Abstract
In the process of natural precursors examining for the carbon dots (CDs) synthesis, a bio-friendly and highly luminescent CDs synthesis is being reported herein. For the first time, we are reporting CDs synthesis using Selenicereus grandiflorus plant materials without any use of additional oxidizing agents like ethanol which was used in the earlier mentioned reports. Ultraviolet-visible spectroscopy (UV-Vis), X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, high-resolution transmission electron (HR-TEM) microscopy and Raman spectroscopy. This method is entirely safe to use in all biological practices because no toxic chemicals were used in this method. Further, we studied the toxicity of CDs against chondrocytes obtained from the knee joint. In order to inquire about the feasible harmful effects of exposing CD on the knee cells, we carried out CD treatment in SW-1353 chondrocytes. Cytotoxicity outcomes displayed a dosage-dependent reduction of cell viability. Therefore, we studied the toxic effects of CDs on the knee, indicating the important prospectives of CDs in future knee therapy.
Keywords:
Introduction
In the past few decades, because of the luminescent quantum dots (QDs) identical electronic and optical nature which is caused due to the edge effects and quantum confinement, QDs have captured a substantial interest of the hopeful candidates in replacing the traditional phosphor materials [Citation1]. Either way, the conventional semiconductor QDs depending on the metallic elements like Ag2S, CdS, PbSe and CdSe almost suffers from the drawback of high cost, toxicity and hydrophobicity which objects their practical implementation. Being a unique variety of carbon family the carbon dots (CDs) were discovered to be a fascinating QDs for various implementations due to their promising luminescent nature, biocompatibility, high chemical reliability, easy functionalization and low toxicity [Citation2]. Excited with these top class benefits, various works are being performed based on effortless synthesis methods and in-depth studies of photoluminescence (PL) nature. Since its first production from the carbon nanotubes in the mechanism of electrophoresis which was in 2004 [Citation3], electrochemical exfoliation [Citation4], various approaches of synthesis, along with pyrolysis [Citation5], partial combustion of oxidation [Citation6], oxidation in presence of concentrated acids [Citation7], laser ablation [Citation8], green hydrothermal methods [Citation9], microwave approaches [Citation10] and plasma-based synthesis [Citation11] in the recent years were developed for the preparation of CQDs with multiple green precursors like egg [Citation11,Citation12], GO [Citation13] and citric acid [Citation14]. Also, green biomolecules have been reported [Citation15]. In spite of various splendid advances still, it is urgent and desirable to synthesize rapidly CQDs of high-quality by a simple and environment-friendly method using low-cost resources. A while ago, as a conventional method of preparing soft chemical, the synthesis method of hydrothermal which is related on the water system is taken into consideration as one of the most cost efficient and simplest methods due to its simple manipulation, cheap apparatus, good selectivity and can be prepared in one step without any complexity in controlling. Many researchers have used this method for the preparation of CQDs. For example, Liu and the co-workers have reported the CQDs preparation with a quantum yield of about 6.2% by the grass treatment using hydrothermal [Citation16]. Fluorescent CQDs were produced by Zhang et al. with 11% of PL quantum yield with the help of BSA protein and precursor of carbon with the passivation of decane diamine [Citation17].
In the process of natural precursors examining for the CDs synthesis, a bio-friendly and highly luminescent CDs synthesis is being reported by us. For the first time, we are reporting CDs synthesis using Selenicereus grandiflorus plant material without any use of additional oxidizing agents like ethanol, which was used in the earlier mentioned reports. This method is entirely safe to use in all biological practices because no toxic chemicals were used in this method. It is well known the biocompatibility of green synthesized nanomaterials prepared using natural biomolecules [Citation12,Citation18–21]. We selected Selenicereus grandiflorus as the carbon source because of the green and cost-effective approach. Further, to inquire the feasible harmful effects of exposing CD on the knee cells, we carried out CD treatment in SW-1353 chondrocytes.
Experiments
Preparation of carbon dots
Nano-pure water was used in performing all the investigation. The flowers from the plant Selenicereus grandiflorus were gathered and saturated for 30 min in the cold water. In the distilled water of 500 ml, we added crushed flower petals of 50 g and then centrifuged for obtaining the pure light pink extract. Finally, for CDs synthesis, the plant extract of 100 ml was boiled at 90 °C for 2 h until the mixture turned greenish brown. The final mixture was centrifuged for 20 min at 5000 rpm and dissolved in 5 ml of 1 N NaOH for CDs solubilizing and also increasing the fluorescence capacity. For purifying the CDs, the above solution of 3 ml was dialyzed against (MW = 12 kD) nano-pure water for about 24 h. Pure CDs yellowish suspension was noticed which expressed extreme green fluorescence under the UV-light.
Animal experiments
BALB/c male rats were acquired from Vital River Laboratories, Beijing, China. The rats used in the experiments are of about seven weeks old along with approximate 25 g body weight. Rats were reproduced at a specific pathogen-free facility (SPF). CD samples that are pre-sonicated were supervised through intratracheal injection (IT) at a body weight of 2.0 mg/kg (with only one injection of 50 μl) and exposed for a period of 2 or 7 d. After sacrificing the rats in the final stage of the experimental period, joint knees from the rats were separated and subjected in 4% of PBS-buffered formaldehyde. Later, subjected tissues of the knee were placed for decalcification with the pure decalcifying solution containing 10% of Ethylenediaminetetraacetic acid (EDTA), following sectioning and embedding.
Cell culture and cellular treatment with CDs
The cell line of human chondrocyte (SW-1353) and the cell line of rat macrophages (J774.A1) were acquired from Shanghai Cell Bank. Cells are cultured using the standard protocol. In PBS, the stock CDs are diluted further for the cellular treatment at different concentrations. Other untreated cells also received the PBS of the same volume only.
Cell viability assay
At different concentrations, with CDs the cells were treated for 24 h, following the determination of cell viability with the help of a Cytotoxicity/Viability Dead/Live Kit (Thermo Fisher Scientific Inc., Waltham, MA; Invitrogen, Carlsbad, CA). Under the microscope, the phase-contrast morphology of the cells was imaged accordingly.
Characterization
High-resolution electron transmission microscopy (JEOL 3010; JEOL Ltd., Tokyo, Japan) which is operated at a value of 300 kV was used for analyzing CD and morphology. Zeta potential and particle size were calculated after proper dilution of CD solution at 25.0 ± 0.5 °C, by powerful laser light dissipation by using particle size analyzer (Nano ZS90; Malvern Instruments, Malvern, UK). For examining the crystalline phase, maestro Pro Philips X-ray diffractometer was used. The prepared samples’ Raman spectrum was noticed using Via Reflex Spectrometer (UK made) at ambient temperature in Renishaw (Renishaw plc, Wotton-under-Edge, UK). The spectra of Fourier transform infrared spectroscopy (FTIR) were calculated using Thermo Nicolet Nexus FTIR of model 870 spectrometer (Triad Scientific, Manasquan, NJ) with KBr pellet strategy ranging from 400 to 4000 cm−1. UV–Vis spectrophotometer of Shimadzu 220 V (E) (Shimadzu Corp., Kyoto, Japan) was used for observing UV–Vis absorption spectra.
Results and discussion
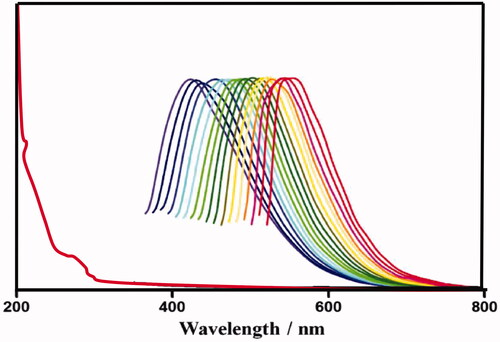
shows the CDs emission spectra that are wide, varying from (400 nm) blue to (600 nm) red, depending on the wavelengths excitation. CDs PL is because of the quantum effect including various emissive traps on the surface of the CD. The wide PL emission profiles moreover confirm that CDs size distribution is wide, as assisted by TEM image. By the increase in excitation wavelength the emission peak location shifts to higher wavelengths with a decrease in intensity. The PL occurred stronger at 440 and 365 nm when excited, with 3.8% value.
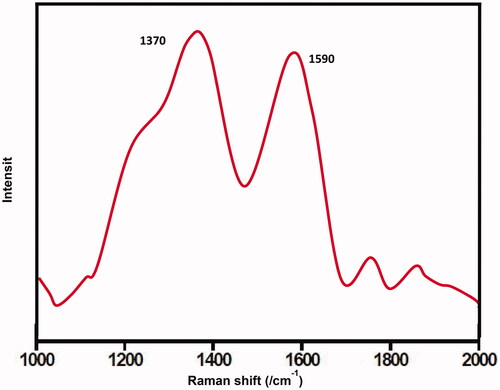
The CDs’ Raman spectrum () shows two wide peaks at about 1590 and 1370 cm−1 that could be assigned to the G (sp3 carbon) and D (sp2 carbon) bands accordingly. The presence of other bands indicated the amorphous nature of synthesized CDs. The band D corresponds with the carbon atoms vibrations with tumbling bonds in the terminating plane of jumbled glassy carbon or graphite. The band G is similar to the mode E2g of graphite and in accordance with the pulsation of sp2-carbon in the hexagonal lattice of 2D carbon.
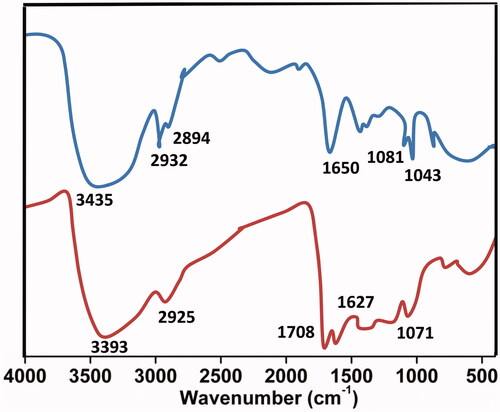
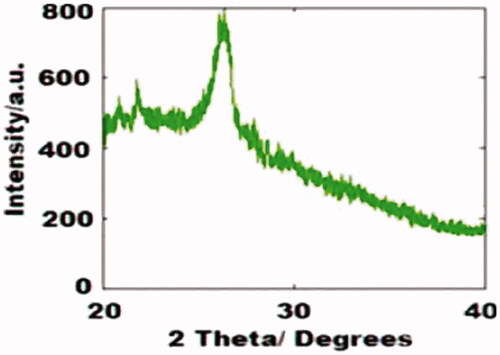
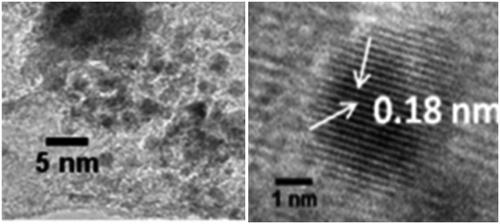
The existence of COOH, C–O, C=C bonds indicates clearly that the CDs were functionalized with carboxylic, hydroxyl, C=O and epoxy acid functionalities revealed by FTIR (. The existence of the above groups, communicates exceptional solubility in the aqueous medium without any chemical changes. XRD pattern () represents Bragg’s reflection at 2θ = 26.36 that is allotted to an interlayer spacing of ∼3.77 Å which is larger than the one between planes (002) in the bulk graphite (3.44 Å). This represents CDs graphitic property as per earlier studies. Moreover, HR-TEM morphological results () disclose the formation of monodisperse, spherical shaped and also have a distribution of size from 2 to 4 nm with most of the particles are at 2.5 nm. represents the lattice spacing of 0.18 nm which is corresponding to feature (102) of graphitic carbon.
Toxicity of CDs on chondrocytes
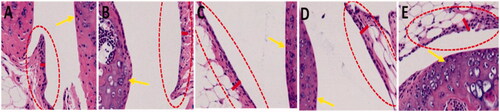
Later, we focused to find out the possible effect on the knee by CD exposure. At this point, the legs of hind were gathered for the purpose of histological examination from the rat after treating with CDs using the I.T. route of exposure for about 7 d. indicates no remarkable deficiencies were described for the articular cartilage layer on femurs (as shown by yellow marks). Surprisingly, synovial membrane thickness increase has been noticed inside the knee joints within CD-treated rats, precisely for C824455 and C1864 treated rats (measured in red ovals indicated with red lines). The synovial membrane thickness increase which is in reply to CD management that is accredited to the pro-inflammatory cells infiltration in the synovial membrane.
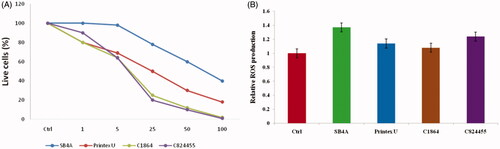
In order to inquire about the feasible harmful effects of exposing CD on the knee cells, we carried out CD exposure to SW-1353 chondrocytes. The cytotoxicity outcomes displayed a dosage-reliant on reduction of viable SW-1353 cells for all the CDs (ranging from 1 to 100 μg/ml (). In resemblance to cells of J774.A1, C824455 and C1864 exposed higher toxicity to the cells of SW-1353 than the Printex U and SB4A (p < .001, as shown in ). To illuminate the technique under contrasting induced toxicity by the CDs, subsequently, we calculated the ROS generation in the cells of SW-1353. As represented in , all the CDs persuaded ROS production, precisely for the C824455 with closely 30% growth of intracellular ROS when compared to the untreated cells. Where this set of data later explained higher toxicity of the SW-1353 cells when induced by the C824455.
Chondrocyte activation by CDs exposure
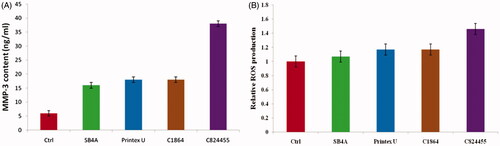
For answering either CD exposure modified cellular homeostasis of the cells of SW-1353 under the concentrations of sublethal, we analyzed the differences in a member of MMP family representative, which is MMP-3 in the cells of SW-1353. As indicated in , we noticed the induction of 12–37% in the expression of MMP-3 mRNA which is from the cells of SW-1353 on exposing all the CDs, as distinguished by the analysis of qRT-PCR. In order to support these results, the protein levels of MMP-3 produced from the cells of SW-1353 into culture media were assessed by using ELISA. Relaying with the data of qRT-PCR, excreted MMP-3 was intensified in the cells responding to the CDs, differentiated to the cells that are untreated ((p < .05, ). Simultaneously, this data indicates that these chondrocytes activated by CDs by stimulating MMP expression suggest a risk of exposing CD in joint disorder. As shown in , CD induced ROS generation, particularly for C824455 with nearly 30% increase of intracellular ROS relative to untreated cells (p < .001). These results exhibited higher toxicity to SW-1353 cells induced by C824455. Anyway, our current data demonstrated that CB exposure altered the priming state of chondrocytes, resulting in activation of chondrocytes, representing a condition as observed in joint disorders, such as OA and RA. Hence, this study deciphered an unrecognized effect of CDs on knee cells.
Conclusions
In the process of natural precursors examining for the CDs synthesis, a bio-friendly and highly luminescent CDs synthesis is being reported herein, without any use of additional oxidizing agents. Further, we studied the toxicity of CDs against chondrocytes obtained from the knee joint. In order to inquire about the feasible harmful effects of exposing CD on the knee cells, we carried out CD treatment in SW-1353 chondrocytes. Cytotoxicity outcomes displayed a dosage-dependent reduction of cell viability. Therefore, the toxic effects of CDs on the knee indicated the important prospectives of CDs in future knee therapy.
Acknowledgements
Authors are thankful to Luoyang Central Hospital affiliated to Zhengzhou University for providing support to do this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Reshma VG, Mohanan PV. Quantum dots: applications and safety consequences. J Lumin. 2019;205:287–298.
- Krishanu G, Ashis G. Carbon dots: the next generation platform for biomedical applications. Mater Sci Eng C. 2019;96:887–903.
- Xu XY, Ray R, Gu YL, et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc. 2004;126:12736–12737.
- Liu M, Xu Y, Niu F, et al. Carbon quantum dots directly generated fromelectrochemical oxidation of graphite electrodes in alkaline alcohols and the applications for specific ferric ion detection and cell imaging. Analyst. 2016;141:2657–2664
- Cao X, Wang J, Deng W, et al. Photoluminescent cationic carbon dots as efficient non-viral delivery of plasmid SOX9 and chondrogenesis of fibroblasts. Sci Rep. 2018;8:7057.
- Liu HP, Ye T, Mao CD. Fluorescent carbon nanoparticles derived from candle soot. Angew Chem Int Ed Engl. 2007;46:6473–6475.
- Wang F, Wang S, Sun Z, et al. Study on ultrasonic single-step synthesis and optical properties of nitrogen-doped carbon fluorescent quantum dots. Fullerenes, Nanotubes, Carbon Nanostruct. 2015;23:769–776
- Reyes D, Camacho M, Camacho M, et al. Laser ablated carbon nanodots for light emission. Nanoscale Res Lett. 2016;11:424.
- Chen W, Hu C, Yang Y, et al. Rapid synthesis of carbon dots by hydrothermal treatment of lignin. Materials. 2016;9:184.
- Roshni V, Divya O. One-step microwave-assisted green synthesis of luminescent N-doped carbon dots from sesame seeds for selective sensing of Fe (III). Curr Sci. 2017;112:385–390.
- Wang J, Wang CF, Chen S. Amphiphilic egg-derived carbon dots: rapid plasma fabrication, pyrolysis process, andmulticolor printing patterns. Angew Chem Int Ed. 2012;124:9431–9435.
- Maddinedi SB, Mandal BK, Anna KK, et al. Diastase induced green synthesis of bilayered reduced graphene oxide and its decoration with gold nanoparticles. J Photochem Photobiol B. 2017;116:252–258.
- Wang Q, Zheng H, Long Y, et al. Microwave–hydrothermal synthesis of fluorescent carbon dots from graphite oxide. Carbon. 2011;49:3134–3140.
- Dong Y, Shao J, Chen C, et al. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon. 2012;50:4738–4743.
- Das R, Bandyopadhyay R, Pramanik P. Carbon quantum dots from natural resource: a review. Mater Today Chem. 2018;8:96–109.
- Liu S, Tian J, Wang L, et al. Hydrothermal treatment of grass: a low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform forlabel-free detection of Cu(II) ions. Adv Mater. 2012;24:2037–2041.
- Zhang Z, Hao J, Zhang J, et al. Protein as the source for synthesizing fluorescent carbon dots by a one-pothydrothermal route. RSC Adv. 2012;2:8599–8601.
- Maddinedi SB, Mandal BK, Maddili SK. Biofabrication of size controllable silver nanoparticles - A green approach. J Photochem Photobiol B, Biol. 2017;167:236–241.
- Maddinedi SB, Mandal BK, Anna KK. Environment friendly approach for size controllable synthesis of biocompatible Silver nanoparticles using diastase. Environ Toxicol Pharmacol. 2017;49:131–136.
- Maddinedi SB, Mandal BK, Anna KK. Tyrosine assisted size controlled synthesis of silver nanoparticles and their catalytic and in-vitro cytotoxicity evaluation. Environ Toxicol Pharmacol. 2017;51:23–29.
- Maddinedi SB, Sonamuthu J, Yildiz SS, et al. Silk sericin induced fabrication of reduced graphene oxide and its in-vitro cytotoxicity, photothermal evaluation. J Photochem Photobiol B Biol. 2018;186:189–196.