department of Pathogenic Biology and Immunology, Yanbian University (Yanji, China). DSS (M.W. = 36,000–50,000) was acquired from MP Biomedicals (Solon, OH). Trizol, M-MLV first strand kits, Fast HiFidelity PCR kits and relevant primers were bought from Promega (Madison, WI). The Total Protein Extraction kit (BC3710) was bought from Solarbio (Beijing, China). STAT3 (124H6) mouse mAb, Phospho-STAT3 (Tyr705) (D3A7) mAb, STAT1 (D1K9Y) rabbit mAb, Phospho-STAT1 (Tyr701) (58D6) mAb were commercially available in Cell Signaling Technology (Beverly, MA) and anti-ZO-1 tight junction (TJ) protein antibody ab216880, Anti-Occludin antibody [EPR20992] ab216327, Anti-STAT6 antibody [YE361] ab32520, Anti-STAT6 (phospho Y641) antibody ab28829 were purchased from Abcam (Cambridge, UK). Peroxidase-conjugated goat anti-rabbit IgG (H + L) and anti-mouse IgG (H + L) were commercially available in Zsbio (Beijing, China). PMA/Ionomycin Mixture (250X) CS1001, BFA/Monesin Mixture (250X) CS1002 and FIX/PERM kits (GAS003) were purchased from Mulin Sciences (Hangzhou, China). Foxp3 Staining Buffer Set, FITC-Anti-Foxp3 antibody, PE-Anti-CD25 antibody, PE-Anti-IL-4 antibody, APC-Anti-CD4 antibody and FITC-Anti-CD4 antibody were commercially available in eBiosciences (San Diego, CA). APC-Anti-IL-17A antibody and PerCP-Cy5.5-Anti-IFN-γ antibody were commercially available in Biolegend (San Diego, CA).
High-performance liquid chromatography (HPLC) characterization for IOP
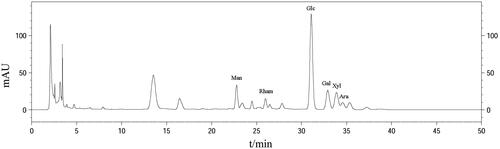
The Chromaster liquid chromatography system (HITACHI, Tokyo, Japan) was used to characterize the monosaccharide composition of IOP [Citation19]. A mixture was prepared by adding 1 ml of 4 mol/l trifluoroacetic acid (TFA) into 1 ml of 5 mg/ml IOP ultrapure water solution in a centrifuge tube which was full of N2, followed by hydrolysation at 110 °C for 3 h and cooling down to room temperature. Six hundred microlitres hydrolysate was added with 600 μL methanol solution and fully mixed, which was then dried with N2 and this process was repeated for three cycles to discard TFA. Then, the methanol solution supplemented with 1-phenyl-3-methyl-5-pyrazolone (PMP; 250 μL; 0.5 mol/l) and distilled water (400 μL) were further introduced and maintained for 100 min at 70 °C. This mixture was left cooling down to room temperature. Afterwards, neutralization and extraction were performed with addition of HCl (0.3 mol/l; 500 μL) and chloroform solution (500 μL) in turn. This step was repeated for three times, and the chloroform was discarded. Finally, the prepared liquid was filtered with microporous membrane (0.45 μm), followed by analysis using HPLC. Calibration was carried out by monosaccharides Man, Rha, Glc, Gal, Xyl and Ara.
Mice
Male BALB/c mice (weight, 18–20 g; age, 6 weeks) were commercially available in Yanbian University, and acclimatized for one week before experimentation. Under controlled conditions at a humidity of 70–75% and stationary temperature of 24–25 °C, these mice were housed in the Animal Laboratory Centre of Yanbian University (Yanji, China), with a light/dark cycle of 12 h and these test mice were provided with standard diet of ad libitum and water. The experimental protocol won approval from the Animal Care Ethics Committee of Yanbian University.
Chronic colitis induction
These selected mouse samples were classified into five groups, i.e. control group, model group (DSS group), DSS plus IOP (100, 200 and 300 mg/kg) groups, with 10 mice in each group. Experimental induction of colitis was performed by DSS application as described by Wirtz et al. [Citation16]. In brief, a DSS solution was prepared by dissolving DSS into drinking water, with the concentration adjusted at 3% (w/v). These mice were given five-day 3% DSS and then 14-day water for the initial two cycles. Then these test mice were only given five days of DSS in the 3rd cycle. The 1st day and the last day of the DSS treatment were marked day 1 and day 43. On the 6th day, mice developed colitis. Intragastric administration of IOP was provided to DSS plus IOP groups. Water gavage was supplied to DSS and control groups.
Clinical disease scores
Mice were monitored disease activity index (DAI) every day, which consisted with three factors: body weight loss, stool consistency and hematochezia. The scoring standards of DAI are as follows: (a) body weight loss (0: no loss; 1: 1–5% loss; 2: 5–10% loss; 3: 10–15% loss; 4: over 15% loss); (b) stool consistency (0: normal; 1: loose but shaped; 2: very loose; 3: diarrhoea); (c) hematochezia (0: no hematochezia; 1: positive haemoccult; 2: blooding in stool; 3: rectal bleeding) [Citation23]. DAI was computed with the sum of these scores.
Sample collection
At 44th day, mice were sacrificed, spleens and mesenteric lymph nodes were collected. The colon is separated from the proximal colonic pubic symphysis, length was measured and segmentation was done according to the following method: the distal 1.5 cm of colon was fixed in 4% Formaldehyde solution, stained with haematoxylin–eosin (H&E) and immunohistochemical (IHC), the next 1.5 cm of colon was snap frozen with liquid nitrogen, detected with reverse transcription-polymerase chain reaction (RT-PCR), the rest of colon was snap frozen using liquid nitrogen for western blot (WB) assay.
Haematoxylin and eosin staining
In order to evaluate the colon injury and inflammation, colon tissues were embedded into paraffin, and sections (thickness, 4 μm) were prepared, followed by H&E staining for histological analysis. These sections were observed under a microscope at ×20 magnification. Histology was scored according to Sun et al. [Citation24]: epithelia: normal morphology, 0; lost goblet cells, 1; lost goblet cells in large areas, 2; lost crypts, 3; lost crypts in large areas, 4; and infiltration: no infiltration, 0; infiltrating crypt basis, 1; infiltrating muscularis mucosa, 2; extensively infiltrating muscularis mucosa with thickened mucosa and obvious swelling, 3; infiltrating submucosa, 4. The total score was calculated based on addition of the two scores.
Immunohistochemistry (IHC) staining
The paraffin-embedded colon tissues were deparaffinized and hydrated, and then put into 0.01 M of sodium citrate (pH 6.0) preheated to boiling by microwave for 15 min, Endogenous peroxidase blocking solution (50 μL) was added dropwise to each section of colon tissues and stand at ambient temperature followed by PBS washing. After the addition of specific antibodies from rabbit against ZO-1, Occludin, p-STAT1, p-STAT3, p-STAT6, they were maintained overnight at 4 °C. These sections were washed on the 2nd day, and then introduced with peroxidase-conjugated goat anti-rabbit IgG for 0.5 h. Afterwards, these sections were treated by DAB solution in the dark for 8 min, and then counterstained with haematoxylin. Finally, the prepared sections were dehydrated, transparent and sealed. A microscope with ×20 magnification was used for observation.
RT-PCR
From these colon tissues, the total RNA extraction was performed using TRIzol, which was then reversely transcribed to cDNA by M-MLV first strand kits. Then, PCR amplification was carried out using Fast HiFidelity PCR kits and relevant primers. The primer sequences are listed below:
β-actin forward primer (F): 5′-TCTGTCGTACCACAGGCAT-3′
reverse primer (R): 5′-CGCTCGTTGCCAATAGTGAT-3′;
TNF-α F: 5F-GGCAGGTCTACTTTGGAGTCATTG-3′,
R: 5′-ACATTCGAGGCTCCAGTCAATTCGG-3′;
IL-17 F: 5-1GCTCCAGAAGGCCCTCAGA-3′,
R: 5′CAGCTTTCCCTCCGCATTGA-3′;
IFN-γ F: 5N-GCCACGGCACAGTCATTGAAA-3′,
R: 5′-CATAACTGTGTTCCCGAGGTGTC-3′;
T-bet F: 5′-CCAGTATCCTGTTCCCAGCC-3′,
R: 5′-CATAACTGTGTTCCCGAGGTGTC-3′;
GATA-3 F: 5′-GCCTGTGCAAAAGAGATTTCAGAT-3′,
R: 5′-TGATTCACAGAGCATGTAGGCC-3′;
ROR-γt F: 5′-TTTGGAACTGGCTTTCCATC-3′,
R: 5′-AAGATCTGCAGCTTTTCCACA-3′;
Foxp3 F: 5′-CAGCTGCCTACAGTGCCCCTAG-3′,
R: 5′-CATTTGCCAGCAGTGGGTAG-3′;
IL-4: F: 5′-TCATCGGCATTTTGAACGAGGT-3′,
R: 5′-GCATCGAAAAGCCCGAAAGAG-3′;
IL-10 F: 5′-AACTGCACCCACTTCCCAG-3′,
R: 5′-TGGCCTTGTAGACACCTTGG-3′.
The relative expression of mRNA was described as a ratio to that of β-actin.
Western blot assay
Specific proteins expression in colon tissue was performed using WB. Proteins were extracted from colon samples using Total Protein Extraction kits, and BCA Protein Assay kits were used for the concentration determination. Then, these proteins were separated using SDS-PAGE, followed by electrophoretic transferring to PVDF membranes. After 1 h of nonfat skim milk (5%) blocking, the membranes were incubated using diluted primary antibodies from mouse anti-STAT3, and rabbit primary antibodies anti-Occludin, anti-ZO-1, anti-p-STAT3, anti-STAT6, anti-p-STAT6, anti-STAT1, anti-p-STAT1, anti-β-actin in 5% nonfat skim milk (w/v), 1× TBS, 0.1% Tween-20 overnight under gentle shaking at the temperature of 4 °C. Following this, incubation was performed with HRP-coupled secondary antibody from mice or rabbits for 60 min. Finally, bands luminescence was transferred onto the film and observed by Super ECL Detection Reagent in the dark. Proteins expressions were analysed with ImageJ software.
Flow cytometry for Th cells
Spleen and MLN cells separated from mice incubated and stimulated with PMA/Ionomycin Mixture and BFA/Monensin Mixture, cultured at 37 °C, 5% CO2, for 5 h. Surface marker was stained with FITC-anti-CD4 antibody at 4 °C for 0.5 h in dark, then was fixated and permeabilizated with FIX/PERM kit, and next stained intracellularly with PerCP-Cy 5.5-Anti-IFN-γ antibody, PE-Anti-IL-4 antibody, APC-Anti-IL-17A antibody for 0.5 h. Finally, cells were detected by flow cytometry.
Flow cytometry for Treg cells
Spleen and MLN cells were separated from mice stained surface marker with APC-Anti-CD4 antibody, PE-Anti-CD25 antibody at 4 °C for 0.5 h in dark, then were fixated and permeabilizated with Foxp3 Staining Buffer Set at 4 °C for 0.5 h, and then stained with intranuclear with FITC-Anti-Foxp3 antibody for 30 min. Finally, cells were analysed by flow cytometry.
Statistical analysis
SPSS 20.0 was employed for statistical analysis (SPSS Inc., Chicago, IL). The results were represented as mean ± SEM. One-way analysis of variance (ANOVA) was used for data analysis. In addition, the intergroup comparison was analysed using Student's two-tailed t-test. p < .05 indicated significant difference.
Results
Monosaccharide composition of IOP
IOP was characterized by chromatogram, as shown in . The purified fractions of IOP included Man, Rham, Glc, Gal, Xyl and Ara (molar ratio: 9.2:4.4:46.6:11.5:11.1:4.3, respectively).
IOP ameliorated disease progress of DSS-induced colitis mice
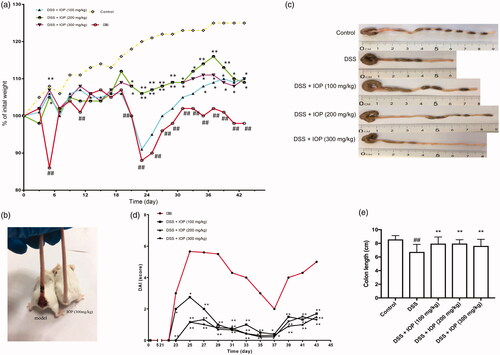
DSS-induced colitis models of mice were used to investigate the therapeutic effect of IOP. From the 5th day of DSS treatment, compared with the control group, the model group began to exhibit serious symptoms of dramatic body weight loss (p < .01). However, oral administration of IOP obviously prevented the body weight loss in comparison to the model group (p < .05) (). Mice’s rectal bleeding was compared with inflammation development period (). The mice in the model group had severe intestinal haemorrhage, and the randomly selected IOP (300 mg/kg) treated mice did not show visual bleeding. DAI, which reflected the levels of body weight loss, rectal bleeding and stool consistency, is a reliable marker of colon inflammation [Citation25]. DAI was obviously decreased in the IOP groups compared to the model group, where p < .05 (). The DSS-treated colon was shortened, which is a key characteristic of colitis. The colon of mice in the model group was substantially reduced by 6.8 cm compared to the normal group, where p < .01. This phenomenon was remarkably alleviated with the administration of IOP when compared to the DSS group, where p < .01, as shown in . The alleviation on DSS-induced colitis was also observed in mice treated with 5′-ASA (Supplementary figure 1), the body weight loss after 5′-ASA treatment was less severe than model group, and treatment with IOP or 5′-ASA did not present significant differences. Whereas 5′-ASA (100 mg/kg) and IOP (100 mg/kg) treatment exerted differentiated effect on DAI (Supplementary figure 2, p < .05), yet dose increase has not substantially enhanced this effect. In colon length evaluation, we found that IOP (100 mg/kg) treatment markedly alleviated the damage of DSS, and 5′-ASA demonstrated similar effect at larger doses (Supplementary figure 3). Both treatment exhibited marked restoration for the damage of colon induced by DSS treatment, and IOP had better effect on the colon length.
Figure 2. IOP ameliorated DSS-induced colitis in mice. Colitis were induced by orally giving DSS (3%), in the first two cycles, mice were given DSS (3%) for five days and followed by water for 14 days, and the third cycle, mice were just given DSS (3%) for five days, modelling 43 days in total. (a) Body weight loss. (b) Rectal bleeding in mice. (c) DAI score of mice. (d,e) Colon length of each group. *p < .05, **p < .01 compared with model group; #p < .05, ##p < .01 compared with control group.
IOP ameliorated the injury in the colon tissues of DSS-induced colitis mice
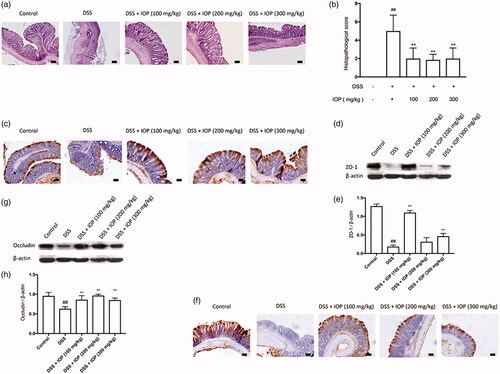
For the mice treated by DSS, the colon samples showed a series of serious pathological changes, including the inflammatory cell infiltration, mucosal damages and necrosis, etc., as revealed by H&E staining. These facts suggested severe injury in colon tissues by DSS. The colon tissue injury was largely prevented by IOP. 100, 200 and 300 mg/kg of IOP showed average histological scores of 2.12, 1.85 and 2.08, respectively, which were all lower than those of the model group (5.03) (). WB and IHC staining were also carried out to evaluate expressions of the tight junction protein, so as to explore the influence of IOP on the epithelial destruction. The protein levels of ZO-1 and Occludin were sharply reduced in the DSS group in comparison to the control group, as shown in as well as , respectively. Further, the decrease in these two proteins was sharply reversed after concomitant IOP treatment compared to the group without IOP treatment, where p < .01, respectively. Therefore, IOP can attenuate DSS-induced colon tissues injury and tight junction protein deficiency.
Figure 3. IOP alleviates colon tissue injury caused by DSS and restores the loss of tight junction (TJ) proteins. Colitis was induced by orally giving DSS (3%), in the first two cycles, mice were given DSS (3%) for five days and followed by water for 14 days, and the third cycle, mice were just given DSS (3%) for five days, modelling 43 days in total. (a) Histopathological changes and inflammation of colon. (b) Histopathological score. (c) ZO-1 expression in colon tissue was examined with immunohistochemical staining. (d,e) ZO-1 expression in colon tissue detected with western blot. (f) Occludin expression in colon tissue tested with immunohistochemical staining. (g,h) Occludin expression in colon tissue examined by western blot. Microphotographs were taken at ×20 magnification; bars = 50 µm. *p < .05, **p < .01 compared with model group; #p < .05, ##p < .01 compared with control group.
Effects of IOP on signature cytokines and transcription factors of CD4+T subsets for DSS-induced colitis mice
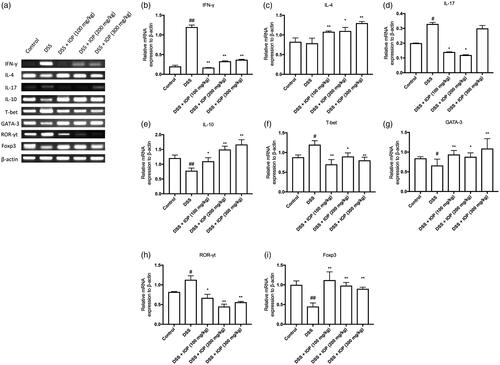
Th1-, Th2-, Th17- and Treg-related cytokines, IFN-γ, IL-4, IL-17, IL-10, as well as transcription factors, T-bet, GATA-3, ROR-γt, Foxp3 were analysed after extracting the total mRNA of colon tissues using RT-PCR, so as to study the mechanism of how IOP regulated the differentiation of CD4+T cells in colitis mice after DSS treatment. Significantly increased IL-17 and IFN-γ expressions were found after DSS administration in comparison with the control group, where p < .05 (). Nevertheless, IOP significantly decreased the mRNA expression of these two kinds of cytokines (). However, mRNA levels of IL-4 and IL-10 decreased in the model group and IOP-treatment showed a significantly promoting effect (). These results suggested that IOP could improve alterations of Th17-, Th2-, Th1-, Treg-related cytokines in colitis colon tissues. mRNA expressions of the related transcription factors were consistent with those of the signature cytokines observed in our study. In the colitis colon tissues, the ROR-γt and T-bet showed decreased mRNA expressions after IOP treatment, which increased the mRNA expressions of Foxp3 and GATA-3 (). These results indicated that IOP prevented the colon tissue inflammation by increasing the mRNA expressions of Th2-, Treg-related cytokines and transcription factors.
Figure 4. IOP regulated mRNA levels of Th1-, Th2-, Th17-, Treg-related cytokines and transcription factors in colon tissue. (a) Related cytokines and transcription factors mRNA expression. (b,f) Statistics of Th1-related IFN-γ and transcription T-bet. (c,g) Statistics of Th2-related IL-4 and transcription factors GATA-3. (d,h) Statistics of Th17-related IL-17 and transcription factor ROR-γt. (e,i) Statistics of Treg-related IL-10 and transcription factor Foxp3. *p < .05, **p < .01 compared with model group; #p < .05, #p < .01 compared with control group.
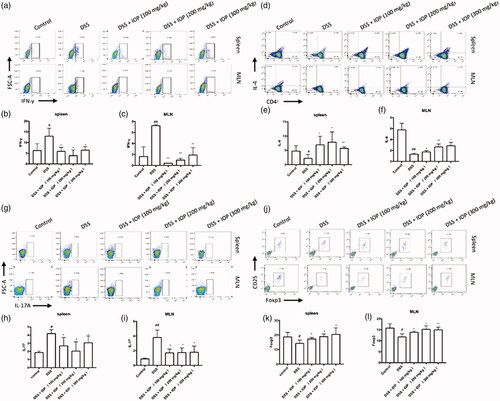
Effects of IOP on Th1-, Th2-, Th17- and Treg-related cells’ immunophenotyping in spleen and MLN of DSS-induced colitis mice
Surface CD4+ staining and intranuclear IFN-γ, IL-4, IL-17A, Foxp3 staining were carried out with lymphocytes isolated from MLN and spleen, so as to investigate the influence of IOP treatment on the number of Th1-, Th2-, Th17-, Treg-related cells (IFN-γ-, IL-4- and IL-17A-, Foxp3-positive cells) in vivo. In comparison to the water control group, the MLN and spleen of the model group showed sharply up-regulated production ratios of IFN-γ+T cells (Th1; n = 3) and IL-17A+T cells (Th17; n = 3), as shown in , respectively. The increase was then remarkably reversed by the administration of IOP. On the contrary, DSS mice showed a totally different trend in Th2 (n = 3) and Treg (n = 3) cells (, respectively), which were sharply down-regulated in comparison with the water control. However, these down-regulated ratios of Th2 and Treg cells were significantly increased after IOP treatment. These results suggested that IOP could regulate the Th1/Th2 and Th17/Treg balance in colitis mice by increasing the Treg and Th2 expressions and inhibiting the Th17 and Th1 expressions in spleen and MLN.
Figure 5. Effect of IOP on Th1, Th2, Th17, Treg differentiation in spleen and MLN examined with flow cytometry. (a–c) The effect of IOP on differentiation ratio of IFN-γ+T cells (Th1). (d–f) Effect of IOP on differentiation ratio of IL-4+T cells (Th2). (g–i) Effect of IOP on differentiation ratio of IL-17+T cells (Th17). (j–l) Effect of IOP on differentiation ratio of Foxp3+T cells (Treg). *p < .05, **p < .01 compared with model group; #p < .05, ##p < .01 compared with control group.
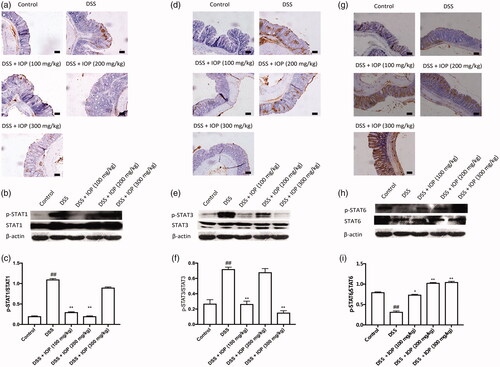
Effects of IOP on JAK-STAT signalling activation in colon tissue of DSS-induced colitis mice
The JAK-STAT pathway has a potential role in IOP bioactivity and regulates the balance of Th1/Th2 and Th17/Treg. Therefore, JAK-STAT was also investigated to ascertain the mechanisms of IOP activity. Expressions of p-STAT1 () and p-STAT3 () were up-regulated in DSS-treated mice, yet remarkably down-regulated after IOP administration. However, IOP administration led to no change in total STAT1 and STAT3 expressions in colon tissues. After DSS treatment, down-regulated expression of p-STAT6 is observed in . This decrease was sharply up-regulated after administration of IOP, which, however, led to no change in total STAT6 expressions. Taken together, these results indicated that the changes of JAK-STAT signalling pathway could, at least partially, explain the effects of IOP activity on the responses of Th1/Th2 and Th17/Treg in DSS-induced colitis mice.
Figure 6. Effect of IOP on the activation JAK-STAT signalling pathway in colon tissue examined with immunohistochemical and western blot. (a–c) STAT1 and p-STAT1 expression in colon tissue. (d–f) STAT3 and p-STAT3 activation of colon tissue. (g–i) STAT6 and p-STAT6 activation of colon tissue. *p < .05, **p < .01 compared with model group; microphotographs were taken at ×20 magnification; bars = 50 µm. #p < .05, #p < .01 compared with control group.
Discussion
IBD is a recurrent inflammatory disease of the gastrointestinal tract with unclear causes, including UC and CD. UC is also called non-specific UC. Its principal manifestations are abdominal pain, diarrhoea and mucopurulent bloody stool. The lesions are mainly limited in the mucosal layer and submucosa of the large intestine. Pathological changes often develop from the end of the rectum to the proximal retrogradely and may invade parts or entirely of colon [Citation26]. CD is a chronic granulomatous inflammation, of which mainly manifestations are abdominal pain and diarrhoea. Most of the lesions are distributed in various parts of the gastrointestinal tract segmental and asymmetrical [Citation27].
In this study, DSS-induced experimental chronic colitis models were successful and perfectly mimic the corresponding clinical features. Typical symptoms of experimental chronic IBD include weight loss, diarrhoea, hematochezia, and followed colon histological damage. We found that at the end of the first cycle of DSS treatment (day 5), weight loss was observed in the model group, whereas it was observed on the 20th day in the IOP (200 mg/kg) group at the second cycle of DSS treatment. Meanwhile, the body weight index was still higher than 100% in the IOP (100 and 300 mg/kg) groups. However, DAI index and colon histological score in model group were significantly higher than IOP groups and colon length was significantly lower than IOP groups. It suggested that IOP treatment can relieve the symptoms of DSS-induced chronic colitis in mice. The decreased intestinal epithelial barrier function and increased intestinal permeability were associated with the pathological process of UC which was an intestinal mucosal injury disease [Citation28]. TJ protein is located between the adjacent intestinal epithelial cells and is the major connection between the intestinal epithelial cells. It plays a major role in intestinal epithelial barrier function and intestinal permeability [Citation29]. ZO-1 and Occludin were two major TJ proteins in Intestine, which may associate with diarrhoea and blood in the stool in UC [Citation30–32]. In our current study, histological evaluation showed that there were obvious colonic epithelial cell destruction and typical UC clinical symptoms in the DSS group. Contrarily, IOP groups maintain the intestinal integrity by reducing the loss of ZO-1 and Occludin, and the tissue injury, diarrhoea and bloody stools are significantly relieved. Especially in the 100 mg/kg IOP group, TJ protein loss was the least, showing excellent mucosal protective effect, suggesting that 100 mg/kg IOP may be the optimal dose for mucosal protection of colitis mice, and further research is needed. All of these data indicate that IOP can serve as a potentially effective agent for the treatment of IBD.
The subpopulation of CD4+ T cells can consist of four major subpopulations of Th1, Th2, Th17 and Treg cells according to differentiation and different biological functions. JAK/STAT pathway can regulate the inflammatory pathways which are mainly characterized by an imbalanced effector T cells in IBD [Citation33]. Recent studies have demonstrated that CD4+ T cells, Th1/Th2 and Th17/Treg, the immune imbalance between these cells may be the most direct and most important factor in the pathogenesis of IBD. The normal intestinal mucosal immune system maintains intestinal balance by maintaining a network balance of proinflammatory cytokines and anti-inflammatory cytokines. Once the balance between them is broken, it may lead to excessive proliferation of effector cells or a decrease in the function of regulatory cells, causing and aggravating the inflammation of the mucosa. The proportion of Th1/Th2 cells imbalance is related to the immune escape of tumours, microbial infections such as bacteria and viruses, and is involved in allergic diseases, autoimmune diseases and transplant rejection reactions. It also plays a role in mediating the occurrence of UC in the body [Citation13]. Due to the huge aggregation of Th1 cells, along with the relative absence of Th2 cells, the IFN-γ (pro-inflammatory cytokine) showed an excessive secretion, and the IL-4 (anti-inflammatory cytokine) exhibited an insufficient secretion, which finally led to increased mucosa damage. The main feature of Th1 cells has been found to be the activation of IFN-γ/STAT1 pathway via acting on p-STAT1 [Citation34,Citation35]. The Th2 cells are differentiated by IL-4 dependent on STAT6, which further activates the transcription factor GATA-3 [Citation36]. Our research shows that the imbalance of Th1/Th2 in spleen and MLN were tending balance in IOP groups mice compared with DSS group mice. IHC and WB analysis for colon tissue in our study demonstrated that IOP regulation Th1/Th2 function by inhibiting STAT1 phosphorylation and improve p-STAT6 activation, respectively. Meanwhile, compared with the DSS group, the IOP treated mice showed obviously up-regulated Th2-related GATA-3 and IL-4 mRNA expressions, as well as down-regulated Th1-related T-bet and IFN-γ mRNA expressions. IOP is effective in controlling Th1/Th2 balance in immune organs or colon tissues. Th17 and Treg are two recently discovered CD4+ T lymphocyte subsets, which are different from Th1 cells and Th2 cells. The imbalance of Th17/Treg is involved in the occurrence and development of many diseases. In recent years, many studies have confirmed that Thl7 and Treg are directly related to the pathogenesis of IBD. Treg cells secreted anti-inflammatory cytokine IL-10, with the expression of the transcription factor Foxp3; however, the pro-inflammatory cytokine IL-17 could be produced by Th17 cells, where the transcription factor ROR-γt would show a significant expression [Citation37,Citation38]. Colitis mice treated with IOP inhibit the percentage of Th17 cells and unregulated the percentage of Treg cells both in the spleen and MLN by FCM analysis. The activation of crucial transcription factor STAT3 in Th17 responses and ROR-γt was inhibited by IOP. Treg-related cytokine IL-10 and Foxp3 were improved in IOP treated mice. Thus, it is suggested IOP may regulate STAT3 to balance Th17 and Treg to stabilize intestinal homeostasis.
The current work reported IOP treatment obviously relieved the inflammatory symptoms of the DSS-induced colitis mice. IOP was found to balance the Th1/Th2, Th17/Treg functions by regulating JAK-STAT signalling pathway and down-regulating the pro-inflammatory cytokine levels of activated T cells. Therefore, our study suggested that IOP is an effective natural with potential for the treatment of IBD.
Supplemental Material
Download ()Supplemental Material
Download ()Supplemental Material
Download ()Disclosure statement
The authors claim that there are no conflicts of interest.
Additional information
Funding
References
- Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045.
- Wang X, Sun Y, Zhao Y, et al. Oroxyloside prevents dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB pathway through PPARγ activation. Biochem Pharmacol. 2016;106:70–81.
- Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12:6102–6108.
- Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753.
- Burger D, Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology. 2011;140:1827–1837.
- Olivera P, Danese S, Peyrin-Biroulet L. Next generation of small molecules in inflammatory bowel disease. Gut. 2016;66:199–209.
- Roda G, Jharap B, Neeraj N, et al. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7:e135.
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;325:417–429.
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533.
- Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199–207.
- Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767.
- Shale M, Schiering C, Powrie F. CD4+ T-cell subsets in intestinal inflammation. Immunol Rev. 2013;252:164–182.
- Bai A, Lu N, Zeng H, et al. All-trans retinoic acid ameliorates trinitrobenzene sulfonic acid-induced colitis by shifting Th1 to Th2 profile. J Interferon Cytok Res. 2010;30:399–406.
- Hundorfean G, Neurath MF, Mudter J. Functional relevance of T helper 17 (Th17) cells and the IL-17 cytokine family in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:180–186.
- Zhang H, Hu X, Liu X, et al. The Treg/Th17 imbalance in Toxoplasma gondii-infected pregnant mice. Am J Reprod Immunol. 2012;67:112–121.
- Li YH, Xiao HT, Hu DD, et al. Berberine ameliorates chronic relapsing dextran sulfate sodium-induced colitis in C57BL/6 mice by suppressing Th17 responses. Pharmacol Res. 2016;110:227–239.
- Tao F, Qian C, Guo W, et al. Inhibition of Th1/Th17 responses via suppression of STAT1 and STAT3 activation contributes to the amelioration of murine experimental colitis by a natural flavonoid glucoside icariin. Biochem Pharmacol. 2013;85:798–807.
- Choi SY, Hur SJ, An CS, et al. Anti-inflammatory effects of Inonotus obliquus in colitis induced by dextran sodium sulfate. J Biomed Biotechnol. 2010;2010:943516.
- Hu Y, Sheng Y, Yu M, et al. Antioxidant activity of Inonotus obliquus polysaccharide and its amelioration for chronic pancreatitis in mice. Int J Biol Macromol. 2016;87:348–356.
- Song Y, Hui J, Kou W, et al. Identification of Inonotus obliquus and analysis of antioxidation and antitumor activities of polysaccharides. Curr Microbiol. 2008;57:454–462.
- Chen H, Yan M, Zhu J, et al. Enhancement of exo-polysaccharide production and antioxidant activity in submerged cultures of Inonotus obliquus by lignocellulose decomposition. J Ind Microbiol Biotechnol. 2011;38:291–298.
- Mishra SK, Kang JH, Kim DK, et al. Orally administered aqueous extract of Inonotus obliquus ameliorates acute inflammation in dextran sulfate sodium (DSS)-induced colitis in mice. J Ethnopharmacol. 2012;143:524–532.
- Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Investig. 1993;69:238–249.
- Sun X, Somada S, Shibata K, et al. A critical role of CD30 ligand/CD30 in controlling inflammatory bowel diseases in mice. Gastroenterology. 2008;134:447–458.
- Nagib MM, Tadros MG, ElSayed MI, et al. Anti-inflammatory and anti-oxidant activities of olmesartan medoxomil ameliorate experimental colitis in rats. Toxicol Appl Pharmacol. 2013;271:106–113.
- Hayashi Y, Narumi K, Tsuji S, et al. Impact of adrenomedullin on dextran sulfate sodium-induced inflammatory colitis in mice: insights from in vitro and in vivo experimental studies. Int J Colorect Dis. 2011;26:1453–1462.
- Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–1605.
- Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153.
- Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13:11–18.
- Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564.
- Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23:379–383.
- Sanders DS. Mucosal integrity and barrier function in the pathogenesis of early lesions in Crohn’s disease. J Clin Pathol. 2005;58:568–572.
- Coskun M, Salem M, Pedersen J, et al. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res. 2013;76:1–8.
- Afkarian M, Sedy JR, Yang J, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat Immunol. 2002;3:549–557.
- Szabo SJ, Kim ST, Costa GL, et al. A novel transcription factor. T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669.
- Zhu J, Guo L, Watson CJ, et al. Stat6 is necessary and sufficient for IL-4’s role in Th2 differentiation and cell expansion. J Immunol. 2001;166:7276–7281.
- Gálvez J. Role of Th17 cells in the pathogenesis of human IBD. ISRN Inflamm. 2014;2014:928461.
- Yang XO, Panopoulos AD, Nurieva R, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363.