?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The pathogenic bacteria delay wound healing due to their interaction in the wound area. This study is aimed to evaluate the efficiency of topical rosemary essential oil (REO) loaded into the nanostructured lipid carriers (NLCs) on in vitro antibacterial activity and in vivo infected wound healing process in the animal model. REO-NLCs morphology, size and in vitro antibacterial activity were done. Two circular full-thickness wound (each 6 mm) were made on the back of each mouse and each wound was infected with a solution containing 107 CFU Staphylococcus aureus and Pseudomonas aeruginosa. Animals were divided into four groups including control, Mupirocin® and two treated groups with a gel containing REO and REO-NLCs. For this purpose, tissue bacterial count, histological assessment, serum level of IL-3, IL-10, VEGF and SDF-1α were evaluated. REO-NLCs showed antibacterial activity against Staphylococcus epidermidis, Staphylococcus aureus, Listeria monocytogenes, Escherichia coli and Pseudomonas aeruginosa. Moreover, REO-NLCs could reduce the rate of tissue bacterial colonization and wound size, while they increased the vascularization, fibroblast infiltration, re-epithelialization, collagen production, IL-3, IL-10, VEGF and SDF-1α serum levels. Our finding revealed the REO-NLCs have antibacterial properties and accelerated infected wound healing, and so that confirming their potential clinical uses for the treatment of infected wounds.
Introduction
Wound healing is a complicated process that contains different interdependent stages, including hemostasis, inflammation, proliferation and remodeling. Colonization and replication of bacteria in the wound site delay the wound healing process. The use of topical antimicrobial agents such as silver sulfadiazine can decrease the risk of infection during wound healing; however, microbial resistance to antibiotics causes public health problems [Citation1,Citation2]. The use of natural antimicrobial agents that are isolated from the essential oils of plants can be an appropriate strategy for preventing the bacterial growth and promoting the wound healing process. Essential oils are known as a volatile, lipophilic liquid or semiliquid compounds, which are produced by plants as secondary metabolites [Citation3]. However, their usage has been faced to the major challenges due to some difficulties in the extraction, formulation and application [Citation4]. Essential oils not only are very decomposable but are also sensitive to environmental variables [Citation5]. Encapsulation is an efficient strategy for preventing the degradation of the essential oils [Citation6]. Encapsulation could protect beneficial effects and release of active compounds [Citation7]. There is an increased interest in the application of nanostructures in material science, pharmacology and medicine [Citation6]. Interest in the use of nanostructured lipid carriers (NLCs) is because of their ability of being incorporated in lipophilic/hydrophobic drugs, controlling drug release, enhancement of drug stability, bioavailability and safety [Citation3].
The essential oil of Rosemary (Rosmarinus officinalis L.) is known to have antioxidant and antimicrobial properties, which are mainly attributed to its flavonoids and terpenes [Citation8–10]. Rosemary essential oil is able to decrease the levels of inflammatory cytokines showing the anti-inflammatory activity and promote the wound healing process in diabetic animal [Citation11,Citation12]. Rosemary essential oil (REO) also has controlling effects on lipoperoxidation of skin lipids [Citation3]. Thus, this study was undertaken to investigate the effects of Rosemary essential oil loaded into the nanostructured lipid carriers (REO-NLC) for the dual application (in-vitro antibacterial and in-vivo infected wound healing activity) as endpoints.
Materials and methods
Poloxamer® 407 and Glyceryl Palmitostearate (Precirol ATO-5®) were purchased from Sigma-Aldrich Company (Steinheim, Germany) and Gattefossé Company (Cedex, France), respectively. Miglyol® 812 was prepared from Sasol Company (Witten, Germany). Other chemical materials were prepared in analytical grade.
Preparation of REO-NLCs
REO-NLCs was prepared by hot melt homogenization, based on Ghodrati et al. method [Citation13]. For this purpose, 200 μg REO was dissolved in 200 mg Miglyol and then, the obtained mixture was mixed with 1.8 g melted (by 70 °C) Poloxamer (1.5 g) and subsequently dissolved in water. Next, the mixture was added into the oil phase, under stirring at 20000 rpm. The mixture was then heated (70 °C) and stirred for 15 min and finally, allowed to cool down to 24 °C. Ultimately, the NLC structures had both phases of solid lipid (e.g. Precirol®), as well as liquid lipid (e.g. Miglyol®).
Characterization of REO-NLCs
The polydispersity index (PDI) was used to evaluate the distribution of nanoparticle population using the laser diffraction technique (Malvern Instruments, UK). Zeta potential was also used to determine the dispersion stability of REO-NLCs using Zeta Sizer (Nano ZSP, Malvern Instruments Corp., Worcestershire, UK). The electric charge on the particle surface was measured by determining the electrophoretic mobility using Malvern Zeta Sizer (Nano ZS, Model Zen 3600; Malvern Instruments Ltd., UK). The morphological characteristics of REO-NLCs were evaluated using a scanning electron microscope (SEM) (KYKY-EM3200). NLC samples were diluted 20 times with water and then one drop dried on a coverslip. Then, the samples were imaged at a voltage of 26 kV after gold coating. The entrapment efficiency (EE%) and drug loading (DL) were determined as reported previously (Equation (1) [Citation14].
Where Wencapsulated is the total amount of loaded REO and Winitial is the amount initial REO used for loading in the NLC system, Wlipid is the amount of the lipid matrix (Precirol) used in the preparation of NLC.
Investigation of antibacterial activity by disc diffusion method
Investigation of antibacterial activity was performed as previously reported by Ghodrati et al. [Citation13]. The bacteria were set in 106 and 0.10 ml it was used on media culture and cultured using cotton swabs. About 1% of the inoculum suspension strains were regularly seeded on the solidified Mueller Hinton agar plates by sterile cotton swabs. Then, 100 µg REO and REO-NLCs were placed on the seeded plates and then incubated at 37ºC for 7 days. Sterile discs impregnated with gentamicin, penicillin and mupirocin antibiotics were considered as a positive control, while sterile discs loaded with DMSO 10% and NLC were considered as without essence (blank) as a negative control. To evaluate the antibacterial properties, antibiotics, DMSO and NLC disc were placed on Mueller–Hinton agar. The inhibition zones were produced around the discs and then compared with the positive control inhibition zone [Citation15]. The inhibition zone was evaluated using digital caliper and data were reported as mm. Antibacterial activities were evaluated against Staphylococcus aureus (ATCC 25923), Staphylococcus epidermidis (ATCC 12228) and Streptococcus pneumoniae (ATCC 49819), Escherichia coli (ATCC 25922), Bacillus anthracis (ATCC 14578), Listeria monocytogenes (ATCC 19133), Pseudomonas aeruginosa (ATCC 27853), Salmonella typhimurium (ATCC 14028).
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
Microdilution assay was used to assess the minimum inhibitory concentration (MIC) of REO and REO-NLCs [Citation16]. As a summary, wells of 96-well microplate were filled with 100 µl of bacteria. Then, 100 µl of REO and REO-NLCs were added into wells of the two columns and mixed properly. Next, 100 µl of the wells that contained samples transferred to wells in the second column and this transfer was repeated. All wells per column received nanoparticles by half of the previous column. For all bacteria, the inoculum of 106 CFU/ml was tested. Then, plates were incubated during 24 h at 37 °C. The microorganism growth was followed by measurement of the absorbance before and after incubation at 620 nm in a Power Wave Microplate Spectrophotometer (BioTek Germany). The well with least concentration, lack of turbidity, was determined as the MIC value. All assays were performed in triplicate. For MBC test, 50 µl from the wells of the MIC assay was removed, spread on nutrient agar plates and incubated at 37 °C for 24 h. After the incubation time, the plates were examined for growth. The least concentrations of REO and REO-NLCs were considered as MBC value.
Gel preparation
Gels were prepared as described by others [Citation3]. In summary, the rheological additive (0.8% w/w) was included and dispersed in the aqueous phase, under mild agitation. In order to solubilize the REO, a solubilizing compound was added to the REO to obtain aqueous gels containing different percentages of EO. All gel formulations were produced by deionized water having 0.35% w/w imidazolidinyl urea, 0.05% w/w methylchloroisothiazolinone and methylisothiazolinone as preservatives.
Induction of infection and dressing
Induction of infection wound was performed as we previously reported [Citation1,Citation2]. All mice were anaesthetized by intramuscular administration of ketamine/xylazine (ketamine 60 and xylazine 20 mg/kg) cocktail and then the skin was scrubbed by a shaver. Two circular full-thickness skin wounds induced by 6 mm biopsy punch and then infected by an aliquot of 25 × 107 cells P. aeruginosa (strain ATCC 27853) and S. aureus (strain ATCC 25923). All animals were randomly divided into four groups including: (1) control, received 0.5 g gel (lack of therapeutic compounds); (2) control standard, received 0.5 g Mupirocin® ointment; (3) REO-treated group, received 0.5 g gel containing rosemary essential oil and (4) REO-NLCs-treated group, received 0.5 g gel containing rosemary essential oil loaded into the NLC, respectively. The gels were topically applied once a day, 24 h after induction of the wound. Six animals in each group were euthanized at 3, 7 and 14 days post-operation. Following that, the wound tissue samples were collected from the cutaneous lesions for histopathological examination and bacterial count analysis.
Bacteriological investigation of granulated tissue
The granulated tissues were dissected on days 3, 7 and 14 after wound induction and 0.1 g of samples were homogenized in the sterile mortar (containing 10 ml of sterile saline). Then, the samples were diluted in the tube containing 9 ml of sterile saline. Following dilution, the samples were superficially cultured on plate count agar (Merck KGaA, Darmstadt, Germany) and then duplicated. Next, the culture plates were incubated at 37ºC for 24 h and 48 h. Finally, all the colonies were counted and the data were reported as CFU/g of granulation tissue [Citation1,Citation2,Citation13].
Histopathological analyses
On 3, 7 and 14 days after wound creation, six animals in each group were selected and the full thickness samples were collected from the wound area. The collected specimens were firstly fixed in 10% neutral-buffered formalin, stained with Hematoxylin-eosin (H&E) and Masson’s trichrome and investigated by an ordinary light microscope (Olympus CX31RBSF attached to a camera, Tokyo, Japan). Three parallel sections were obtained from each specimen. Cellular infiltration (inflammatory cells and fibroblasts/one mm2 of the tissue) and collagen deposition were evaluated. The results for collagen intensity in Masson’s trichrome staining were shown in semi-quantitative (for all days) and quantitative (for day 14 after wound induction). Edema in all groups was analyzed and graded as negative (-), mild (+), mild to moderate (++), moderate (+++) and intensive (++++). All parameters were analyzed in high-power fields (HPFs). The epithelial thickness was finally evaluated by a morphometric lens (Olympus, Tokyo, Japan) and reported in µm [Citation1]. The collagen intensity was analyzed by using Image pro-insight software (version 9.00, Cyber media, USA) . In addition, other histological parameters, including vascularization, fibroblast infiltration and granulation tissue were assessed by a four-point scale as follows: (0: none; 0.5: few; 1: moderate; 2: many and 3: considerable). The evaluations were performed by two-blinded pathologists [Citation1,Citation2].
Immuno-fluorescent staining for fibroblast
Immuno-fluorescent staining was conducted as previously reported by our previous paper [Citation13]. Prior to staining, the tissue slides were heated in a hot air oven (Venticell, MMM, Einrichtungen, Germany) at 60 °C for 25 min. Next, the sections were the de-paraffinized (in xylene, 2x) and then rehydrated in ascending gradients of alcohol (96, 90, 80, 70, 50%). Thereafter, the antigen retrieval process was conducted (in 10 mM sodium citrate buffer PH: 7.2) and the sections then underwent the peroxidase blocking process (0.03% hydrogen peroxide containing sodium azide, 5–10 min). Thereafter, the sections were incubated with biotinylated primary fibroblast antibody (Rabbit/anti-mouse, 1:500) for 15 min. Following a soft rinse in PBS (PH:7.00), the sections were incubated with treptavidin-HRP (streptavidin conjugated to horseradish peroxidase in phosphate-buffered saline), for 15 min. The sections were softly rinsed in PBS and a DAB chromogen was included on tissue sections (5 min). Finally, the sections were counterstained with hematoxylin for 5 s. Positive reaction for interested protein was marked fluorescent green under a fluorescent microscope.
Sample collection and inflammatory mediators
On 3, 7 and 14 days after induction of wound, the blood samples were collected from six animals per group and transferred into the heparinized tubes. Then, they were centrifuged at 2000 rpm for 10 min and stored at −80 °C for further analyses. The samples were evaluated for the plasma concentration of IL-3 (Cat. No.: ab113345), IL-10 (Cat. No.: ab108870), vascular endothelial growth factor or VEGF (Cat. No.: ab9530) and SDF-1α (Cat. No.: ab100741) by the specified ELISA kits of Abcam, Cambridge, UK.
Image cytometry analyses
To evaluate histological parameters, 10 sections from each sample (NO = 6 samples for each group) were analyzed in 10 random microscopic fields of each section. The pixel-based intensity for immunoreactivity was analyzed by Image-Pro Insight software (version 9.00) per 50000 × 50000 µm [Citation13]
Statistical analysis
All analyses for wound healing were performed by PASW version 18.0 (California, USA). Model assumptions were evaluated by examining the residual plot. Two-way ANOVA was used to analyze the results. Dunnett's test for pair-wise comparisons was used to assess the effect of time and treatments. Data for antimicrobial activity were statistically analyzed by one-way analysis of variance based on Tukey's HSD test using SPSS 23.0 software package (Chicago, USA). Differences were considered as significant at p < .05.
Results
Characterization of REO-NLCs
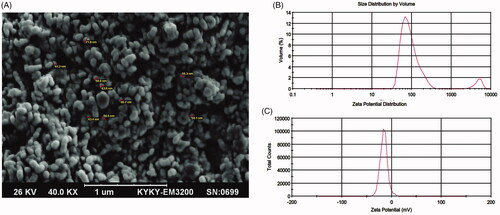
The obtained information revealed that REO-NLCs had a smooth surface, spherical and uniformly distributed around (). The Zeta potential (ZP) value of REO-NLCs was found to be -15.7 mv (). Particle size (PS) analyses () showed that REO-NLCs had a range size from 100 to 250 nm with narrow PDI = 0.335. The mean of EE and DL for REO-NLC were estimated to be 92.14 ± 1.3% and 9.5 ± 0.12%, respectively.
Antimicrobial activity
The results showed that REO-NLCs and REO had the similar activity against the tested bacteria () (p > .05), but both had smaller inhibition zone for all bacteria strains versus gentamicin, penicillin and mupirocin (p > .05). As it is listed in , REO-NLCs and REO had the most antibacterial activity against positive bacteria, including S. epidermidis, S. aureus and L. monocytogenes (). The mean for MIC and MBC were a range between 4.9 and 20 µg/ml ().
Table 1. The mean of diameter zone (mm) in experimental groups.
Table 2. The data for MIC and MBC in REO and REO-NLCs groups.
Wound closure rate
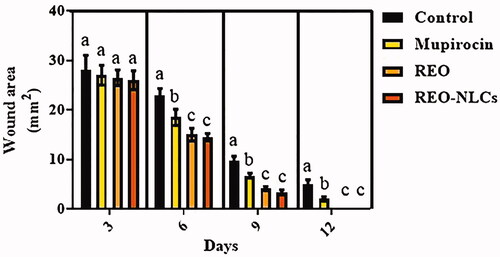
The treated animals showed lower wound area in comparison to the control group (p < .05) (). There was no significant difference between animals treated with REO-NLCs and REO on post-operation (p > .05).
Total tissue bacterial count
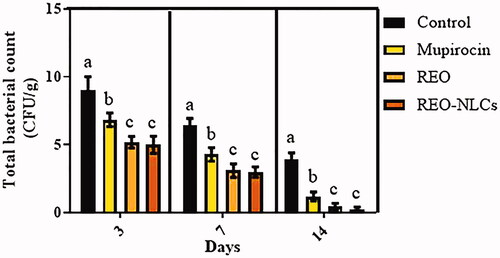
Topical administration of REO-NLCs, REO and mupirocin significantly reduced the rate of total bacterial count in comparison to the control group on all days post wound creation () (p < .05).
Histopathological analysis
The data for histological parameters are listed in . Edema score was reduced in the REO-NLCs-treated animals in comparison to the control group from 3 to 14 days post-wounding. Our histological findings showed a remarkable up-regulation in neovascularization in the treated animals. Remarkable neovascularization (p < .05) was also observed in the REO-NLCs-treated groups in comparison to the control group from 3 to 14 days post-wounding (). Moreover, our finding showed well-formed granulation tissue in REO-NLCs-treated group in comparison to the control group (). Complete epithelialization was observed in the REO-NLCs-treated animals on day 12 after wounding and in the mupirocin-treated and control animals on day 16 (). Immuno-fluorescent staining for fibroblast distribution to wound area indicated an intensive fibroblast distribution in REO-NLCs-treated group in comparison to other groups ( and ). Collagen deposition was raised in the REO-NLCs-treated animals in comparison to the control animals (). Moreover, the 3 D plot area analyses for fibroblast distribution showed that the wounds in the REO-NLCs-treated group exhibited remarkable fibroblast distribution ( and ).
Figure 4. Histological section from wound area on day 3 after wound induction; immuno-fluorescent staining for fibroblast are presented in first row (A1–D1). See that REO-NLCs and the REO significantly stimulated the fibroblasts infiltration. Figure A2–D2 are representing the granulation tissue generation in different groups. See well-formed granulation tissue in REO and REO-NLCs-treated groups versus mupirocin and control animals. The software analyses for fibroblast distribution are represented in Figure A3-D3. See intensive fibroblast distribution in REO-NLCs-treated group (A: Control, B: Mupirocin-treated, C: REO-treated and D: REO-NLCs-treated).
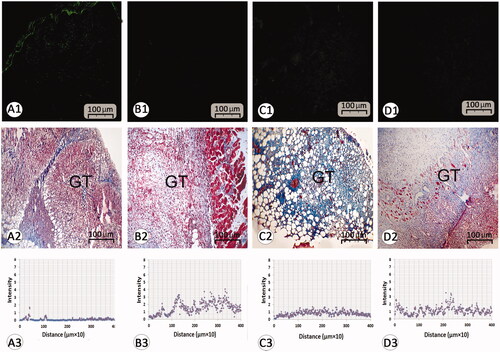
Figure 5. Histological section from wound area on day 14 after wound induction; (A1–D1) immuno-fluorescent staining for fibroblast are presented in first row (A1–D1). See that REO-NLCs and the REO significantly stimulated the fibroblasts infiltration. Figure A2–D2 are representing Masson-trichrome staining techniques for collagen deposition in dermis (DS). See the well-formed re-epithelialization in REO and REO-NLCs-treated groups compared with mupirocin-treated and control animals. See hair follicle sprouting (HF) and sebaceous glands (SG) in REO and REO-NLCs-treated cross sections. The software analyses for fibroblast distribution are represented in Figure A3–D3. See intensive fibroblast distribution in REO-NLCs-treated group (A: Control, B: Mupirocin-treated, C: REO-treated and D: REO-NLCs-treated).
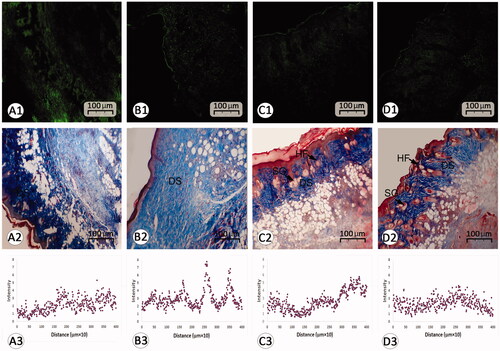
Table 3. Effect of REO and REO-NLCs gels on histological scorings on different days.
Inflammatory cytokines analysis
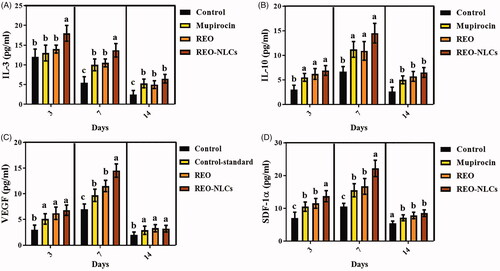
The treated animals with mupirocin, REO and REO-NLCs (especially REO-NLCs-treated animals) showed a higher concentration of IL-3, IL-10, VEGF and SDF-1α in the plasma compared to the control group on days post-operation () (p < .001).
Discussion
The particle size of NLCs is known as an essential factor because of its importance in drug release and drug absorption. Smaller particle size shows higher interfacial surface area for drug absorption. Rohini and Bose have reported that ideally, PDI < 0.25 is assumed as narrow size distribution, while the particles with PDI < 0.5 can be considered as suitable particle size distribution [Citation17]. In this study, the prepared NLCs had a mean size of 100–250 nm. This value was calculated to be 0.335 for REO-NLCs. Mitrea et al. reported that type and concentration of lipid, bioactive compounds and preparation procedures can have major effects on the size and structural properties of NLCs [Citation18]. A study revealed that NLCs that contain plant essential oils with PDI values 0.271–0.468 had a wide size distribution and small size [Citation19]. In this study, the PDI value was measured to be 0.335 for REO-NLCs, which implicates appropriate compatibility among lipids. The ZP value of REO-NLCs was found to be in a range of -10 to -15 mv. The obtained ZP values show that the REO-NLCs had electrical charges on the surface. Negative charges on the surface can be due to REO contents [Citation20]. Higher contents of the essential oils can decrease the electrokinetic potentials [Citation20]. To avoid the aggregation or precipitation of nanoparticles prepared with small ZP, NLCs were embedded in a gel matrix. The advantage of neutral zeta potential is the non-toxicity and biocompatible characteristic which is quite important in reactive sub-100 nm particles. The mean of EE% was measured to be 92.14 ± 1.3% for REO-NLC, which is optimum. The solubility of core material in the lipid matrix, compaction of lipid structure, type and concentration of surfactant, liquid oil type and environmental variables could influence EE% [Citation19].
REO-NLCs and REO similarly showed maximum efficiency against Gram-positive bacteria. Borges et al. [Citation21] showed that Gram-positive bacteria were more susceptible to nanoemulsions of essential oil than Gram-negatives. This could be attributed to the fact that the lipophilic ends of lipoteichoic acids in the cell wall of Gram-positives facilitate the penetration of hydrophobic compounds [Citation21]. Nanoparticle properties may enhance the passive cellular absorption mechanisms and also decrease mass transfer resistances [Citation22]. The NLCs can penetrate bacteria cell, disrupt biomembranes, release polypeptides into the medium, decrease adenosine triphosphate content of the cell and finally destroy membrane [Citation21]. DL index is more critical and informative than EE explaining the amount of carrier carrying the active ingredient. Generally, DL 10% and more can be assumed as a suitable capacity for carrier [Citation13].
The obtained results showed that the administration of REO-NLCs and REO gels promoted wound healing process in the infected mice model by lowering tissue edema, tissue bacterial count on the first few days after the wound creation and the increment of IL-3, IL-10 and VEGF levels, neovascularization, fibroblast infiltration, collagen deposition and re-epithelization in the seven days after the wound creation.
The acute inflammation is responsible for the production of essential wound healing factors such as cytokines at the early stages. Inflammation as a main step of the wound healing process is important for elimination of the microorganisms such as Staphylococcus aureus and Pseudomonas aeruginosa, however, partial deletion of the microorganisms in the wound area is associated with delay of healing process and destruction of extracellular matrix [Citation1,Citation2]. It was shown that inhibition of wound remodeling and matrix synthesis could significantly delay wound closure [Citation23]. Previously, it was confirmed that Rosemary officinalis essential oil has an antimicrobial activity of Rosemary officinialis essential oil has been attributed to a wide range of antibacterial compounds such as 1,8-Cineole and α-Pinene, especially against Pseudomonas aeruginosa and Staphylococcus aureus. The obtained results showed that the application of REO-NLCs considerably reduced bacterial growth and colonization in the treated animals, this could be attributed to its bacterial effects. Also, it was reported that 1, 8 cineol (Rosemary) is able to inhibit the production of pro-inflammatory cytokines, which are positively related to tissue inflammation in different wounds, including diabetic wounds [Citation24]. Therefore, it has been suggested that 1, 8 cineol has anti-inflammatory and antibacterial properties.
Previous studies reported that IL-10 [Citation25,Citation26] and IL-3 [Citation27] could have anti-inflammatory properties and decrease edema in the wound tissue. It was also shown that they could increase neovascularization and promote healing rate [Citation28]. In this regard, our results revealed that the levels of IL-3 and IL-10 were increased in both REO-NLCs and REO-treated animals. In addition, histological assessment indicated that tissue edema was reduced in the treated animals. It seems that REO and REO-NLCs can reduce bacterial count, inflammatory responses and tissue edema could be attributed to the compounds.
REO-NLCs gel also improved neovascularization and cellularity. Angiogenesis as an important stage of wound healing can supply essential nutrients and oxygen during the wound healing process and also promote granulation tissue formation [Citation29]. Vascular endothelial growth factor (VEGF) is known as a cytokine responsible for the induction of angiogenesis. VEGF promotes cell migration, proliferation and synthesis of extracellular matrix proteins [Citation30]. In addition, the enhanced levels of IL-10 are correlated with angiogenesis [Citation31]. Our findings for ELISA indicated an increase in VEGF level in the REO-NLCs and REO-treated animals. Parallel to these facts, our histological results showed an increase in neovascularization in the granulation tissue of the REO-NLCs-treated animals. It can be argued that REO-NLCs induce angiogenesis by up-regulation of VEGF level.
Fibroblasts are known as the key effectors in the cutaneous wound healing process through secretion of the growth factors and production of the extracellular matrix/collagen [Citation32]. It is well known that exposure of mesenchymal stem cells with IL-3 could increase cell migration and wound closure. On the other hand, Barhanpurkar-Naik showed that IL-3 stimulates cell migration in the mesenchymal stem and also fastens wound closure [Citation33]. In this context, the level of the IL-3 was higher in the REO-NLCs-treated animals. Parallel to these facts, the immuno-fluorescent analysis indicated high fibroblast cell migration to the wound area in REO and REO-NLCs-treated animals.
It is reported that stromal cells can provide an essential microenvironment in order to overlay the epithelium. In line with this issue, there is a direct association between fibroblasts number and keratinocyte growth [Citation34]. REO-NLCs increased SDF-1α in comparison to the control group. It is accepted that SDF-1α is significantly increased in the injured tissues and facilitates the migration and engraftment of circulation of CXCR4-positive cells [Citation33]. With regards to increasing the level of IL-3 and SDF-1α in REO-NLCs-treated animals, it can be claimed that therapeutic gels helped to trigger the wound healing.
Fibroblasts trigger wound contraction by the synthesis of extracellular matrix proteins such as collagen [Citation35,Citation36]. Fibroblasts firstly contract the loose fibrin matrix and subsequently, apply the contracted matrix as a surface for migration and tissue remodeling. It is believed that strengthening collagen fibrils and organization of collagen fibers fasten wound contraction [Citation32]. There is a positive correlation between collagen and fibroblast function. Our histological and immuno-fluorescent findings fibroblast infiltration and collagen deposition were increased in REO-NLCs-treated animals. It can be concluded that fibroblasts helped to synthesize the collagen, thereby decreasing the wound size. In addition, the increment of collagen deposition in the dermis and ameliorated epithelialization in the REO-NLCs-treated groups could be due to the increased wound contraction.
Conclusions
This study prepared REO loaded into NLCs with high encapsulation efficiency and high physical stability, the REO-NLCs which showed in-vitro antibacterial properties. Moreover, REO-NLCs also accelerated the infected wound healing process in the animal models by different mechanisms such as reducing the tissue bacterial colonization, inflammation period, and also increasing neovascularization, cellularity and wound contraction ratio.
Ethical approval
The applied procedures were approved by the Ethical Committee of Faculty of Veterinary Medicine, Islamic Azad University, Urmia Branch (No. IAUU, 1106). All the ethical principles and management were in agreement with National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Acknowledgement
This work was done in Veterinary Faculty of Urmia Islamic Azad University as a DVM thesis of Mr. Keyvan Khezri. The authors are grateful to Dr. Maryam Mohammadi for help in NLC preparation. This study was the result of a thesis research project and was supported by author’s own work.
Disclosure statement
The authors declare that there are no conflicts of interest.
References
- Modarresi M, Farahpour MR, Baradaran B. Topical application of Mentha piperita essential oil accelerates wound healing in infected mice model. Inflammopharmacol. 2018;6:1–7.
- Farahpour MR, Vahid M, Oryan A. Effectiveness of topical application of ostrich oil on the healing of Staphylococcus aureus- and Pseudomonas aeruginosa-infected wounds. Connect Tissue Res. 2017;31:1–10.
- Montenegro L, Pasquinucci L, Zappalà A, et al. Rosemary essential oil-loaded lipid nanoparticles: in vivo topical activity from gel vehicles. Pharmac. 2017;9:48.
- Almadiy AA, Nenaah GE, Al Assiuty BA, et al. Chemical composition and antibacterial activity of essential oils and major fractions of four Achillea species and their nanoemulsions against foodborne bacteria. LWT – Food Sci Technol. 2016;69:529–537.
- Hosseini SF, Zandi M, Rezaei M, et al. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohydr Polym. 2013;95:50–56.
- Feyzioglu GC, Tornuk F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT – Food Sci Technol. 2016;70:104–110.
- Fang Z, Bhandari B. Encapsulation of polyphenols–a review. Trends Food Sci Technol. 2010;21:510–523.
- Gad AS, Sayd AF. Antioxidant properties of Rosemary and its potential uses as natural antioxidant in dairy products-a review. FNS. 2015;6:179–193.
- Jiang Y, Wu N, Fu YJ, et al. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ Toxicol Pharmacol. 2011;32:63–68.
- Lo AH, Liang YC, Lin-Shiau SY, et al. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-κB in mouse macrophages. Carcinogen. 2002;23:983–991.
- Juhás Š, Bukovská A, Čikoš Š, et al. Anti-inflammatory effects of Rosmarinus officinalis essential oil in mice. Acta Vet Brno. 2009;78:121–127.
- Abu-Al-Basal MA. Healing potential of Rosmarinus officinalis L. on full-thickness excision cutaneous wounds in alloxan-induced-diabetic BALB/c mice. J Ethnopharmacol. 2010;131:443–450.
- Ghodrati M, Farahpour MR, Hamishehkar H. Encapsulation of peppermint essential oil in nanostructured lipid carriers: in-vitro antibacterial activity and accelerative effect on infected wound healing. Colloid Surface A Physiochem Eng Asp. 2019;564:161–169.
- Babazadeh A, Ghanbarzadeh B, Hamishehkar H. Novel nanostructured lipid carriers as a promising food grade delivery system for rutin. J Funct Food. 2016;26:167–175.
- Fazeli MR, Amin G, Attari MM, et al. Antimicrobial activities of Iranian sumac and avishan-e shirazi (Zataria multiflora) against some food-borne bacteria. Food Control. 2007;18:646–649.
- Varona S, Rojo SR, Martín Á, et al. Antimicrobial activity of lavandin essential oil formulations against three pathogenic food-borne bacteria. Indust Crops Prod. 2013;42:243–250.
- Rohini R, Bose S. Electromagnetic interference shielding materials derived from gelation of multiwall carbon nanotubes in polystyrene/poly (methyl methacrylate) blends. ACS Appl Mater Interfaces. 2014;6:11302–11310.
- Mitrea E, Ott C, Meghea A. New approaches on the synthesis of effective nanostructured lipid carriers. Rev Chim. 2014;65:50–55.
- Nahr FK, Ghanbarzadeh B, Hamishehkar H, et al. Food grade nanostructured lipid carrier for cardamom essential oil: preparation, characterization and antimicrobial activity. J Funct Food. 2018;40:1–8.
- Lacatusu I, Badea N, Niculae G, et al. Lipid nanocarriers based on natural compounds: an evolving role in plant extract delivery. Eur J Lipid Sci Technol. 2014;116:1708–1717.
- Borges RS, Keita H, Ortiz BL, et al. Anti-inflammatory activity of nanoemulsions of essential oil from Rosmarinus officinalis L.: in vitro and in zebrafish studies. Inflammopharmacol. 2018;5:1–24.
- Weiss J, Gaysinsky S, Davidson M, et al. Nanostructured encapsulation systems: food antimicrobials. Global Issues Food Sci Tech. 2009;24:425–479.
- Eming SA, Brachvogel B, Odorisio T, et al. Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem. 2007;42:115.
- Juergens UR, Dethlefsen U, Steinkamp G, et al. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir Med. 2003;97:250–256.
- Ip WE, Hoshi N, Shouval DS, et al. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356:513–519.
- King A, Balaji S, Le LD, et al. Regenerative wound healing: the role of interleukin-10. Adv Wound Care (New Rochelle). 2014;3:315–323.
- Yogesha SD, Khapli SM, Srivastava RK, et al. IL-3 inhibits TNF-alpha-induced bone resorption and prevents inflammatory arthritis. J Immunol. 2009;182:361–370.
- Verma SK, Krishnamurthy P, Barefield D, et al. IL 10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via STAT3 dependent inhibition of NFκB. Circul. 2012;126:418–429.
- Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222.
- Manzuoerh R, Farahpour MR, Oryan A, et al. Effectiveness of topical administration of Anethum graveolens essential oil on MRSA-infected wounds. Biomed Pharmacotherap. 2019;109:1650–1658.
- Krishnamurthy P, Thal M, Verma S, et al. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circ Res. 2011;109:1280–1289.
- Tracy LE, Minasian RA, Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care. 2016;5:119–136.
- Barhanpurkar-Naik A, Mhaske ST, Pote ST, et al. Interleukin-3 enhances the migration of human mesenchymal stem cells by regulating expression of CXCR4. Stem Cell Res Ther. 2017;8:168.
- Quan C, Cho MK, Shao Y, et al. Dermal fibroblast expression of stromal cell-derived factor-1 (SDF-1) promotes epidermal keratinocyte proliferation in normal and diseased skin. Protein Cell. 2015;6:890–903.
- Shinde AV, Humeres C, Frangogiannis NG. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta. 2017;1863:298–309.
- McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol. 2007;39:666–671.