Abstract
Development of novel methods is needed for the synthesis of nanoparticles. Attention on such particles has elevated disquiet about the eco-friendly manner of their fabrication methods. In the present study, we equipped and synthesized gold nanoparticles (AuNPs) from Panax notoginseng; investigated as an environmentally friendly and non-toxic substrate. Amalgamation of AuNPs was distinguished by numerous studies such as UV-absorbance and it shows peak values in the range of 520–550 nm. Nanoparticles sizes are confirmed by dynamic light scattering analysis and it shows 100 nm. Moreover, transmission electron microscopy (TEM) and energy dispersive X-ray analysis (EDX) confirmed the shape of the Au particles present in the synthesized materials. FTIR analysis studies showed the active molecules positioned in the plane of the synthesized particles. The anticancer potential of AuNPs was evaluated in PANC-1 cells. Additionally, AuNPs efficiently induced cytotoxicity, ROS and apoptosis by intonating intrinsic apoptotic gene expressions in PANC-1 cells. Finally, our study confirmed the synthesis of AuNPs from Panax notoginseng, which showed anticancer effects in an environmentally friendly manner.
Introduction
Nanotechnology has triggered the augmentation of resuscitation medicine, and with the progress in nanotechnology, the established design has resulted in improved resuscitative properties of tissues. The functions and interaction between the cells exist at a nanolevel; modern altered systems can amend the functions and behaviour, which results in enhanced cellular level functions that can imitate indigenous organs and tissues [Citation1]. Characteristics of nanomaterials exhibit different properties like morphology, distribution and size [Citation2]. Nanodelivery systems are proficient of, in particular, targeting tumour tissues, which consent to exploit the improved dispersion and withholding effect [Citation3]. Plentiful chemicals are used to extend as nanoparticles for the beneficial effect. Conversely, those resources are highly cost-effective and hazardous to the environment; consequently, uses of metal nanoparticles are measured as eco-friendly and economical [Citation4,Citation5].
Nanoparticles size ranges from 100 to 1000 times lighter than a human cell; as a result nanoscale devices can enter the cellular environment with no trouble and act together with, proteins, DNA, enzymes and receptors [Citation6]. Numerous nanoparticle-based drug delivery systems have been intended to accomplish the constantly emergent needs of the basic and clinical research fields. Both in Indian and Chinese culture gold is used as health adjuvant in the form of “gold bhasma” for treatment of a variety of health issues and deficiencies like mounting imperative power and for the treatment of male impotence [Citation7]. Gold has exclusive properties like limpness, harmless to the cellular environment and is a valuable and striking material for the biomedical researchers in addition to significance in healing and imaging systems [Citation8–9].
Pancreatic cancer is the fourth foremost cause of cancer-associated transience with predictable 43,140 cases in line with 36,800 mortality in 2010 in America [Citation10]. A recent study showed that the 5-year survival rate for patients is 33% who underwent surgery for stage-I pancreatic cancer [Citation11]. Surgery remains the better way for the treatment of pancreatic cancer. Nonetheless, the majority of patients diagnosed are waiting for the tumour to attain a highly developed stage, and prognosis also deprived subsequent re-sectioning of the tumour. Radiation-oriented management is usually unsuccessful and fabricates only subsidiary survivals [Citation12]. Furthermore, the uses of chemotherapy and radiotherapy result in prominent and considerable side effects on patients [Citation13]. Numerous plants have been effectively used for the proficient and speedy extracellular synthesis of gold nanoparticles. Consequently, a plant-mediated amalgamation of nanoparticles have the utmost and ultimate loom for embattled delivery on cancer cells and avert the unwanted side effects. A number of medicinal plants have been used at the side of the conservative treatment schedule [Citation14].
Nanoparticles from plants could be more beneficial since it does not need complicated processes such as intracellular synthesis and numerous decontamination steps [Citation15,Citation16]. Panax notoginseng is an extremely cherished ginseng species in genus Panax, Araliaceae family [Citation17]. Panax notoginseng is considered as a medication for averting blood loss and recovering from injury and also used broadly in the form of foods or drugs. This species is used in Chinese traditional medicine, which possesses the capacity to protect liver injury that was induced by D-galactosamine in mice [Citation18]. Panax notoginseng and its active components seem to be efficient against colorectal cancer [Citation19,Citation20]. Ethanol extracts of Panax notoginseng also restrain spleen tumour and liver metastasis [Citation21]. Previous reports have shown that Panax notoginseng extracts are effective against skin, lung, prostrate and liver cell lines [Citation22–25].
In this study, we produced gold nanoparticles (AuNPs) from the ethanolic extract of Panax notoginseng and it was exemplified by numerous studies such as high resolution-transmission electron microscope (HR-TEM), UV-visible absorbance spectrum, dynamic light scattering analysis (DLS), FTIR, and energy-dispersive X-ray analysis (EDX). Furthermore, synthesized AuNPs were oppressed in the anticancer prospective studies by apoptotic regulation in pancreatic cancer cells (PANC-1).
Materials and methods
Chemicals, antibodies and solvents
Dulbecco’s modified eagles medium (DMEM), Foetal bovine serum (FBS), and trypsin-EDTA were purchased from GIBCO (California, USA). Antibodies such as Bax, Bcl-xl, Bcl-2, β-actin, caspase-3 and goat anti-mouse IgG-HRP polyclonal antibody were purchased from Santacruz, USA. 3–(4, 5-dimethyl-2-thiaozolyl)-2, 5-diphenyl-2H tetrazolium bromide (MTT), 2, 7-diacetyl dichlorofluorescein (DCFH-DA), acridine orange (AO), propidium iodide (PI) were purchased from Sigma Chemical Co., St. Louis, MO, USA. Other fine chemicals and solvents were used in the analytical grade.
Gold nanoparticles (AuNPs) synthesis and purification
Panax notoginseng leaves were worn out to make the aqueous extract. Cleaned Panax notoginseng leaves were weighing 25 g and rinsed with double-distilled water. Then, it was allowed to dry and firmed into 100 ml disinfected distilled water and it was filtered by Whatman No.1 filter paper (25 μm). For the synthesis of gold, the digestive growing method was used to generate gold nanoparticles from polyscattering nanoparticles by using digestive agents. Followed by heating colloidal suspension at elevated temperatures (∼138 °C) for 2 min followed by reducing of temperature in the presence of alkanethiols. Temperature plays an imperative role in scheming the size allotment of the gold colloids [Citation26].
Characterization of AuNPs synthesized from Panax notoginseng
Following synthesis and refinement of AuNPs, they were characterized by several experiments, which confirmed the size, shape and morphological characteristics of nanoparticles. The produced AuNPs were inveterated by ultraviolet-visible absorbance spectrum studies at wavelength ranges of 300–700 nm (Shimadzu UV spectrophotometer). Size and morphological distinctiveness of AuNPs were recognized and confirmed by transmission electron microscopy (TEM) images. Nanoparticles nature and stability were analyzed by energy-dispersive X-ray (EDX). Surface morphology and size of the nanoparticles were determined by dynamic light scattering (DLS). FTIR spectroscopy measurements were performed to examine the active compounds present on the synthesized AuNPs. The FTIR spectra of AuNPs were recorded in the range of 4000 and 1000 cm−1 in KBr pellets using FTIR spectrophotometer. Atomic force microscopy (AFM) was used to determine the film with or without heat treatment using an Agilent 5500 AFM with silicon cantilever model and typical resonance frequency 2.7 N/m and 80 kHz, respectively, and tip radius <10 nm. Experiments were made in non-contact mode (topography and phase contrast). Gwyddion 2.10 software was used to analyze the AFM images.
Cell culture
Human pancreatic cancer cell lines (PANC-1) were used for this study. Cells were procured from Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). Cells were preserved in DMEM medium enhanced with 10% foetal bovine serum (FBS) and 1% antibiotics at 37 °C in a humidified incubator at 5% CO2. After 96 h, the culture was conquered with around 80% confluence and the cells were harvested with 1 ml of trypsin-EDTA solution. Finally, cells were ready to use for further studies.
Cell viability assay
After collecting PANC-1 cells, 5000–10,000 cells were added into 96-well plates and incubated for 24 h at 37 °C. Then, amalgamated AuNPs from Panax notoginseng were treated with various concentrations (5–100 µg/ml) and incubated for 24 h at 37 °C. After incubation, the cells were exposed with MTT reagent (1 mg/mL) in all the wells. Finally, the culture plate was incubated at 37 °C for 2–3 h to form purple formazan crystals. 200 μL of DMSO was added to each well to suspend formazan crystals, and then, the optical density was measured at 570 nm in a microplate reader.
Intracellular ROS measurement
AuNPs intervened ROS generation in PANC-1 cells was deliberated by fluorescent probe, 2,7-diacetyl dichlorofluorescein (DCFH-DA) staining [Citation27]. Shortly, an aliquot of isolated PANC-1 cells (1 × 106 cells/ml) was equipped and loaded in six-well plates. Then, the cells were indulged with 25 and 50 µg/ml concentrations of AuNPs at 24-h incubation. Then, 1 μl of DCFH-DA (1 mg/mL) was added to all the wells and incubated at 37 °C in a CO2 incubator under dark condition. The fluorescent intensity was calculated by spectrofluorimeter with excitation and emission correspondingly at 485 ± 10 and 530 ± 12.5 nm.
Fluorescent terminal deoxynucleotidyl transferase (TdT)-meditated dUTP-fluorescein nick end-labelling (TUNEL)
AuNPs interceded nuclear fragmentation was examined by Sigma TUNEL assay kit. After treatment of AuNPs, the PANC-1 cells were cleansed with PBS twice, then fixed in paraformaldehyde (2%) for 30 min and permeabilized with 0.1% Triton X-100 for 30 min. Then, they incubated with TUNEL reaction buffer at 37 °C for 1 h in the dark, and again rinsed twice with phosphate buffer saline. Finally, cells were stained with DAPI, and it was visualized under a Nikon fluorescence microscope.
Apoptotic studies for acridine orange/ethidium bromide
AuNPs arbitrated apoptotic changes in PANC-1 cells were evaluated by acridine orange and ethidium bromide (AO/Et) staining. Shortly, 1 × 105 cells were seeded in a 6-well plate, and then the cultured cells were mixed with 25 and 50 µg/ml concentrations of AuNPs incubation for 24 h. When incubation was over, the cells were cleaned with PBS (ice-cold) then discoloured with 20 µL of AO/PI staining (10 µL/mg AO and 10 µL/mg Et) solution at 37 °C for 15 min. The discoloured apoptotic and viable cells were observed using a fluorescent microscope.
Caspase-8, 9 and 3 activity assay
Caspase-8, 9 and 3 activities were deliberated by caspase assay kit, the methodology was followed as per manufacturer’s instruction. Shortly, the PANC-1 cells were loaded in 6-well plate and treated with AuNPs then allowed to incubate for 24 h at CO2 incubator. Then, the cells were allowed to treat with suitable caspase-8, 9 and 3 reagents and then incubated for 2 h in dark room. Then, the optical density for caspase-8, 9 and 3 were measured using a microplate reader at 400 or 405 nm.
Western blot analysis
Apoptotic markers such as, Bcl-xl, Bax, Bcl-2 and caspase-3 were examined by western blotting. Shortly, 1 × 106 cells were uncovered with AuNPs for 24-h incubation. Then, the proteins were extorted from cells using with RIPA buffer which contains cocktail protease. The protein concentration was measured using the Thermo scientific nanodrop instrument (USA). Then, the proteins were positioned to 10% SDS-PAGE and the gel was reassigned to PVDF membranes. The membrane was blocked with 5% bovine serum albumin for 3 h. The membranes were combined with a suitable primary antibody and kept for 24-h incubation at 4 °C. Additionally, it was incubated with suitable conjugated horseradish peroxidase secondary antibodies for 1 h. Bands were identified by using improved chemiluminescent substrates and band intensity was analyzed using Image J software.
Statistical analysis
All experiments were carried out in three self-determined experiments and the results were articulated as the mean ± standard deviation (Mean ± SD) by using one-way analysis of variance (ANOVA). Values of p < .05 were indicative of significant differences.
Results and discussion
Since splendid metal nanoparticles, especially gold nanoparticles, are extensively used in the human environment, there is a rising need to expand eco-friendly practices of nanoparticle synthesis using toxic free chemicals. Consequently, the organic process of nanoparticle production using plant extracts, by adding up with microorganisms, have been recommended as a promising eco-friendly substitute to both physical and chemical methods. By means of plants for nanoparticle, separation can be beneficial over other organic methods by eradicating the intricate process of preserving cell cultures [Citation28]. Reasons for these kinds of nanosyntheses are their cost-effectiveness and eco-friendliness and their numerous biomedical functions [Citation29,Citation30]. In the present study, we have verified the preparing of gold nanoparticles from Panax notoginseng and it was illustrated by UV-visible absorbance spectrum, dynamic light scattering analysis (DLS), high-resolution transmission electron microscope (HR-TEM), FTIR, energy-dispersive X-ray analysis (EDX). Furthermore, production of AuNPs has exploited the anticancer potential studies by apoptotic regulation in pancreatic cancer cells (PANC-1).
Characterization of gold nanoparticles (AuNPs) synthesized from Panax notoginseng
The synthesis of AuNPs from Panax notoginseng was primarily indicated by the colour exchange and the formation of ruby-red colour indicated the formation of the AuNPs (). The colour change was specified to excitation of surface Plasmon resonance synthesized AuNPs. UV- spectroscopy was used at dissimilar time indication while fixing the concentration of the Panax notoginseng and the aqueous solution of gold chloride. The ocular sign of the colour replacement and the formation of ruby-red colour indicated the formation of the AuNPs; that directs the formation of gold nanoparticles (). This related data were previously proved by numerous researches on synthesis of gold nanoparticles [Citation31].
Figure 1. UV–visible spectrum absorption pattern and Dynamic Light Scattering of gold nanoparticles synthesized from Panax notoginseng. (A) UV-visible absorption spectrum of synthesized AuNPs. (B) Dynamic light scattering (DLS) images of AuNPs synthesized from Panax notoginseng and the size of the nanoparticles is 128 nm.
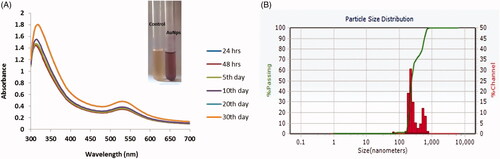
Moreover, we established the Panax notoginseng arbitrated produced AuNPs by UV visible absorbance. A mounting concentration of Panax notoginseng extracts leads to increased intensity of absorption. The UV-visible spectra recorded after different time intervals such as 24, 48, 72 and 92 h from the initiation of reaction with different amounts of plant extract. This finding coincides with previous studies in which UV-Vis spectroscopy was used to examine the shape and size-controlled nanomaterials in the aqueous suspensions [Citation32]. In the present study, the synthesized AuNPs displays the highest absorbance, which was found to be ∼540 nm at 96 h prepare solution. The maximum absorbance bands were raised at a dissimilar concentration of AuNPs solution in different time points. When compared to other metal nanoparticles predominantly AuNPs have free electrons which contribute to SPR absorption bands due to the united vibration of electrons of metal nanoparticles in resonance with the lightwave [Citation33]. Size distributions of synthesized gold nanoparticles from Panax notoginseng extract is done by dynamic light scattering (DLS) method. Herein histogram image indicated that the particle size of the Au nanoparticles from Panax notoginseng is 128 nm (). Furthermore, particle aggregation was not observed that results in the precision of particle size and allocation. Additionally, morphological uniqueness of AuNPs synthesized from Panax notoginseng was further considered and studied by HR-TEM analysis ().
Figure 2. HR-Transmission electron microscopy (HR-TEM) and energy-dispersive X-ray analysis (EDX) of gold nanoparticles synthesised from Panax notoginseng. (A) Transmission electron microscopy (HR-TEM) and (B) Energy-dispersive X-ray (EDX) and analysis AuNPs synthesised from Panax notoginseng.
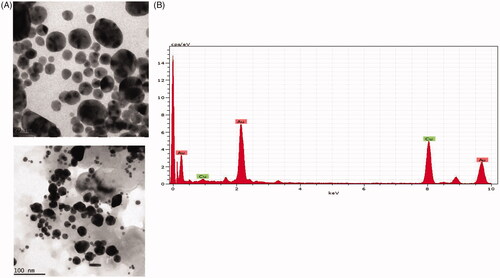
HR-TEM image shows that the morphological structure of AuNPs which are observed in, hexagonal, spherical, oval and triangular with average sizes between 80 to 12 nm. This finding supports the statistically interrelated with DLS images for AuNPs produced from Panax notoginseng. EDX studies were examined to assess the shape of the particles synthesized from Panax notoginseng extract gold metal (). Moreover, the TEM figures afford high-density AuNPs synthesized by the Panax notoginseng extract in additional, which confirms the gold nanostructures development. In the present study, sturdy signals were experienced in the gold region, which confirms the AuNPs formation. In our study, we obtained the strong signals of Au (1.4–2 and 2.5–3 keV). Due to surface plasmon resonance, the distinctive visual absorption for metallic crystal was generally found to be 3 KeV [Citation34], which supports our finding. The topographical structure of biosynthesized AuNP’s was analyzed by atomic force microscopy (.
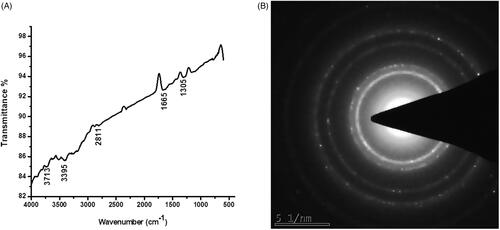
Figure 3. Atomic force microscopy analysis of gold nanoparticles synthesized from Panax notoginseng.
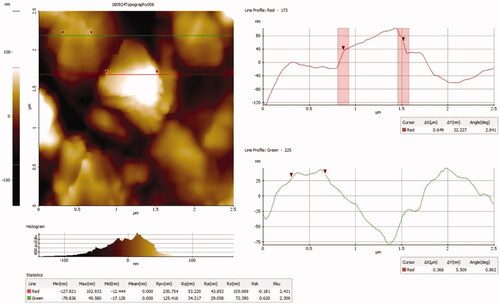
The AFM analysis confirmed the nanosphere shapes of the gold nanoparticles which correlate with the TEM image. FTIR studies used to recognize the numerous functional groups present in the synthesis of AuNPs from Panax notoginseng extract by obtaining different spectrum values. In present study FTIR showed that 3713 cm1, 3395 cm1, 2811 cm1, 1665 cm1, and 1305cm1 which specify the hydroxyl(3550–3200 cm1), aromatic(1310–1200 cm1), carboxyl(1500–2000 cm1), amide and alkyl (2000–1650 cm1) groups that present on the surface of AgNPs (). shows the selected area electron diffraction (SAED) pattern of biosynthesized gold nanoparticles exhibits ring-like structure which indicates the crystalline nature of the nanoparticles. Our finding correlates with previous studies in which the synthesis of gold nanoparticles from different plants extracts has numerous functional groups [Citation35].
Anticancer potential of AuNPs from Panax notoginseng pancreatic cancer cell lines (PANC-1)
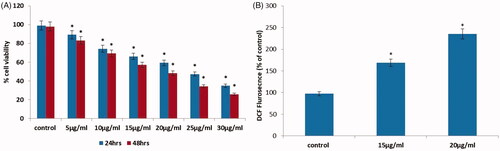
AuNPs were tested for their anticancer activity against pancreatic cancer cells (PANC-1). Consequently, the anticancer potential of AuNPs was studied by MTT cytotoxicity assay. In this assay, the raised concentration of AuNPs considerably restrained the PANC-1 cells growth for 24 and 48 h incubation (. Hence, we found that 15 and 20 µg/ml concentrations of AuNPs revealed prominent cell death in PANC-1 cells. Consequently, we preferred 15 and 20 µg/ml concentrations of AuNPs for apoptotic studies. Synthesized AuNPs from Dendropanax morbifera leaf extracts shows significant anticancer activity HaCaT cells [Citation36].
Intracellular reactive oxygen species (ROS) in a system induces oxidative stress which results in apoptosis [Citation37]. Therefore, AuNPs-mediated ROS production was examined by DCFH-DA fluorescent assay. In the present study, we confirmed that AuNPs produced from Panax notoginseng induces ROS generation in pancreatic PANC-1 cells (. In addition, ROS overproduction leads to nuclear fragmentation and mitochondrial depolarization that leads to oxidative stress-mediated apoptosis [Citation38–40] which supports our finding.
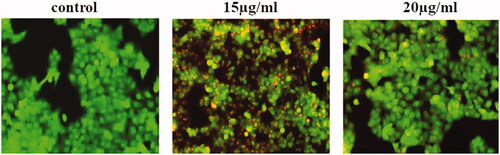
Tumour progress and development and resistance to the majority of oncologic treatment results mainly due to deficiency of the apoptotic stimuli. Apoptosis is a crucial controller of tissue homeostasis [Citation41,Citation42]. Consequently, apoptosis is a painstaking and important pathway for cancer treatment. AuNPs synthesized Panax notoginseng provoke ROS mediated apoptosis were evaluated by AO/PI dual staining. In the present study, control cells show acridine orange-stained green fluorescent which signifies viable cells. While treatment with AuNPs synthesized from Panax notoginseng showed augmented apoptotic cells by observing EtBr stained red fluorescent cells (.
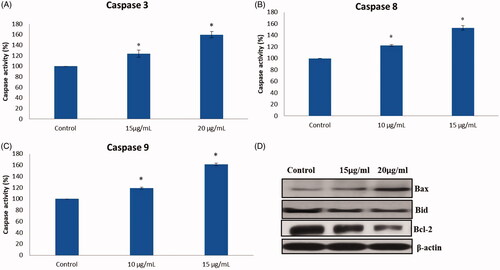
AuNPs mediated apoptosis makers such as Bax, Bcl-2, Bcl-xl, Caspase-3, 8 and 9 were examined by caspase assay kit and western blotting. shows treatment with AuNPs synthesized from Panax notoginseng downregulated the expression of Bcl-2, Bcl-xl and upregulated the expression of Bax, caspase-8, caspase-9 and caspase-3 in PANC-1 cells. This data directly related to acridine orange/v staining. Finally, with these findings, AuNPs synthesized from Panax notoginseng successfully persuaded apoptosis by amending the intrinsic apoptotic pathway. Previous studies support our present findings in which gold nanoparticles persuade apoptosis in MCF-7 human breast cancer cells.
Conclusion
In this study, we illustrate an effortless, rapid, and reproducible method for the eco-friendly synthesis of AuNPs without the need for classy reducing agents. The potential of AuNPs synthesized from Panax notoginseng were well demonstrated. The physio-chemical distinctiveness of synthesized AuNPs from Panax notoginseng is established by UV-absorbance, DLS, HR-TEM, EDX and FTIR. AuNPs successfully induces cytotoxicity, ROS and apoptosis by accenting intrinsic apoptotic gene expressions in PANC-1 cells. Finally, our findings confirm that the synthesis of AuNPs from Panax notoginseng demonstrates antimicrobial and anticancer effects.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chaudhury K, Kumar V, Kandasamy J, et al. Regenerative nanomedicine: current perspectives and future directions. Int J Nanomed. 2014;9:4153–4167.
- Kaviyarasu K, Manikandan E, Kennedy J, et al. A comparative study on the morphological features of highly ordered MgO: AgO nanocube arrays prepared via a hydrothermal method. RSC Adv. 2015;5:82421–82428.
- Mokhtarzadeh A, Hassanpour S, Vahid ZF, et al. Nano-delivery system targeting to cancer stem cell cluster of differentiation biomarkers. J Control Release. 2017;266:166–186.
- Gan PP, Ng SH, Huang Y, et al. Green synthesis of gold nanoparticles using palm oil mill effluent (POME): a low-cost and eco-friendly viable approach. Bioresource Technol. 2012;113:132–135.
- Roopan SM, Madhumitha G, Rahuman AA, et al. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crops Prod. 2013;43:631–635.
- Tian Y, Qi J, Zhang W, et al. Facile, one-pot synthesis, and antibacterial activity of mesoporous silica nanoparticles decorated with well-dispersed silver nanoparticles. ACS Appl Mater Interfaces. 2014;6:12038–12045.
- Bhattacharya R, Patra CR, Earl A, et al. Attaching folic acid on gold nanoparticles using noncovalent interaction via different polyethylene glycol backbones and targeting of cancer cells. Nanomed Nanotechnol Biol Med. 2007;3:224–238.
- Ilkhani H, Sarparast M, Noori A, et al. Electrochemical aptamer/antibody based sandwich immunosensor for the detection of EGFR, a cancer biomarker, using gold nanoparticles as a signaling probe. Biosensors Bioelectr. 2015;74:491–497.
- Ho-Wu R, Yau SH, Goodson T, III. Linear and nonlinear optical properties of monolayer-protected gold nanocluster films. ACS Nano. 2016;10:562–572.
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
- Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol. 2007;14:1320–1326.
- Yang F, Jin C, Jiang Y, et al. Liposome based delivery systems in pancreatic cancer treatment: from bench to bedside. Cancer Treat Rev. 2011;37:633–642.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197.
- Calixto JB. Twenty-five years of research on medicinal plants in Latin America: a personal view. J Ethnopharmacol. 2005;100:131–134.
- Nabikhan A, Kandasamy K, Raj A, et al. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf B: Biointerfaces. 2010;79:488–493.
- Lee WM, An YJ, Yoon H, et al. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water‐insoluble nanoparticles. Environ Toxicol Chem. 2008;27:1915–1921.
- Yang BR, Cheung KK, Zhou X, et al. Amelioration of acute myocardial infarction by saponins from flower buds of Panax notoginseng via pro-angiogenesis and anti-apoptosis. J Ethnopharmacol. 2016;181:50–58.
- Tung NH, Quang TH, Son JH, et al. Inhibitory effect of ginsenosides from steamed ginseng-leaves and flowers on the LPS-stimulated IL-12 production in bone marrow-derived dendritic cells. Arch Pharm Res. 2011;34:681e5.
- He NW, Zhao Y, Guo L, et al. Antioxidant, antiproliferative, and pro-apoptotic activities of a saponin extract derived from the roots of Panax notoginseng (Burk.) F.H. Chen. J Med Foo. 2012;15:350–359.
- Wang CZ, Xie JT, Fishbein A, et al. Antiproliferative effects of different plant parts of Panax notoginseng on SW480 human colorectal cancer cells. Phytother Res. 2009;23:6–13.
- Chen PF, Liu LM, Chen Z, et al. Effects of ethanol extracts of Panax notoginseng on liver metastasis of B16 melanoma grafted in mice. J Chin Integr Med. 2006;4:500–503.
- Park SC, Yoo HS, Park C, et al. Induction of apoptosis in human lung carcinoma cells by the water extract of Panax notoginseng is associated with the activation of caspase-3 through downregulation of Akt. Int J Oncol. 2009;35:121–127.
- Konoshima T, Takasaki M, Tokuda H. Anti-carcinogenic activity of the roots of Panax notoginseng. II. Biol Pharm Bull. 1999;22:1150–1152.
- Wang Nan WJ-b, Ming-yuen L, Yi-tao W. Advances in studies on Panax notoginseng against atherosclerosis. Chin Tradit Herb Drugs. 2008;5:787–791.
- Toh DF, Patel DN, Chan EC, et al. Anti-proliferative effects of raw and steamed extracts of Panax notoginseng and its ginsenoside constituents on human liver cancer cells. Chin Med. 2011;6:1749–8546.
- Prasad BLV, Stoeva SI, Sorensen CM, et al. Digestive ripening agents for gold nanoparticles: Alternatives to thiols. Chem Mater. 2003;15:935–942.
- Gunaseelan S, Balupillai A, Govindasamy K, et al. Linalool prevents oxidative stress activated protein kinases in single UVB-exposed human skin cells. PLoS One. 2017;12:e0176699.
- Chen TT, Yi JT, Zhao YY, et al. Biomineralized metal–organic framework nanoparticles enable intracellular delivery and endo-lysosomal release of native active proteins. J Am Chem Soc. 2018;140:9912–9920.
- Sarvamangala D, Kantipriya K, Murthy USN, et al. Green synthesis of AgNPs using alternanthera sessilis leaf extract [A Natural Source for Ocular Therapy]. Int J Innovative Res Sci, Eng Technol. 2014;3:15000–15010.
- Rizvi SAA, Saleh AM. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J. 2018;26:64–70.
- Aljabali A, Akkam Y, Al Zoubi M, et al. Synthesis of gold nanoparticles using leaf extract of Ziziphus zizyphus and their antimicrobial activity. Nanomaterials. 2018;8:174.
- Zhang XF, Liu ZG, Shen W, et al. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. IJMS. 2016;17:1534.
- Eustis S, El-Sayed MA. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev. 2006;35:209–217.
- Kaviya S, Santhanalakshmi J, Viswanathan B, et al. Biosynthesis of silver nanoparticles using citrus sinensis peel extract and its antibacterial activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscop. 2011;79:594–598.
- Kuppusamy P, Yusoff MM, Maniam GP, et al. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications - An updated report. Saudi Pharm J. 2016;24:473–484.
- Wang C, Mathiyalagan R, Ju Kim Y, et al. Rapid green synthesis of silver and gold nanoparticles using Dendropanax morbifera leaf extract and their anticancer activities. IJN. 2016;11:3691–3701.
- Ivanova D, Zhelev Z, Aoki I, et al. Overproduction of reactive oxygen species - obligatory or not for induction of apoptosis by anticancer drugs. Chin J Cancer Res. 2016;28:383–396.
- Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biology. 2014;2:702–714.
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950.
- Guo C, Sun L, Chen X, et al. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8:2003–2014.
- Czabotar PE, Lessene G, Strasser A, et al. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49.
- Kressel M, Groscurth P. Distinction of apoptotic and necrotic cell death by in situ labelling of fragmented DNA. Cell Tissue Res. 1994;278:549–556.