Abstract
Loss of the capacities of epidermal stem cells (ESCs) induced by ultraviolet-B (UV-B) irradiation has been widely associated with various skin diseases. Netrin-1, a member of the axonal guidance protein family, has displayed diverse biological functions in different types of cells and tissues, mediated by its specific receptor UNC-5 homolog B (UNC5b). In this study, we examined the physiological functions of netrin-1 and UNC5b in ESCs upon UV-B exposure. Our results indicate that UNC5b is expressed in ESCs, and its expression is upregulated in response to UV-B radiation. We found that treatment with netrin-1 prevented UV-B radiation-induced oxidative stress by reducing the generation of reactive oxygen species (ROS) and expression of NADPH oxidase 4 (NOX-4). Additionally, treatment with netrin-1 improved UV-B radiation-induced mitochondrial dysfunction by increasing mitochondrial membrane potential (MMP) levels and adenosine triphosphate (ATP) production. The presence of netrin-1 attenuated UV-B radiation-induced lactic dehydrogenase (LDH) release. UV-B exposure resulted in the loss of the capacities of ESCs by reducing the expressions of integrin β1 and Krt19, the two major ESC markers. Importantly, this process was prevented by netrin-1. Silencing of UNC5b abolished the effects of netrin-1 on the expression of integrin β1 and Krt19, suggesting that the effects of netrin-1 in maintaining the capacities of ESCs are dependent on UNC5b. Mechanistically, we found that the Wnt/β-catenin signalling may be involved. Our findings suggest that netrin-1 may serve as a therapeutic agent for the treatment of skin diseases.
Introduction
Skin is an important organ that covers the entire surface of the human body, thereby playing a pivotal role in protecting the body against external damage [Citation1]. Studies have shown that skin has a high frequency of cell turnover [Citation2]. Epidermal stem cells (ESCs), a specific type of stem cell present in skin tissue, are essential for maintaining the homeostasis of skin tissue. Due to their pluripotent properties, ESCs are involved in the physiological processes of skin development, wound healing and restructuring [Citation3]. In addition to their self-renewal ability, ESCs possess an unlimited capacity to differentiate to all cell types that comprise the skin [Citation4]. Epidermal development and ESC behaviour are tightly regulated by a variety of cellular signalling pathways, which include Wnt/β-catenin-dependent signalling cascades [Citation5]. Impaired ESC capacities have been linked with a variety of diseases, including skin cancers, non-healing diabetic skin ulcers and skin ageing [Citation6]. Various environmental factors, including Ultraviolet (UV) radiation, can damage skin by causing insults to ESCs. UV-B, defined as a subtype of UV light with a 280–315 nm wavelength, is able to reach the earth’s surface through the ozone layer [Citation7]. ESCs are vulnerable to repetitive exposure to UV-B radiation. UV-B exposure leads to significant damage to ESCs by causing oxidative stress and damage to DNA [Citation8]. Inhibition of UV-B exposure-induced intracellular insult using therapeutic agents has been recognized as an essential approach to sustain the functional capacities of ESCs.
Netrin-1, an important member of the netrins family, was originally identified as an axonal guidance protein involved in the process of embryonic development [Citation9]. Diverse biological functions of netrin-1 have been reported in previous studies. For example, netrin-1 plays an important role in angiogenic processes by interacting with specific receptors on the surface of the endothelium, such as UNC-5 homolog B (UNC5b) [Citation10]. UNC5b is recognized as a dependence receptor of netrin-1, playing an important role in mediating the intracellular biological functions of netrin-1. Activation of UNC5b has been implicated in several physiological processes, including neural development, angiogenesis, inflammatory responses and tumorigenesis [Citation11]. Interestingly, netrin-1 has been recently reported to improve the tissue-regeneration capacity of stem cells [Citation12]. However, less about the physiological role of netrin-1 and UNC5b in UV-B exposure-induced impairment of the capacities of ESCs has been characterized. In this study, we report a new function of netrin-1 and UNC5b by showing that netrin-1 treatment attenuated UVB-induced impairment of the capacities of ESCs, dependent on UNC5b.
Materials and methods
Epidermal stem cell (ESC) isolation and treatment
Eight-week-old C57BL/6 female mice were used to isolate ESCs from skin on the back as described previously [Citation13]. All of the animal experiments in this study were performed in accordance with the approval granted by the Institutional Animal Care and Use Committee at Shandong University. Isolated ESCs were maintained in Dulbecco's Modified Eagle Medium (DMEM)/F12 medium supplemented with 15% embryonic stem cell screened fetal bovine serum (FBS), 1% glutamine, 1% penicillin/streptomycin and 4 ng/ml fibroblast growth factor-basic (bFGF). To examine the effects of UV-B on the expression of UNC5b, ESCs were exposed to UV-B at the intensities of 25, 50 and 75 mJ/cm2 for 8 h. ESCs were pre-incubated with netrin-1 (#1109-N1-025, R&D Systems, USA) (200, 400 ng/ml) for 12 h, followed by treatment with UV-B at 50 mJ/cm2 for 8 h. To silence UNC5b, ESCs were infected with lentiviral UNC5b shRNA.
Quantitative real-time PCR analysis
Total RNA was extracted from ESCs using Trizol (Invitrogen, USA). The complementary DNA (cDNA) was synthesized using a First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA) with reverse transcriptase (RT) enzymes and 1 μg RNA. A total of 20 ng cDNA was used for quantitative real-time PCR analysis with SYBR Green I MasterMix (Roche, Switzerland) on a LightCycler 480 PCR platform. The expression of targeted genes was calculated by the 2−ΔΔCt method and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The following primers were used in this study:
mouse UNC5b: 5′-GAGTGGCTATGCTTGTGATTTGG-3′, reverse: 5′-CATAGCAAAGCCTTTCCCTGTG-3′;
mouse integrin β1: forward: 5′-ACACCGACCCGAGACCCT-3′, reverse: 5′-CAGGAAACCAGTTGCAAATTC-3′;
mouse Krt19: forward: 5′-GCACTACAGCCACTACTACACGA-3′, reverse: 5′-CTCATGCGCAGAGCCTGTT-3′;
mouse Wnt1: forward: 5’-CAT-CTT-CGC-AAT-CAC-CTC-CG-3′, reverse: 5’-GTG-GCA-TTT-GCA-CTC-TTG-G-3’;
mouse MYC primers: forward: 5′-ACCAGATCCCGGA GTTGGAA-3′, reverse:
5′-CGTCGTTTCCGCAACAAGTC-3′;
mouse cyclin D1: 5′-TAGGCCCTCAGCCTCACTC-3′, reverse: 5′-CCACCCCTGGGATAAAGCA-3′k
mouse GAPDH, forward: 5′-AGAAGGCTGGG-GCTCATTTG-3′; reverse: 5′-AGGGGCCATCCACAGTCTTC-3′.
Western blot analysis
After the indicated treatment, cells were washed three times with PBS and lysed with cell lysis buffer containing 1 mM phenylmethylsulfonylfluoride (PMSF) and cocktail protease inhibitor. A total of 20 μg isolated protein was run on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. After washing three times with tris buffered saline Tween (TBST) solution, membranes were blocked with 5% skim milk for 1 h at room temperature (RT). Then, membranes were sequentially incubated with appropriate primary antibody overnight at 4 °C and horseradish peroxidase (HRP)-conjugated secondary antibody for 2 h at RT. The immunoreactive bands were detected using enhanced chemiluminescence (Thermo Fisher Scientific, USA). The following antibodies were used in this study: Rabbit anti-UNC5b monoclonal antibody (#13851, Cell Signaling Technology, USA); Rabbit anti-Nox-4 monoclonal antibody (#ab109225, Abcam, USA); Rabbit anti-cyclin D1 monoclonal antibody (#2922, Cell Signaling Technology, USA); Rabbit anti-Myc polyclonal antibody (#ab39688, Abcam, USA); Rabbit anti-Wnt1 polyclonal antibody (#ab15251, Abcam, USA); Mouse anti-β-actin monoclonal antibody (#3700, Cell Signaling Technology, USA).
Measurement of ROS
The level of intracellular ROS in ESCs was determined by 2,7-dichlorofluorescin diacetate (DCFH-DA) (Sigma-Aldrich, USA) staining. Briefly, cells were seeded into 24-well plates and subjected to the indicated treatments. The culture was then loaded with DCFH-DA at a final concentration of 1 μM and incubated at 37 °C for 2 h. The supernatant was removed, and the wells were washed three times in PBS. Fluorescence signals were visualized using a DM500 fluorescence microscope (Leica Microsystems, Germany). The fluorescence density of each sample was analyzed using Image J software (NIH, USA). Regions of interest (ROI) were defined in the fluorescent images and the average number of cells present in the ROI was determined. The integrated density value (IDV) of the ROI was evaluated. The IDV was divided by the average number of cells and the quotient determined the index average level of intracellular ROS.
Determination of mitochondrial membrane potential (MMP)
MMP levels in ESCs were measured using tetramethylrhodamine methyl ester (TMRM) (Thermo Fisher Scientific, USA) staining in accordance with the manufacturer’s instructions. Briefly, cells were seeded into 24-well plates and subjected to the indicated treatments. Cultures were then loaded with TMRM at a final concentration of 20 nM and incubated at 37 °C for 1 h. Fluorescence signals were visualized using a DM500 fluorescence microscope (Leica Microsystems, Germany). The fluorescence density of each sample was analyzed using Image J software (NIH, USA). Regions of interest (ROI) were defined in the fluorescent images and the average number of cells present in the ROI was determined. The integrated density value (IDV) of the ROI was evaluated. The IDV was divided by the average number of cells and the quotient determined the index average level of intracellular MMP.
Measurement of adenosine triphosphate (ATP) levels
Intracellular levels of ATP in cultured ESCs were determined by a bioluminescence assay using a commercial kit (#A22066, Thermo Fisher Scientific, USA). Briefly, ESCs were lysed with a lysis buffer, followed by centrifugation at 10,000 × g for 10 min at 4 °C. Equal volumes (100 μl) of supernatant and luciferin/luciferase reagent were mixed together. Light output was immediately detected using a microplate luminometer.
Determination of lactate dehydrogenase (LDH)
After the necessary treatment, the release of LDH from ESCs into the supernatant was measured using a commercial LDH kit (Sigma-Aldrich, USA) in accordance with the manufacturer’s instructions [Citation14]. Briefly, 100 µl assay medium was collected and transferred into each well, then incubated for 30 min at RT. The OD value at 490 nm was measured to quantify the LDH level.
Measurement of cell viability
The reagent 3–(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) (Sigma-Aldrich, USA) was used to assess the cell viability of ESCs. After the indicated treatment, ESCs were washed three times with phosphate buffered solution (PBS). MTT was then added to the culture medium without FBS at a final concentration of 5 mg/ml. After incubation in darkness for 2 h at 37 °C, the reaction products were dissolved in dimethyl sulfoxide (DMSO). Absorbance measured at 570 nm was used to represent cell viability.
Statistical analysis
Results are displayed as means ± standard error of mean (SEM). All experiments were conducted at least three times. Data were analyzed using analysis of variance (ANOVA) followed by Bonferroni post-test comparisons in SPSS 20 software (SPSS, USA). Differences between groups were considered statistically significant when the p value was less than .05.
Results
Unc5b is an important mediator of netrin-1 on the surface of cells. First, we assessed the expression patterns of UNC5b in ESCs. RAW264.7 cells were used as a positive control. The RT-PCR results in reveal that UNC5b was expressed in ESCs at the gene level. The western blot results in reveal that UNC5b was expressed in ESCs at the protein level. We then investigated the expression of UNC5b in response to UV-B radiation. The real-time PCR results in indicate that UV-B exposure significantly increased the expression of UNC5b at the gene level in an intensity-dependent manner (25, 50, 75 mJ/cm2). Consistently, the western blot results in reveal that UV-B exposure significantly increased the expression of UNC5b at the protein level in an intensity-dependent manner (25, 50, 75 mJ/cm2).
Figure 1. UNC5b is expressed in epidermal stem cells (ESCs). (A) RT-PCR analysis revealed that UNC5b is expressed in ESCs; (B) Western blot analysis revealed that UNC5b is expressed in ESCs. RAW264.7 cells were used as a positive control. Experiments were performed in triplicate.
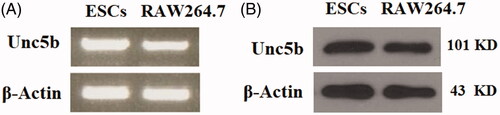
Figure 2. Expression of UNC5b is increased in response to exposure to ultraviolet-B (UV-B) in ESCs. ESCs were exposed to UV-B at the intensity of 25–75 mJ/cm2 for 8 h. (A) Expression of UNC5b at the gene level was determined by real-time PCR analysis; (B) Expression of UNC5b at the protein level was determined by western blot analysis (ANOVA:*, #, $, p < .001 vs previous column group, n = 4–5).
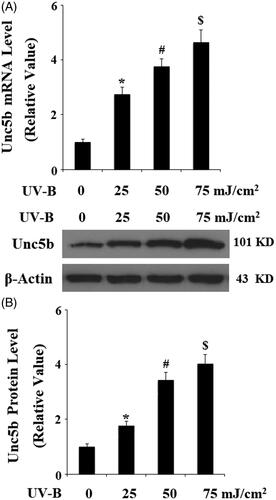
The above findings implicate that UNC5b may play an important role in UV-B radiation-induced insult in ESCs. Therefore, we treated ESCs with netrin-1, an agonist of UNC5b. The DCFH-DA results in indicate that netrin-1 reduced UV-B radiation-induced production of ROS in a dose-dependent manner. The mitochondria-derived enzyme NAD(P)H oxidase homolog (NOX-4) is a main source of intracellular ROS. As expected, we found that netrin-1 treatment significantly prevented UV-B radiation-induced expression of NOX-4. The TMRM staining results in demonstrate that UV-B radiation significantly reduced the level of mitochondrial membrane potential (MMP), which was prevented by the presence of netrin-1 in a concentration-dependent manner. Consistently, bioluminescence assay showed that UV-B radiation-induced reduced ATP was prevented by treatment with netrin-1 (). These results suggest that netrin-1 may have an important protective effect against UVB- induced mitochondrial dysfunction.
Figure 3. The UNC5b agonist netrin-1 attenuates ultraviolet-B (UV-B) exposure-induced oxidative stress in ESCs. ESCs were preincubated with netrin-1 (200, 400 ng/ml) for 12 h, followed by treatment with UV-B at 50 mJ/cm2 for 8 h. (A) Intracellular ROS was determined by DCFH-DA; Scale bar, 100 μM; (B) Western blot analysis of NOX-4 (ANOVA:*, #, $, p < .001 vs previous column group, n = 4–5).
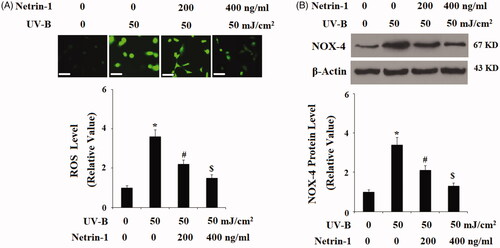
Figure 4. The UNC5b agonist netrin-1 protected ESCs against ultraviolet-B (UV-B) exposure-induced mitochondrial dysfunction. ESCs were preincubated with netrin-1 (200, 400 ng/ml) for 12 h, followed by treatment with UV-B at 50 mJ/cm2 for 8 h. (A) Mitochondrial membrane potential (MMP) determined by tetramethylrhodamine methyl ester (TMRM) staining; Scale bar, 100 μM; (B) Intracellular levels of Adenosine triphosphate (ATP) determined by the bioluminescence assay (ANOVA:*, #, $, p < .001 vs previous column group, n = 4–5).
UV-B radiation has been reported to cause cell damage by promoting the release of LDH. The release of LDH from ESCs into the supernatant was measured using an LDH cytotoxicity assay kit. As shown in , UV-B radiation significantly promoted LDH release from ECSs, however, the presence of netrin-1 significantly inhibited the release of LDH. The cell viability of ESCs was determined by MTT assay. The results in indicate that UV-B radiation significantly reduced the cell viability of ESCs, which was prevented by netrin-1 in a dose-dependent manner. These findings suggest that netrin-1 possesses a protective effect against UV-B radiation-induced cell death of ESCs.
Figure 5. The UNC5b agonist netrin-1 protected ESCs against ultraviolet-B (UV-B) exposure-induced LDH release. ESCs were preincubated with netrin-1 (200, 400 ng/ml) for 12 h, followed by treatment with UV-B at 50 mJ/cm2 for 8 h. LDH release was determined by a commercial kit (ANOVA:*, #, $, p < .001 vs previous column group, n = 5).
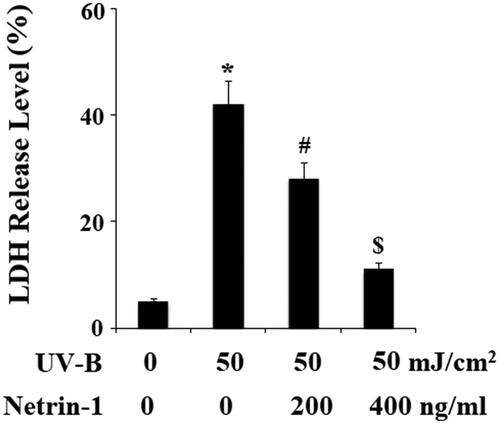
Figure 6. The UNC5b agonist netrin-1 prevented ultraviolet-B (UV-B) exposure-induced reduced cell viability. ESCs were preincubated with netrin-1 (200, 400 ng/ml) for 12 h, followed by treatment with UV-B at 50 mJ/cm2 for 8 h. Cell viability was determined by MTT assay (ANOVA:*, #, $, p < .001 vs previous column group, n = 5).
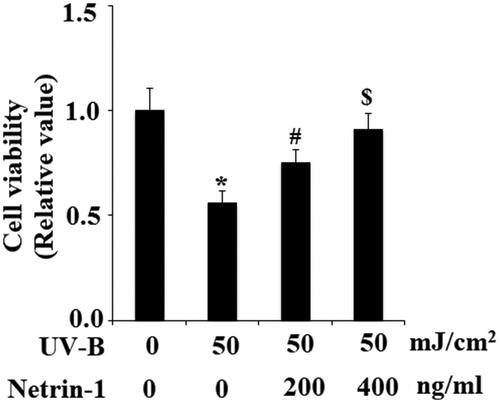
UVB radiation leads to loss of the capacities of stem cells. Integrin β1 and Krt19 are two important ESC markers. Therefore, we then examined whether treatment with netrin-1 had an impact on the expression of integrin β1 and Krt19 against UV-B radiation. The expression of integrin β1 and Krt19 at the gene level was determined using real-time PCR analysis. The results in demonstrate that UV-B exposure significantly decreased both the expression of integrin β1 and Krt19. However, the presence of netrin-1 significantly prevented this process. Most of the intracellular biological functions of netrin-1 are mediated by UNC5b. Hence, we knocked down the expression of UNC5b in ESCs. Successful knockdown of UNC5b is shown in . As expected, the results in indicate that silencing of UNC5b abolished the effects of netrin-1 on promoting expression of integrin β1 and Krt19. These findings suggest that the effects of netrin-1 in maintaining the capacities of ESCs are mediated by its specific receptor UNC5b.
Figure 7. The UNC5b agonist netrin-1 prevented ultraviolet-B (UV-B) exposure-induced impairment in the capacities of ESCs. ESCs were preincubated with netrin-1 (200, 400 ng/ml) for 12 h, followed by treatment with UV-B at 50 mJ/cm2 for 8 h. Expression of integrin β1 and Krt19 at the gene level was determined by real-time PCR analysis (ANOVA:*, #, $, p < .001 vs previous column group, n = 4–5).
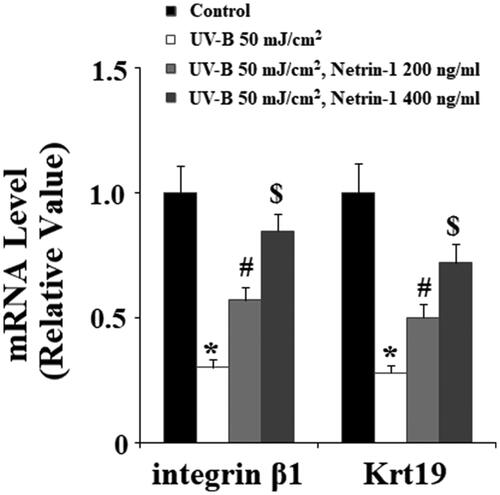
Figure 8. Knockdown of UNC5b abolished the protective effects of netrin-1 against ultraviolet-B (UV-B) exposure-induced impairment in the capacities of ESCs. ESCs were infected with lentiviral UNC5b shRNA. Cells were pre-incubated with netrin-1 (200, 400 ng/ml) for 12 h, followed by treatment with UV-B at 50 mJ/cm2 for 8 h. (A) Western blot analysis revealed the successful knockdown of UNC5b; (B) Real-time PCR analysis showed that knockdown of UNC5b abolished the effects of netrin-1 in integrin β1 and Krt19 expressions (ANOVA:*, #, $, p < .001 vs previous column group, n = 4–5).
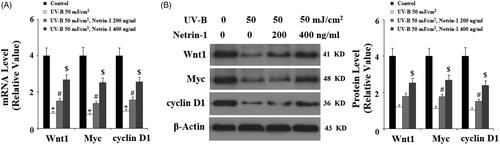
The Wnt/β-catenin signalling pathway plays an important role in supporting epidermal development and ESC behaviour. Here, we examined whether treatment with netrin-1 had an influence on Wnt/β-catenin signalling. The real-time PCR analysis and western blot analysis results shown in indicates that UV-B exposure significantly reduced the expression of Wnt1, Myc, and cyclin D1 at both the mRNA and protein levels, which were suppressed by the presence of netrin-1, suggesting that netrin-1 might maintain the capacities of ESCs through controlling Wnt/β-catenin signalling cascades.
Figure 9. The UNC5b agonist netrin-1 ameliorated ultraviolet-B (UV-B)-induced reduced Wnt pathway proteins. Cells were pre-incubated with netrin-1 (200, 400 ng/ml) for 12 h, followed by treatment with UV-B at 50 mJ/cm2 for 8 h. (A) Real-time PCR analysis of Wnt1, Wnt3a, Myc, cyclin D1; (B) Western blot analysis of Wnt1, Wnt3a, Myc, cyclin D1 (ANOVA:*, #, $, p < .001 vs previous column group, n = 4–5).
Discussion
UV light irradiation can be divided into three subtypes according to their wavelength: UV-A (320–400 nm), UV-B (280–320 nm), and UV-C (200–280 nm) [Citation15]. Among them, UV-B irradiation has been recognized as a key environmental mutagen and carcinogen of epidermal cells. ESCs are an important component of the epidermal basal layer. Cells present in this layer bring about all epidermal structures, including a striated epidermis and epidermal appendages. All epidermal keratinocytes, keratinocyte stem cells and ESCs are vulnerable to attack by UV-B [Citation16]. Repetitive exposure to UV-B irradiation accelerates premature ageing of the skin by disturbing cell turnover homeostasis and loss of the capacity of stem cells. UV-B acts as an important inducer of DNA damage in skin. UV-B irradiation-induced DNA damage causes undesirable dysregulation of the skin stem cell pool [Citation17]. Impairment of stem cell capacities in ESCs gives rise to the failure of self-renewal and differentiation [Citation18]. Loss of the capacities of ESCs interferes with the processes of skin homeostasis, wound healing and rebuilding. Importantly, loss of the capacities of ESCs has been linked with a variety of diseases, including unsuccessful diabetic wound repair [Citation19]. In the current study, we aimed to investigate the effects of netrin-1 and its receptor UNC5b in the stem cell capacities of ESCs under the condition of UV-B irradiation.
Netrin-1 signalling is tightly controlled through interactions with its major receptors on the cell surface, including UNC5b [Citation20]. Previous studies have shown that UNC5b is mainly expressed in the nervous and cardiovascular systems, where it acts as a guidance receptor through binding to netrin-1 and activating intracellular signalling cascades [Citation21]. In the current study, for the first time, we report that UNC5b is expressed in ESCs. Also, we found that UNC5b expression is increased in response to UV-B irradiation in ESCs, implicating the possible involvement of UNC5b in UV-B-induced stress. UV-B irradiation can cause gene mutation in ESCs through the excessive generation of intracellular ROS [Citation22]. Here, we found that netrin-1 treatment reduced UV-B-induced ROS overproduction and NOX-4 expression, suggesting a novel antioxidant capacity of netrin-1. Consistently, it has been recently shown that netrin-1 displays an UNC5b-dependent anti-inflammatory effect in endothelial cells through suppressing TNF-α-induced production of the cytokines MCP-1, IL-1β and IL-6 as well as expression of the vascular adhesion molecules VCAM-1, ICAM-1 and E-selectin [Citation23]. Exacerbation of cell death and mitochondrial dysfunction has been linked to decline in ESC function in response to UV-B radiation. Here, we found that netrin-1 ameliorated UV-B-induced mitochondrial dysfunction and LDH release. Indeed, netrin-1 treatment has been shown to attenuate cell death and neuronal apoptosis in cultured primary cortical neurons after oxygen-glucose-deprivation (OGD) injury [Citation24]. It should be noticed that UV-B exposure debilitated the features of ESCs through reducing the expression of integrin β1 and Krt19, two important ESC markers. However, this inhibitory effect is mitigated by netrin-1 treatment, suggesting that netrin-1 can maintain the capacities of ESCs. Consistently, a recent study has shown that UNC5b is expressed on the surface of human adipose-derived stem cells (hASCs) and is required for osteogenic differentiation [Citation25]. Mechanistically, we found that netrin-1 could activate Wnt/β-catenin signalling in ESCs. The Wnt/β-catenin pathway is crucial for the fate of endothelial cells. Additionally, Wnt/β-catenin signalling plays a role in a wide range of physiological functions, including cell survival, cell growth and embryonic development. The effects of netrin-1 on Wnt/β-catenin activation suggest that netrin-1 may exert an effect on various cellular functions in ECSs.
Collectively, we have found a novel biological function of netrin-1 and its receptor UNC5b in maintaining the stem cell capacities of ESCs under insult from UV-B irradiation. Our results suggest that netrin-1 may have therapeutic potential for the treatment of skin diseases.
Authors contribution
Dr Peng Wu and Dr Yibing Wang conceived the hypothesis of this article; Dr Peng Wu drafted the article; Dr Yibing Wang revised the article; Dr Peng Wu, Dr Yongqian Cao and Dr Ran Zhao performed the experiments and analyzed the data; Dr Yibing Wang applied for the grant and supplied the materials.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Hirabayashi T, Murakami M, Kihara A. The role of PNPLA1 in ω-O-acylceramide synthesis and skin barrier function. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:869–879.
- Rognoni E, Watt FM. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol. 2018;28:709–722.
- Fuchs E. Skin stem cells in silence, action, and cancer. Stem Cell Rep. 2018;10:1432–1438.
- Li Y, Zhang J, Yue J, et al. Epidermal stem cells in skin wound healing. Adv Wound Care (New Rochelle). 2017;6:297–307.
- Veltri A, Lang C, Lien WH. Concise review: Wnt signaling pathways in skin development and epidermal stem cells. Stem Cells. 2018;36:22–35.
- Yokouchi M, Kubo A. Maintenance of tight junction barrier integrity in cell turnover and skin diseases. Exp Dermatol. 2018;27:876–883.
- Panich U, Sittithumcharee G, Rathviboon N, et al. Ultraviolet radiation-induced skin aging: the role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016;2016:7370642.
- Mei XL, Lian S. Ultraviolet B light-induced apoptosis in human keratinocytes enriched with epidermal stem cells and normal keratinocytes. Chin Med J (Engl). 2011;124:591–598.
- Xu K, Wu Z, Renier N, et al. Neural migration. Structures of netrin-1 bound to two receptors provide insight into its axon guidance mechanism. Science. 2014;344:1275–1279.
- Prieto CP, Ortiz MC, Villanueva A, et al. Netrin-1 acts as a non-canonical angiogenic factor produced by human Wharton's jelly mesenchymal stem cells (WJ-MSC). Stem Cell Res Ther. 2017;8:43.
- Bhat SA, Gurtoo S, Deolankar SC, et al. A network map of netrin receptor UNC5B-mediated signaling. J Cell Commun Signal. 2018;13:121–127.
- Lee SS, Lee SJ, Lee SH, et al. Netrin-1-induced stem cell bioactivity contributes to the regeneration of injured tissues via the lipid raft-dependent integrin α6β4 signaling pathway. Sci Rep. 2016;6:37526.
- Yang RH, Qi SH, Shu B, et al. Epidermal stem cells (ESCs) accelerate diabetic wound healing via the Notch signalling pathway. Biosci Rep. 2016;36:e00364.
- Sheng B, Gong K, Niu Y, et al. Inhibition of gamma-secretase activity reduces Abeta production, reduces oxidative stress, increases mitochondrial activity and leads to reduced vulnerability to apoptosis: implications for the treatment of Alzheimer's disease. Free Radic Biol Med. 2009;46:1362–1375.
- Häder DP, Sinha RP. Solar ultraviolet radiation-induced DNA damage in aquatic organisms: potential environmental impact. Mutat Res. 2005;571:221–233.
- Damiani E, Ullrich SE. Understanding the connection between platelet-activating factor, a UV-induced lipid mediator of inflammation, immune suppression and skin cancer. Prog Lipid Res. 2016;63:14–27.
- Plikus MV, Van Spyk EN, Pham K, et al. The circadian clock in skin: implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J Biol Rhythms. 2015;30:163–182.
- Velarde MC, Demaria M, Melov S, et al. Pleiotropic age-dependent effects of mitochondrial dysfunction on epidermal stem cells. Proc Natl Acad Sci USA. 2015; 112:10407–10412.
- Morasso MI, Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell. 2005;97:173–183.
- Ranganathan P, Mohamed R, Jayakumar C, et al. Guidance cue netrin-1 and the regulation of inflammation in acute and chronic kidney disease. Mediators Inflamm. 2014;2014:1–525891.
- Rubina KA, Tkachuk VA. Guidance Receptors in the Nervous and Cardiovascular Systems. Biochemistry Mosc. 2015;80:1235–1253.
- Goyal S, Amar SK, Srivastav AK, et al. ROS mediated crosstalk between endoplasmic reticulum and mitochondria by Phloxine B under environmental UV irradiation. J Photochem Photobiol B. 2016;161:284–294.
- Lin Z, Jin J, Bai W, et al. Netrin-1 prevents the attachment of monocytes to endothelial cells via an anti-inflammatory effect. Mol Immunol. 2018;103:166–172.
- Chen J, Du H, Zhang Y, et al. Netrin-1 prevents rat primary cortical neurons from apoptosis via the DCC/ERK pathway. Front Cell Neurosci. 2017;11:387.
- Hu X, Liu Y, Zhang M, et al. UNC-5 netrin receptor B mediates osteogenic differentiation by modulating bone morphogenetic protein signaling in human adipose-derived stem cells. Biochem Biophys Res Commun. 2018;495:1167–1174.
