Abstract
Objective
Cyclin D1 was an important molecular involved in the pathological process of osteoarthritis (OA). The purpose of this study was to identify the effect and potential mechanism of Cyclin D1 for the proliferation and apoptosis of OA chondrocytes.
Methods
We used polymerase chain reaction (PCR) method to identify the expression levels of Cyclin D1 and down-stream Wnt/β-catenin pathway-related genes in OA chondrocytes according to the grade of OA. Small interfering RNA (siRNA) or overexpression of Cyclin D1 were used to identify the role of Cyclin D1 in cell proliferation and apoptosis. Next, we used XAV-939 to inhibit the Wnt/β-catenin pathway and explore the relevant mechanism.
Results
Cyclin D1 was significantly decreased with OA grade (p < .05). After siCyclin D1 transfection, the expression level of WNT3 and nuclear β-catenin were significantly increased, while Wnt10a and total β-catenin were not obviously changed. Co-cultured with XAV-939 and siCyclin D1 abolished the effects of siCyclin D1 on proliferation and apoptosis of OA chondrocytes (p < .05).
Conclusions
Cyclin D1 regulated chondrocyte proliferation and apoptosis through Wnt3/β-catenin instead of Wnt10a/β-catenin signalling pathway.
Introduction
Osteoarthritis (OA) has been considered as the most common disease in elderly people with great influence on disability and deformity [Citation1,Citation2]. Many drug therapies were used for controlling the clinical symptoms and delay the progression of OA [Citation3]. A complicated combination of metabolic and genetic factors participated in the pathogenetic process of OA [Citation4].
Previous reports indicated that Wnt/β-catenin signalling pathway has been involved in regulating chondrocyte hypertrophic differentiation during OA [Citation5,Citation6]. Inhibition Wnt/β-catenin signalling pathway has a beneficial role in delaying the ageing process [Citation7,Citation8]. Overexpression of Wnt/β-catenin relevant molecular was associated with articular cartilage damage [Citation9]. Primary effectors implicated in OA development include two Wnt proteins (Wnt3 and Wnt10a) [Citation10,Citation11]. However, the Wnt/β-catenin signalling pathway regulation in OA needs further investigation.
Cyclin D1, a member of the cyclin protein family has been identified as an indispensable factor for regulating the cell cycle [Citation12]. Cyclin D1, through binding to cyclin-dependent kinase4/cyclin-dependent kinase6 (CDK4/CDK6), promotes phosphorylation of retinoblastoma protein (pRB). Zhu et al. [Citation12] collected 76 healthy controls and 154 OAs and found that Cyclin D1 was significantly decreased in OAs group than healthy controls. Zan et al. [Citation13] revealed that Cyclin D1 gene silencing could suppress the proliferation and induces apoptosis of rat chondrocytes in IL-1β-induced OA. There were several shortcomings in the above article. First, they use the rats’ chondrocytes and the gene difference between humans should be noted. Second, they only performed the in-vitro study. Third, overexpression of Cyclin D1 was not performed. Last, the potential mechanism was not explored. And, whether Cyclin D1 could activate the Wnt/β-catenin signalling pathway and prevent chondrocyte apoptosis was unclear.
The purpose of this study was to assess the differential expression of Cyclin D1 in OA chondrocyte and healthy controls according to different OA grading. Then, we separated the OA chondrocyte and used RNA interference to identify the potential mechanism for Cyclin D1 regulated OA chondrocyte apoptosis. Furthermore, we investigate how Cyclin D1 and Wnt/β-catenin signalling pathway regulated OA chondrocyte apoptosis.
Patients and methods
Reagent
XAV-939 was purchased from MedChem, Express (Princeton, NJ) and dissolved in dimethyl sulfoxide (DMSO) with final concentration at 5 mM. Primers were designed and synthesis by Aocheng company of Beijing, China. All antibodies were purchased from Santa Cruz (Santa Cruz, CA).
Clinical samples
OA and normal articular cartilage samples (OA = 20, each grade of cartilage = 5, normal cartilage sample = 5) were obtained the department of The Second Xiangya Hospital of Central South University between November 2016 and 2017. OA samples were classified into four groups according to Kellgren–Lawrence score by two authors (Yi-yue Chen and You Chen) independently [Citation14]. The grading was assessed by a surgeon based on preoperative X-ray radiograph. If there was disagreement between the authors, consensus was reached by discussion. This study had obtained approval from the Institute Review Ethics Committee of The Second Xiangya Hospital of Central South University and all participants consented to participate in this research.
Cell culture
OA cartilage tissues and normal articular cartilage tissues (load bearing) were washed by phosphate buffer solution (PBS) and cut into small pieces. Then the pieces were digested with 0.15% collagenase for nearly 30 min. Then, we used 10 ml foetal bovine serum (FBS) to suspend the digestion. Strainer (×100) was used to remove the mass tissues. Then, all of the cells were enclosed in a centrifuge tube and centrifuged at 3000 rpm for 5 min. The obtained chondrocytes were seeded into a 6-pore plate and cultured in α-DMEM with 10% FBS.
Cell transfection for knocking-down cyclin D1 or overexpression cyclin D1
Briefly, 5 × 104 OA chondrocytes were harvested and seeded into a 6-well plate. When chondrocytes reached 95% confluence and transfected with the recombinant virus vectors (Lenti-KD™ or Lenti-OE™), the knockdown or overexpress Cyclin D1 sequence was as follows: Lenti-KD™ carrying Cyclin D1 small interfering RNA (siRNA) (sequence: ACCTCCCGAATTTGCTGCCTGAGAT). Lenti-OE™ carrying Cyclin D1 was designed by Jikai Company (Beijing, China). The multiplicity of infection (MOI) value was selected according to the manufacturer instruction (MOI = 40). After treatment for 12 h, we changed the normal media. At the end of the experiment (72 h), we collected the cells for the next experiment.
QRT-PCR
Cells were harvested for extraction of total RNA by Trizol reagent (Invitrogen, Carlsbad, CA). Then, 1 μg of total RNA was reversely transcribed into cDNA using ReverTra Ace Qpcr RT Kit (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. The qRT-PCR analysis was carried out using SYBR1 Green Real-time PCR Master Mix (Toyobo Co. Ltd., Osaka, Japan). Housekeeping gene (β-actin) was used as internal control. The primer sequences are listed in .
Table 1. Primes used in qRT-PCR.
Western blot
The protein expression of Wnt3, Wnt10a, total β-catenin and nuclear β-catenin were measured. Total protein was extracted by radioimmunoprecipitation assay (RIPA) lysis buffer. BCA protein assay kit (Beyotime Biotechnology, Shanghai, China) was used for measuring the protein concentration. According to the protein concentration of the protein and equal amounts of protein were separated on 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). Subsequently, SDS-PAGE was transferred onto polyvinylidene fluoride membranes (PVDF; Millipore, Billerica, MA).
Then the PVDF was blocked by 5% defatted milk for 3 h and incubated with primary antibody at 4 °C overnight. Taking them out of the refrigerator and then rinsed with TBST three times every 15 min, the member was incubated with the corresponding secondary antibody at room temperature for 3 h. Finally, PVDF membranes were washed three times with TBST and electrochemiluminescent (ECL) was added. The proteins were visualized by an enhanced chemiluminescence detection system (Millipore, Burlington, NJ).
Apoptosis analysis
The flow cytometry (Life Technologies, Waltham, MA) was applied for the determination of chondrocytes apoptosis in accordance with the manufacturer’s instruction. Briefly, 1 × 106 cells were harvested, suspended and then washed by 400 µl binding buffer and stained by 5 µl Annexin-V-FITC avoiding light at room temperature for 15 min. After being counterstained with 10 µl propidium iodide (PI), the cells were treated with an ice bath avoiding light for 5 min. Chondrocytes apoptosis was analyzed using flow cytometry and a FACScan. Chondrocytes that were positive to both PI and Annexin V were considered apoptotic.
Statistical analysis
All of the data analyses were performed by SPSS version 20.0 (SPSS, Chicago, IL). Each group was expressed as mean ± standard deviation. Variance analysis was used for detecting the difference between the groups and two groups’ difference was detected at p < .05 was identified as a significant difference.
Results
Cyclin D1 was low-expressed in OA cartilages
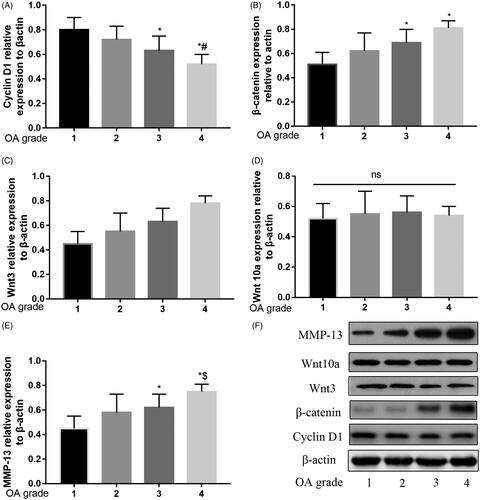
The degree of OA was classified according to Kellgren–Lawrence score [Citation14] and shown in Supplement file 1. As illustrated in , with the OA grade increasing, the relative expression of Cyclin D1 was decreasing. And the relative expression of β-catenin, Wnt3 and MMP-13 were increasing with the OA grade increasing. There was no significant difference between the relative expressions for Wnt10a between the four groups.
Figure 1. Relative expression of β-catenin, Wnt3, Wnt10a, Cyclin D1 and MMP-13 in different OA grade. Relative expression of Cyclin D1 decreased by grade of OA (A). Relative expression of β-catenin (B), Wnt3 (C) and MMP-13 (E), increased by grade of OA. No significant difference in Wnt10a between the different OA grade (D). Western blot analysis over the expression of these proteins by grade of OA (F).
Cyclin D1 promoted OA chondrocytes proliferation
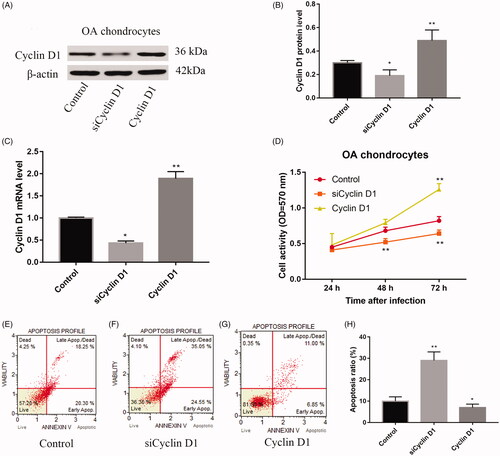
shows that compared with control group, the relative protein expression of Cyclin D1 was significantly lower in siCyclin D1 group and higher in Cyclin D1 group (p < .05). And to further investigate the mRNA level of these groups, we found that the relative mRNA expression of Cyclin D1 was significantly lower in siCyclin D1 group and higher in Cyclin D1 group with a statistical significance (). With infection time extension, cell activity of each group was increased. And the cell activity in Cyclin D1 group was higher than siCyclin D1 and control group at 24 and 48 h (p < .05, ).
Figure 2. The relative expression of protein of Cyclin D1 was decreased when infection with siRNA and increased when over-expression with Cyclin D1 (A,B); the relative expression of mRNA of Cyclin D1 was decreased when infected with siRNA and increased when overexpressed with Cyclin D1 (C); cell activity was up-regulated in Cyclin D1 group than control group and siCyclin D1 group (D); flow cytometry analysis of control (E); siRNA (F) and over-expression (G) with Cyclin D1. Statistical chart for apoptosis ratio for these groups (H).
Cyclin D1 inhibited cell apoptosis and cell cycle stuck of OA chondrocytes
As shown in ), compared with control group, siCyclin D1 significantly increased apoptosis ratio (p < .01). However, Cyclin D1 significantly decreased apoptosis ratio than control group (p < .05).
siCyclin D1 activated Wnt/β-catenin signalling pathway in OA chondrocytes
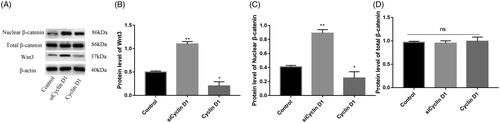
As illustrated in , the mRNA relative expression of Wnt3 and nuclear β-catenin in siCyclin D1 group, were significantly higher than the control group (p < .05, ). When, Cyclin D1 was overexpressed, the relative expression of Wnt3 and nuclear β-catenin was decreased than the control group (p < .05, ). There was no significant difference between the total β-catenin in RNA and protein level in these three groups (p > .05 ). We further analyze the relative protein level expression of Wnt3, Wnt10a and nuclear β-catenin expression in siCyclin D1 and overexpression Cyclin D1 groups. Results are shown in . The protein expression of Wnt3 and nuclear β-catenin was increased in siCyclin D1 group than in control group and decreased in overexpression Cyclin D1 group with significant difference (p < .05, ). There was no significant difference in the total β-catenin between siCyclin D1 group, overexpression Cyclin D1 group and control group (p > .05, ).
Figure 3. The relative expression of Wnt3 (A), nuclear β-catenin (B) and total β-catenin RNA (C) in a control group, siCyclin D1 and Cyclin D1 groups. Compared with control group, siCyclin D1 could promote the expression of Wnt3 and nuclear β-catenin.
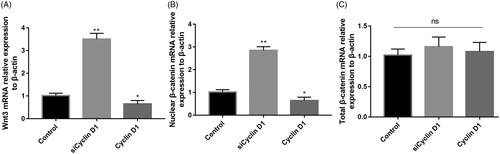
Figure 4. Representative image of Western blot for the expression of Wnt3, nuclear β-catenin and total β-catenin (A); Compared with control group, siCyclin D1 could increase the expression of Wnt3 (B) and nuclear β-catenin (C). No significant difference was found between the total β-catenin expression (D).
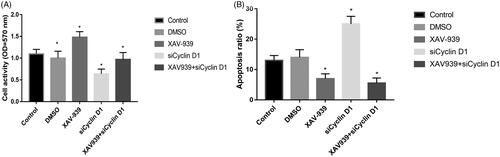
XAV-939 promote OA chondrocytes proliferation and inhibit chondrocytes apoptosis
As shown in , XAV-939 significantly increased the cell activity than the control group. The siCyclin D1 could significantly suppress chondrocytes proliferation and increased apoptosis ratio compared with the control group and XAV-939 group ().
Compared with control group, XAV-939 group decreased the apoptosis ratio with statistically significant (p < .05), while, siCyclin D1 group increases the apoptosis ratio. When combined with XAV-939 and Cyclin D1 siRNA led to significantly lower cell apoptosis than control but no significant difference from XAV-939 group or Cyclin D1 siRNA group ().
Discussion
Our results revealed a direct impact of the Cyclin D1-Wnt3-β-catenin pathway on the pathogenesis of OA based on the following. First, we observed a decreased expression of Cyclin D1 and increased expression of WNT3-β-catenin in OA patients. The expression level of Cyclin D1 increases with the grade of OA. Second, siCyclin D1 could inhibit chondrocyte proliferation and result in the promotion of genes associated with WNT3-β-catenin signalling. Third, inhibition of Wnt-β-catenin signalling by XAV-939 could rescue the chondrocyte apoptosis.
At the end stage of OA, total joint arthroplasty was the final choice [Citation15,Citation16]. Total joint arthroplasty was always associated with an increase in economic costs [Citation17]. Preventing OA progress could relieve knee pain and decrease relevant costs. The aetiology of primary OA remains incompletely understood and in somewhat was controversial [Citation18]. OA is a set of comprehensive diseases and caused by many biological factors and gene regulation. We firstly reported that Cyclin D1 was relatively different expressed in different OA grade patients. Wu et al. [Citation19] identified that when over-expression of Cyclin D1, the proliferation of chondrocyte was increased. Beier et al. [Citation20] further verified the Cyclin D1 in vivo for the promotion of the proliferation of chondrocyte. And, reducing the expression of Cyclin D1 inhibited the chondrocyte proliferation through a delay of the cell cycle and decreased E2F activity.
And, MMP-13 was increased as the grade of OA increased. Cyclin D1, as the central players of cell cycle regulation, plays a vital role in the transition from G1 phase to S phase. Chondrocyte apoptosis was the pathogenic factor of OA. Beier et al. [Citation21] revealed that PTHrP and TGF-beta could activate transcription of the Cyclin D1 promoter to protect the cartilage. Zan et al. [Citation13] found that silenced Cyclin D1 gene could suppress the proliferation and induces apoptosis of rat chondrocytes in IL-1β-induced OA. However, the definite mechanism was not well established. This study used overexpression and gene-silencing methods, aimed to comprehensively analysis the role of Cyclin D1 for OA. Results identified that overexpressing Cyclin D1 could prevent the occurrence of OA. Zheng et al. [Citation21] revealed that there was a connection between Cyclin D1 and Wnt-β-catenin signalling pathway. Expression of β-catenin and Cyclin D1 in the Wnt/β-catenin signalling pathway is inhibited by DKK-1, a Wnt/β-catenin signalling pathway inhibitor.
The downstream molecules of Cyclin D1 were not well understood. In this study, we found that Wnt-3-β-catenin pathway rather than Wnt10a was up-regulated with the OA grade. Previous studies showed that the Wnt pathway participated in a series of cell homeostasis processes, including cell differentiation, proliferation, migration and adhesion [Citation22,Citation23]. Further studies found that the Wnt pathway was inhibited in normal cartilage, whereas its activation promoted OA [Citation23]. Emerging evidence demonstrates that Wnts and Wnt-related molecules are involved in arthritis development [Citation24]. Zhou et al. [Citation24] revealed that Wnt7b is the most closely linked to OA progression. And Honsawek et al. [Citation25] revealed that compared with normal patients, DKK1 levels in patients with knee OA were significantly lower in patients with knee OA, and correlated to the radiographic grading of knee OA. These results were in accordance with our findings. We found that Wnt3a and nuclear β-catenin was correlated to the radiographic grading of knee OA. We used the Western-blot technique to reveal the protein level and the result was more believable.
In conclusion, we found that Cyclin D1 has been involved in the pathogenetic process of OA and the mechanism was through regulating Wnt-β-catenin pathway. Thus, Cyclin D1-Wnt-β-catenin might be considered as a potential therapeutic target Cyclin D1 regulated chondrocyte apoptosis through Wnt3/β-catenin instead of Wnt10a/β-catenin signalling pathway for OA treatment.
Supplement_file_1.tif
Download ()Acknowledgement
We thank Wan-Chuan Wang for molecular analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Quinn RH, Murray JN, Pezold R, et al. Surgical management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2018;26:e191–e193.
- Fan MP, Si M, Li BJ, et al. Cell therapy of a knee osteoarthritis rat model using precartilaginous stem cells. Eur Rev Med Pharmacol Sci. 2018;22:2119–2125.
- Calce SE, Kurki HK, Weston DA, et al. The relationship of age, activity, and body size on osteoarthritis in weight-bearing skeletal regions. Int J Paleopathol. 2018;22:45–53.
- Kabiri S, Halabchi F, Angoorani H, et al. Comparison of three modes of aerobic exercise combined with resistance training on the pain and function of patients with knee osteoarthritis: a randomized controlled trial. Phys Ther Sport. 2018;32:22–28.
- Huang X, Post JN, Zhong L, et al. Dickkopf-related protein 1 and gremlin 1 show different response than frizzled-related protein in human synovial fluid following knee injury and in patients with osteoarthritis. Osteoarthritis Cartilage. 2018;26:834–843.
- Yang X, He H, Gao Q, et al. Pulsed electromagnetic field improves subchondral bone microstructure in knee osteoarthritis rats through a Wnt/beta-catenin signaling-associated mechanism. Bioelectromagnetics. 2018;39:89–97.
- Yuasa T, Otani T, Koike T, et al. Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest. 2008;88:264–274.
- Blom AB, Brockbank SM, van Lent PL, et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60:501–512.
- Funck-Brentano T, Bouaziz W, Marty C, et al. Dkk-1-mediated inhibition of Wnt signaling in bone ameliorates osteoarthritis in mice. Arthritis Rheumatol. 2014;66:3028–3039.
- Nalesso G, Sherwood J, Bertrand J, et al. WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J Cell Biol. 2011;193:551–564.
- Sun Y, Wang F, Sun X, et al. CX3CR1 regulates osteoarthrosis chondrocyte proliferation and apoptosis via Wnt/beta-catenin signaling. Biomed Pharmacother. 2017;96:1317–1323.
- Zhu X, Yang S, Lin W, et al. Roles of cell cycle regulators cyclin D1, CDK4 and p53 in knee osteoarthritis. Genet Test Mol Biomarkers. 2016;20:529–534.
- Zan PF, Yao J, Wu Z, et al. Cyclin D1 gene silencing promotes IL-1β-induced apoptosis in rat chondrocytes. J Cell Biochem. 2018;119:290–299.
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502.
- Dai WL, Lin ZM, Guo DH, et al. Efficacy and safety of Hylan versus hyaluronic acid in the treatment of knee osteoarthritis. J Knee Surg. 2018.
- Liu G, Gong M, Wang Y, et al. Effect of methylprednisolone on pain management in total knee or hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Clin J Pain. 2018;34:1.
- Sun H, Huang Z, Zhang Z, et al. A meta-analysis comparing liposomal bupivacaine and traditional periarticular injection for pain control after total knee arthroplasty. J Knee Surg. 2018.
- Ogata T, Ideno Y, Akai M, et al. Effects of glucosamine in patients with osteoarthritis of the knee: a systematic review and meta-analysis. Clin Rheumatol. 2018;37:2479–2487.
- Wu G, Fan H, Huang Y, et al. Duhuo Jisheng Decoction-containing serum promotes proliferation of interleukin-1β-induced chondrocytes through the p16-cyclin D1/CDK4-Rb pathway. Mol Med Rep. 2014;10:2525–2534.
- Beier F, Ali Z, Mok D, et al. TGFbeta and PTHrP control chondrocyte proliferation by activating cyclin D1 expression. Mol Biol Cell. 2001;12:3852–3863.
- Zheng W, Lin P, Ma Y, et al. Psoralen promotes the expression of cyclin D1 in chondrocytes via the Wnt/β-catenin signaling pathway. Int J Mol Med. 2017;40:1377–1384.
- Held A, Glas A, Dietrich L, et al. Targeting beta-catenin dependent Wnt signaling via peptidomimetic inhibitors in murine chondrocytes and OA cartilage. Osteoarthritis Cartilage. 2018;26:818–823.
- Corr M. Wnt-beta-catenin signaling in the pathogenesis of osteoarthritis. Nat Clin Pract Rheumatol. 2008;4:550–556.
- Zhou Y, Wang T, Hamilton JL, et al. Wnt/β-catenin signaling in osteoarthritis and in other forms of arthritis. Curr Rheumatol Rep. 2017;19:53.
- Honsawek S, Tanavalee A, Yuktanandana P, et al. Dickkopf-1 (Dkk-1) in plasma and synovial fluid is inversely correlated with radiographic severity of knee osteoarthritis patients. BMC Musculoskelet Disord. 2010;11:257.