Abstract
In the current study, photosensitizer effect of carboxylate multiwalled carbon nanotubes (MWCNTs-COOH) on CT26 fibroblastic cells following near infrared (NIR) irradiation was quantized in photo-thermal therapy (PTT). Moreover, it was tried to achieve optimal dose of MWCNTs-COOH and laser exposure time. Characterizations of MWCNTs-COOH were scrutinized using scanner electron microscope (SEM), spectrophotometer, and particle size analyzer. The seeded CT26 cells were treated with nontoxic concentrations of MWCNTs-COOH and then irradiated. Finally, viability (%) of the CT26 cells was determined using MTT assay. The findings revealed that 10, 50, and 80 μg/mL of MWCNTs-COOH have remarkable photosensitizer effects on CT26 cancerous cell lines against NIR irradiation (2.5 W/cm2). It was shown that using the 80 μg/mL concentration of MWCNTs-COOH against 60, 120, 180, 240, and 300 s of NIR irradiation and also, 10 and 50 μg/mL concentration of MWCNTs-COOH against 180, 240, and 300 s of NIR irradiation can lead to significant decrease in mean cell viability (%) by more than 50%. According to the obtained data, it seems that using the PPT with MWCNTs-COOH, as adjunct therapy in CT26 fibroblastic cells, can help to increase therapeutic ratio of main modalities of cancer treatment such as radiotherapy, chemotherapy, and surgery.
Introduction
Cancer is considered as chief cause of death in developed countries and also, it is second chief cause of death in developing countries [Citation1,Citation2]. According to a recent report, there would be an estimation of 18.1 million new cancer cases (other than nonmelanoma skin cancer) and 9.6 million cancer deaths (other than nonmelanoma skin cancer) in 2018 [Citation3]. There are various modalities for the treatment of cancerous tumors such as surgery, radiotherapy, chemotherapy, cryotherapy, and ultrasound with high intensity [Citation4–6]. However, most of these therapeutic modalities are relatively effective, and they may return and invasive. To reduce the adverse side effects of treatment modality and damages of healthy and non-target tissues and also to increase the efficacy of treatment, it is necessary to consider successful therapeutic approaches, such as the complementary treatments which one of these treatments is thermal therapy [Citation7,Citation8].
Thermal therapy as a minimally invasive treatment can be used instead of other methods or in combination with other modalities such as surgery. Diathermia (41 °C), hyperthermia (45–41°C), and thermal ablation (greater than 45 °C) are the three common methods of thermal therapy which are classified according to the temperature and duration time of the heat radiation [Citation7]. In the hyperthermia therapy, the tumoral cells underwent a temperature range of about 45–42 °C or a higher temperature for a few minutes. It makes tumoral cells sensitive against main treatments such as radiotherapy and chemotherapy, and causes the death of a percentage of these cells by heat [Citation8–10]. Hyperthermia prevents the repairing DNA damages of cells induce by radiation which is probably due to its effect on cell proteins [Citation11].
Photothermal therapy (PTT) is a subset of hyperthermia therapy. In PTT, the energy of photons converts to heat and ultimately leads to the damage of cancerous cells. Heat sources in this technique include near-infrared (NIR), radio-frequency (RF), microwave, and ultrasound waves [Citation8,Citation10,Citation12–14]. PTT is performed in three ways: conventional photothermal, photothermal based on dye molecules or natural chromophores, and nanomaterial-based photothermal. Latter method has more treatment efficacy than the other two methods, because of the more useful surface plasmon resonance (SPR) oscillations of nanoparticles to dye molecules and natural chromophores. Therefore, in nanomaterial-based photothermal method, less power and energy is needed to increase a certain amount of temperature than conventional PTT, and the fundamental characteristics of nanoparticles is optical stability [Citation9,Citation10,Citation12,Citation14,Citation15]. In PPT, the plasmonic nanoparticles are delivered to the tumor, and these particles are exposed to laser light. It causes oscillation of the conduction band electrons, which results in the absorption or diffusion of the light radiation. The absorbed light converts to heat and irreversibly affects the surrounding tissues [Citation16].
Carbon nanotubes, as an important element in the nanotechnology with excellent absorption in the optic window area (650–900 nm), can be used as a powerful absorbent agent in photothermal treatments to remove the treatment limitations of deep areas [Citation10,Citation17–19]. These carbon-based materials having tubular structures are made up of complicated graphene sheets with a very small diameter. With regard to the number of pipe walls, these nanomaterials are divided into two categories: (1) multiwalled carbon nanotubes (MWCNTs) and (2) single-walled carbon nanotubes (SWCNTs). Among the carbon nanotubes, MWCNTs have unique physical and chemical properties, including a very large surface, high physical and thermal stability, and excellent conductivity; as because of these benefits, their use has been taken into consideration in recent years [Citation20,Citation21]. MWCNTs are capable of converting NIR radiation to heat and also, they are biocompatible. Studies have shown that MWCNTs can reduce adverse side effects to healthy tissues, leading to increased anticancer effects [Citation22,Citation23].
In this in vitro study, MWCNTs-COOH were used as photosensitizer agents for increasing the efficiency of PTT. The main characteristic of current study is using the carboxylate functional group in MWCNTs; as it contributes to optimal biodispersity [Citation24] in which the optimization of dispersity condition can increase the cellular uptake and finally, biological desirable results [Citation6]. Furthermore, it was tried to achieve optimal results at low doses of MWCNTs-COOH and short laser exposure times in PTT. It is notable that the MWCNTs-COOH effect in PTT was evaluated on CT26 fibroblastic cells; as to the best of our knowledge, this study is the first research on investigating the MWCNTs-COOH effect on these cell lines.
Materials and methods
Preparation of nanoparticles
Carboxylic acid-functionalized multiwalled carbon nanotubes (purity of MWCNTs-COOH >99%, OD: 20–30 nm, ID: 5–10 nm, length: 10–30 µm) were procured from US research nanoparticles. Weight percentage of carboxylated functional group (–COOH) into the MWCNTs-COOH agent was 2.73. MWCNTs-COOH powders were suspended in distilled water and diluted with cell culture medium (RPMI) to obtain the desired concentration of 5, 10, 20, 40, 80, 100, 250, 600 µg/mL. The stocks of MWCNTs-COOH suspension with measured concentration of 600 µg/mL were prepared and maintained for subsequent experiments in the refrigerator.
Characterization of MWCNTs-COOH
The UV-visible absorption spectrum of MWCNTs-COOH suspension was recorded using a UV spectrophotometer (4802 UV/VIS Double Beam Spectrometer) at a 200–1100 nm range. In this process, the deionized water was considered as base line. Also, the size and potential distribution were examined using a particle size analyzer (PSA, Zetasizer, Malvem, UK). Surface morphology of MWCNTs-COOH particles were obtained by scanning electron microscope (SEM, Hitachi, S-4800) with 10 KeV.
Determination of temperature induced by laser irradiation
MWCNTs-COOH suspensions were prepared at the concentrations of 0, 2.5, 5, 10, 25, 35, 50, 65, 80, 100, 250, and 500 µg/mL and then, each sample was irradiated with an 810 nm continuous-wave laser (MDL-2.5 w, CHANGCHUN New Industries OPTOElectronics TECH CO, Spot size = 6 × 8 mm2) in three different fixed powers (1.5, 2, and 2.5 w) for 20 s. Finally, temperature fluctuations of samples were measured by a thermometer (Testo-735).
Sterilization methods
The stocks of MWCNT-COOH particles were sterilized by ethanol treatment. The specimen was dissolved in a specified volume of 70% ethanol, then, it was centrifuged at 4000 rpm for 10 min and air-dried in biosafety cabinet. To achieve a stable and homogeneous suspension, MWCNTs-COOH particles were dispersed in water through ultrasonic bath (D-78224 Singen/Htw) with 40 kHz frequency for 1.5 h.
Splitting of cells
All cellular experiments were implemented with CT26, undifferentiated colon carcinoma cell lines of mouse, which were prepared from the Pasteur institute of Iran (IPI). CT26 cell lines were harvested in Roswell park memorial institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 µg/mL streptomycin, 100 U/mg penicillin, and incubated in an ambient of 95% air and 5% CO2 at 37 °C for facilitation of dispersal. In order to replenish nutrients and keep correct pH, cell medium was changed every two days.
Determination of cytotoxicity
Harvested cells were reached approximately 80% confluent (80% of flask surface covered by cells monolayer), then they were removed by treatment with 0.05% trypsin, and 54 × 105 cells (number of wells in one group = 6; number of cells in each well = 1 × 105) were dispensed in nine groups with different concentrations of MWCNT-COOH suspension (0, 5, 10, 20, 40, 80, 100, 250, and 600 µg/mL) into white-walled 96-well plate. Finally, MTT assay was conducted to obtain a non-toxic concentration of MWCNTs-COOH suspension after 24 h.
Determination of photosensitization effect
With regard to the determination of nontoxic concentration, in the next step of experiments, the cells were treated with different concentrations of MWCNT-COOH particles (0, 10, 50, 80 µg/mL). Prepared microplates were incubated at 37 °C for 3 h. Then, the cell medium was changed and exposed to laser irradiation for 20, 40, 60, 120, 180, 240, and 300 min. Finally, MTT assay was implemented to quantitative of cell viability in different laser irradiation time and MWCNT-COOH suspension concentrations.
Laser irradiation schedule
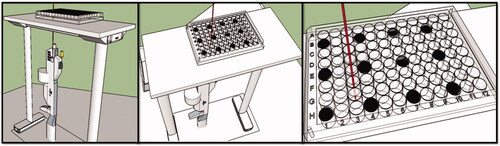
Microplates, after three hours of incubation with different concentrations of MWCNT-COOH suspension, were placed on the designed base with carefully (), so that the bottom of the wells was exposed to laser irradiation. In order to reduce the heat impact of adjacent wells during exposure, the cells were cultured with two wells spaced apart and empty wells were filled with distilled water.
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) assay
MTT dye reduction assay was used to quantitative cytotoxicity of nanoparticles and determination of cell viability during the experiment. Exactly, after seeding period of CT26 cells with or without MWCNT-COOH particles and exposed of laser irradiation, the cell mediums were elicited and added to 20 µL of MTT solution (Sigma M2128, 5 mg/mL) to each well, and microplates were incubated for 4 h at 37 °C in a humidified 5% CO2-air mixture. After configuration of formazan crystals, the MTT solution was discarded from the wells, without perturbing the formazan precipitate. 100 µL/well dimethyl sulfoxide (DMSO 100%; Sigma D 8779 ACS) was added to dissolve formazan. After 10 min, the absorbance of each well was measured with a microELISA reader (EIA Reader, EL307, Biotek Instruments, Burlington, VT) at 570 nm and reference wavelength of 630 nm.
Statistical analysis
In the first step, the Kolmogorov-Smirnov test was used to verify the normality of the data. In the next step, the p values were calculated using one-way ANOVA test and Tukey test for comparing the treatment groups. It should be noted that p < .05 was considered to be statistically significant.
Results and discussion
Characterization of MWCNTs-COOH
The UV-visible absorption spectrum () showed that the absorbance of MWCNTs-COOH in the 200–1100 nm wavelength range is much higher than water. According to this absorption spectrum, maximum absorption occurred at the 277 nm wavelength. These results were in line with the findings of other studies [Citation25,Citation26]. Hence, MWCNTs-COOH suspension increased heat generation and temperature compared to water remarkably. In PTT, temperature increase of MWCNTs is more efficiency than the other nanoparticles such as SWCNTs, carbon nano-horn, gold nano-shells, and gold nano-rods; as MWCNTs have a good absorption spectrum at all wavelengths relatively [Citation9,Citation10,Citation12,Citation27].
Figure 2. UV/VIS absorption spectrum of MWCNTs-COOH using a duplex atomic absorption spectrophotometer (a) and SEM image of MWCNTs-COOH particles (b).
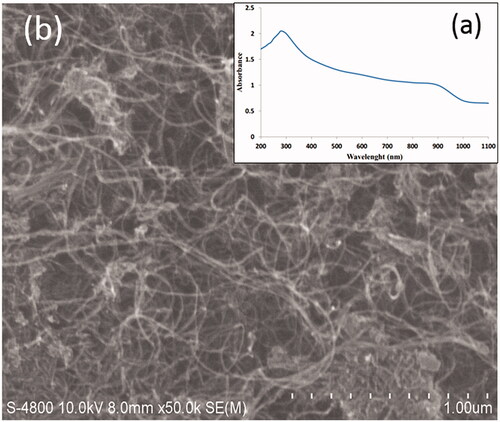
As seen from the (SEM picture at 10 μm dimension), surface morphology of MWCNTs-COOH revealed that these particles were distributed very homogeneously on the distilled water which its reason can be due to cross-link roles of carboxylic acid groups between adjacent MWCNTs [Citation28,Citation29]. Aggregation of MWCNTs particles at the distilled water is an important limitation in the using of them [Citation30,Citation31]. Several methods have been suggested to solve this problem. One of these is to connect the carboxyl group (-COOH) to carbon nanotubes. This connection increases the dispersity of carbon nanotubes in water [Citation31]. With increasing the carbon nanotube dispersion, absorption in the NIR region is appeared and it leads to an increase heat production in photothermal therapy subsequently [Citation30,Citation32,Citation33].
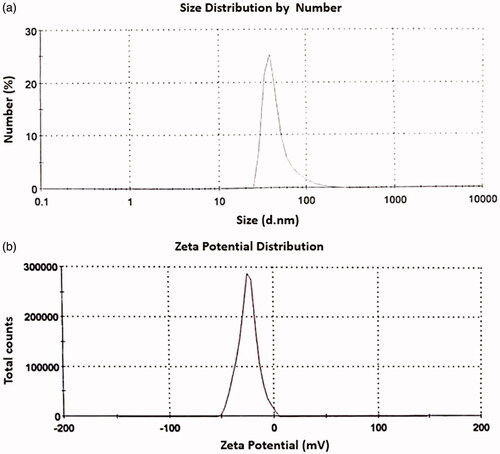
The MWCNTs-COOH size distribution curve was obtained and presented in . According to this curve, the largest number of MWCNTs-COOH has a diameter of about 47 nm. Zeta potential is an important factor in the distribution of nanoparticles. When the zeta potential is high enough (regardless of the negative or positive sign), it will ensure the stability of the nanoparticles’ suspension, nevertheless, low zeta potential leads to accumulation of nanoparticles in suspension [Citation34]. According to the zeta potential distribution curve of MWCNTs-COOH (), the highest number of MWCNTs-COOH particles has a zeta potential of about –24.8 mV. The findings of this part bear little resemblance to other findings of previous studies [Citation35,Citation36]. The reasons for these differences can be as different solvent medium of MWCNTs-COOH particles.
Temperature response of distilled water against laser irradiation
The results () demonstrated that the temperature of distilled water exposed to laser radiation increases after 20 s at the presence of various concentrations of MWCNTs-COOH. An increased water temperature was recorded for three laser powers of 1.5, 2, and 2.5 watts, separately. As shown in , at a concentration more than 80 µg/mL, the temperature begins to decrease smoothly. In addition, this behavior was observed in the all three laser powers (1.5, 2, and 2.5 watts). Thus, a 2.5 watt laser power was used to investigate the photosensitizer effects of MWCNT-COOH, because it created the highest temperature in the aqueous medium ().
Figure 4. The temperature fluctuations of MWCNT-COOH suspension at various concentrations against three power levels (1.5, 2, and 2.5 watts) upon 20 s irradiation time. Temperature was measured by thermometer (Triplicate).
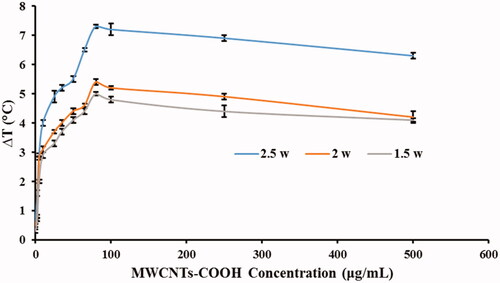
To induce heat in the NIR region, MWCNTs are more efficiency than SWCNTs, because MWCNTs have more electrons in each particle to energy absorbance [Citation30]. In a study by Bruke et al., it was showed that MWCNTs and SWCNTs at 0.1 mg/mL concentration increase the temperature to 28 and 4 °C, respectively. It means that MWCNTs were able to increase the temperature of seven times more than the SWCNTs at a same concentration. Also, MWCNTs at concentration of 100 mg/mL and SWCNTs at concentration of 2000 mg/mL resulted in same temperature increase, which this concentration of the SWCNTs is a very high concentration for in vivo and in vitro studies [Citation32]. In another study, Fisher's et al. reported that to generate the same temperature in to different condition with and without MWCNTs, it should be used a 50.9 w/cm2 laser (without MWCNTs) instead of 15.3 w/cm2 (with MWCNTs), and the radiation exposure time increases from 5 min (with MWCNTs) to 10 min (without MWCNTs). It is clear that photo-thermal therapy is impossible with high radiation power of laser [Citation30].
Cytotoxicity measurements
After 24 h treatment of CT26 cells with different concentrations of MWCNTs-COOH suspensions (5, 10, 20, 40, 80, 100, 250, and 600 μg/mL), the MTT assay results were obtained. The dose–response curve () was plotted to evaluate MWCNTs-COOH cytotoxicity. As indicated in the expression of the data, the total numbers of the CT26 cells in each of the concentrations were normalized to the corresponding control groups. It can observe from which the mean cell viability (%) reduces by increasing the MWCNTs-COOH suspension concentration. The mean cell viabilities (%) in some treatment groups, such as the concentrations of 5, 20, and 40 μg/mL, were about 1–2.5% higher than the control group which their reason may be due to stimulation of cell proliferation in these conditions. Nevertheless, the mean cell viability (%) in the mentioned groups had not significant difference compared to control groups (p values > .05). Only group treated at concentration of 600 μg/mL had a significant difference at the cell viability (%) compared to the control group (p values = .001). In the other treatment groups (5, 20, and 40 μg/mL), the mean cell viabilities (%) were 1 to 2% less than the control group. According to statistical analysis, no significant decrease at the mean cell viability (%) was found after 24-h in the CT26 cells treated by 5, 10, 20, 40, 80, 100, 250 μg/mL of MWCNTs-COOH suspensions compared to control group (p values > .05). The data would seem to suggest that the MWCNTs-COOH suspensions with concentration of <250 μg/mL were nontoxic for CT26 cells.
Figure 5. CT26 cells viability (%) for determination of MWCNTs-COOH cytotoxicity was measured by MTT assay. Percent of toxicity = (ODtest/ODcontrol) × 100. The experiments were repeated three times.
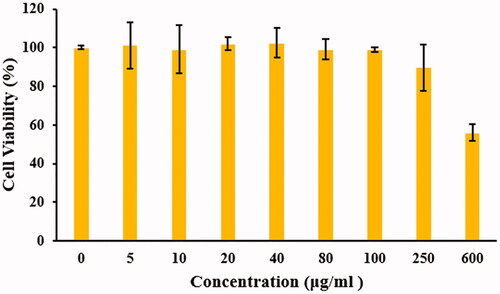
The results of the cytotoxicity in other studies are closely related to our study. Bruke et al. study showed that MWCNTs do not have any toxic effect on the renal cancer cells lines [Citation32]. Fröhlich et al. measured cytotoxicity of MCNTs on different cell lines using formazan bioreduction method. Their results revealed that there are no significant differences in the mean cell viability (%) of MCNTs at concentrations up to 50 μg/mL compared to the vehicle group [Citation37]. Nanoparticle distribution plan, cell lines, and cell viability assay can be the cause of cytotoxicity fluctuation [Citation6,Citation38,Citation39].
Quantification of photosensitizer effect
In order to cancerous cell treatment and to achieve higher therapeutic efficacy, multiple therapeutic methods can be important [Citation7–9]. The main goal of the multiple therapies is to prevent the recurrence of tumor. One of the most important reasons for tumor recurrence is the exist of hypoxic cells in the central regions of the tumors [Citation7,Citation8,Citation40]. The central cells of tumor have fewer PHs than the peripheral cells, causing radiation resistance against X-rays and gamma rays [Citation7,Citation8]. To reduce this problem, some solutions were presented, including (1) using high-oxygen during radiation therapy, (2) using sensitive drugs that specifically affects low-oxygenated cells, (3) using high-level LET rays, (4) hyperthermia therapy [Citation7–10].
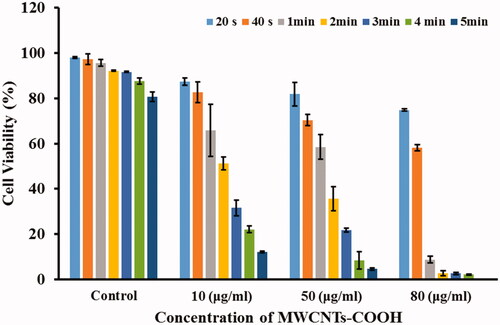
According to the experimental results obtained in the aqueous medium (), the highest laser power of 2.5 watts was selected. At the first, CT26 cells were treated with MWCNTs-COOH suspensions at concentrations of 0, 10, 50, and 80 μg/mL. Then, the cells were irradiated with laser at different period times of 20, 40, 60, 120, 180, 240, and 300 s. The results of these experiments (treated cells with MWCNTs-COOH against laser radiation), as the mean cell viability (%) and standard deviation, were illustrated in and .
Figure 6. Mean cell viability (%) of the CT26 cells treated with different concentrations of MWCNTS-COOH particles against laser radiation. The subgroups were compared to the 0 μg/mL (control) in each group by Tukey test.
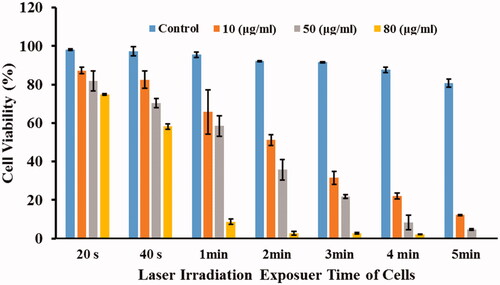
Figure 7. Mean cell viability (%) of the CT26 cells treated with different concentrations of MWCNTS-COOH particles against laser radiation. The subgroups were compared to the 20 s in each group by Tukey test.
As shown in , the mean cell viabilities (%) decrease with increasing of MWCNTs-COOH concentration at each period time of laser irradiation. For example, the mean cell viabilities (%) for concentrations of 0, 10, 50, and 80 μg/mL at the period time of 20 s were 0.98 ± 0.37%, 87.35 ± 1.60%, 81.82 ± 5.13%, and 74.87 ± 0.40%, respectively. These values for 2-min laser irradiation were 92.2 ± 0.34%, 51.2 ± 2.78%, 74.75 ± 5.34%, and 2.7 ± 1.07%, respectively. In fact, at the 80 μg/mL concentration of MWCNTs-COOH, approximately 98% of the cells died with 2 min of laser irradiation. Our experiment agreed with previous results [Citation30,Citation41,Citation42]. Mocan et al. demonstrated that 10 and 50 μg/mL concentrations of MWCNTs-PEG lead to significant cytotoxicity compared to control group against laser irradiation (3 min, 808 nm, 2 W/cm2) [Citation42]. Also, Fisher et al. and Eldridge et al. represented that 10 and 100 μg/mL concentrations of MWCNTs increase cell death compared to NIR alone significantly [Citation30,Citation41].
On the other hand, it was shown that in each concentration of MWCNTs-COOH, the cell viabilities (%) decrease with increasing the radiation exposure time (). For example, at 50 μg/mL concentration, the cell viability (%) for radiation times of 20, 40, 60, 120, 180, 240, and 300 s were 81.82 ± 5.13, 70.37 ± 2.51, 58.5 ± 5.4, 35.47 ± 5.34, 21.58 ± 0.91, 8.39 ± 3.77, and 4.62 ± 0.43, respectively. In other words, in the 50 μg/mL concentration of MWCNTs-COOH with 4 min of laser irradiation, about 92% of the cells died.
In , all groups were compared to control group (0 μg/mL concentration of MWCNTs-COOH). According to , the mean cell viabilities (%) in treated groups with 10, 50, and 80 μg/mL concentrations of MWCNTs-COOH against specific irradiation time increased remarkably compared to the control group. For example, in the group of 2-min laser irradiation and 10, 50, and 80 μg/mL subgroups, p values were .001, <.001, and <.001, respectively.
In , all irradiation time were compared to 20-s irradiation time in each group. According to , the mean cell viabilities (%) in the control group with exposure times of 40 and 60 s had not suppressed significantly (p values > .05). But the mean cell viabilities (%) with exposure times of 120, 180, 240, and 300 s decreased remarkably (p values were .040, .026, .002, and <.001, respectively) in compared with 20 s. In the next group (10 μg/mL of MWCNTs-COOH), the mean cell viabilities (%) with laser irradiation times of 40, 60, 120, 180, 240, and 300 s were significantly low (p values were .034, .002, <.001, <.001, and <.001, respectively). Also, in the other groups (50 and 80 μg/mL of MWCNTs-COOH), the mean cell viabilities (%) with laser irradiation times of 40, 60, 120, 180, 240, and 300 s were significantly low (p values were <.001).
Dong et al. investigated the photothermal effect of MWCNTs/DOX/TC (MWCNTs-COOH conjugated with TAT-chitosan loaded on DOX), MWCNTs/DOX, MWCNTs/TC or MWCNTs/DOC/TC at concentrations of 5 and 10 μg/mL during 5 min NIR irradiation with 1 W/cm2. Their findings demonstrated that MWCNTs/DOX/TC (compared with MWCNTs/DOX, MWCNTs/TC, or MWCNTs) have more efficient photothermal effects and cause to temperature increase of 6.1 °C (5 μg/mL) and 8.0 °C (10 μg/mL) during 5 min of laser irradiation. Furthermore, they showed that MWCNTs/TC (with 5 and 10 μg/mL of CNT) had no significant toxic against Bel7402 hepatoma cells after 72 h incubation either with laser irradiation. However, MWCNTs/DOX/TC or MWCNTs/DOX with laser irradiation demonstrated a smoothly enhanced toxicity in comparison with those of without laser irradiation after 24 and 48 h incubation [Citation43]. In our study, 10 μg/mL of MWCNTs-COOH contribute to temperature increase of 4 °C against 20 s irradiation. Also, using different concentrations of MWCNTs-COOH against the various time duration of laser allowed us to quantify the behavior of MWCNTs-COOH.
In addition, the mean cell viabilities (%) were compared between different groups to achieve optimal time duration of laser irradiation. As shown in , the mean cell viability (%) between 120 and 180 s laser irradiation times did not significantly varied at the groups with concentrations of 0, 10, 50, and 80 μg/mL of MWCNTs-COOH suspension (p values were 1.000, .054, .079, and 1.000, respectively). Hence, increasing the exposure time from 120 to 180 s cannot be justified because it causes further damage to the normal tissues in the laser path without increment of treatment ratio.
Table 1. Results of comparison of the mean cell viability (%) between different irradiation times at the specific concentration of MWCNTs-COOH using t-test.
Based on the obtained results (), the mean cell viabilities (%) did not increase significantly with increment of MWCNTs-COOH concentration from 10 to 50 μg/mL at the 20 and 60 s of laser irradiation times (p-values were 0.306 and 0.694, respectively). However, this increment of MWCNTs-COOH concentration cannot be justified for observation of remarkable results; because it will lead to supplementary cytotoxicity.
Table 2. Results of comparison of the mean cell viability (%) between different concentrations of MWCNTs-COOH at the specific irradiation time using t-test.
In the current study, the temperature increase in water medium by 2.5 w/cm2 laser (irradiation time = 10 min) at groups without and with 80 μg/mL MWCNTs-COOH were 58.2 and 6.3 °C, respectively. This temperature at the presence of MWCNTs-COOH can be harmful to nontarget organisms and leads to heat damage. Therefore, the amount of temperature increase should be adequately controlled to minimize the thermal damages to the nontarget tissues. To solve this problem, new approaches must be designed to reduce the concentration of nanoparticles, radiation exposure time and radiation power in experiments [Citation30,Citation32,Citation33]. Moreover, selectively increase the amount of nanoparticles in the target tissue can lead to reduce the thermal-induced adverse side effects; as it can be achieved with drug delivery system by conjugating nanoparticles to specific molecules and antibodies [Citation44].
With regards to the findings of current study, it seems that investigating the conjugating of various functional groups to multiwalled carbon nanotubes in photothermal therapy for targeted drug delivery in future studies may be helpful. Also, the use of anionic, cationic and biocompatible nonionic surfactants, to increase the dispersity of multiwall carbon nanotubes can play an important role in increasing the therapeutic efficiency. Presumably it is possible to clarify and quantify the relationship between the degree of toxicity of multiwall carbon nanotubes with the amount of cellular uptake.
Conclusion
According to the results of current study, it could be reasonably argued that MWCNTs-COOH can employ as photosensitizer agents with lower concentrations. As detailed, using the 80 μg/mL concentration of MWCNTs-COOH can decrease the viability of CT26 fibroblastic cells by 23.26, 39.06, 86.84, 89.5, 88.96, 85.59, and 80.72% against 20, 40, 60, 120, 180, 240, and 300 s of NIR irradiation, respectively. To achieve desirable results, the irradiation time should not increase, because presence of the 80 of MWCNTs-COOH upon 60 s of NIR irradiation has almost the same results with those of 120, 180, 240, and 300 s. It seems that focusing the thermal energy on cancer cell/tissue using lower concentration of MWCNTs-COOH without increasing of NIR irradiation time can result to favorable therapeutic results.
Declaration statement
No potential conflict of interest was reported by the authors.
Acknowledgements
This article was extracted from a research project in medical physics by the first author. Authors especially thank their personnel for their contribution to this study.
Additional information
Funding
References
- Center M, Siegel R, Jemal A. Global cancer facts & figures. Atlanta (GA): American Cancer Society; 2011. p. 1–52.
- Farhood B, Geraily G, Alizadeh A. Incidence and mortality of various cancers in Iran and compare to other countries: a review article. Iran J Public Health. 2018;47:309–316.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
- Li CJ, Mikule K, Li Y. Compositions and methods for cancer treatment. Google Patents. 2017.
- Bahreyni-Toosi M, Zare M, Ale-Davood A, et al. In-vitro study of photothermal anticancer activity of carboxylated multiwalled carbon nanotubes. J Biomed Phys Eng. 2017;7:317.
- Goushbolagh NA, Farhood B, Astani A, et al. Quantitative cytotoxicity, cellular uptake and radioprotection effect of cerium oxide nanoparticles in MRC-5 normal cells and MCF-7 cancerous cells. BioNanoScience 2018;8:1–9.
- Habash RW, Bansal R, Krewski D, et al. Thermal therapy, part 1: an introduction to thermal therapy. Crit Rev Biomed Eng. 2006;34:459–489.
- Van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13:1173–1184.
- Leung JP, Wu S, Chou KC, et al. Investigation of sub-100 nm gold nanoparticles for laser-induced thermotherapy of cancer. Nanomaterials (Basel). 2013;3:86–106.
- Huang X, El-Sayed MA. Plasmonic photo-thermal therapy (PPTT). Alexandria J Med. 2011;47:1.
- Kampinga H, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol. 2001;77:399–408.
- Huang X, Jain PK, El-Sayed IH, et al. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008;23:217.
- Jin H, Yang P, Cai J, et al. Photothermal effects of folate-conjugated Au nanorods on HepG2 cells. Appl Microbiol Biotechnol. 2012;94:1199–1208.
- Cherukuri P, Glazer ES, Curley SA. Targeted hyperthermia using metal nanoparticles. Adv Drug Deliv Rev. 2010;62:339–345.
- Glazer ES, Curley SA. The ongoing history of thermal therapy for cancer. Surg Oncol Clin N Am. 2011;20:229–235.
- Riley RS, Day ES. Gold nanoparticle‐mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wires Nanomed Nanobiotechnol. 2017;9:e1449.
- Young JK, Figueroa ER, Drezek RA. Tunable nanostructures as photothermal theranostic agents. Ann Biomed Eng. 2012;40:438–459.
- Markovic ZM, Harhaji-Trajkovic LM, Todorovic-Markovic BM, et al. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011;32:1121–1129.
- Zhou F, Da X, Ou Z, et al. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J Biomed Opt. 2009;14:021009.
- Okpalugo T, Papakonstantinou P, Murphy H, et al. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005;43:153–161.
- Zhang D, Kandadai MA, Cech J, et al. Poly(L-lactide) (PLLA)/multiwalled carbon nanotube (MWCNT) composite: characterization and biocompatibility evaluation. J Phys Chem B 2006;110:12910–12915.
- Akhavan O, Ghaderi E, Aghayee S, et al. The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J Mater Chem. 2012;22:13773–13781.
- Zhang Z, Liu S, Xiong H, et al. Electrospun PLA/MWCNTs composite nanofibers for combined chemo-and photothermal therapy. Acta Biomater. 2015;26:115–123.
- Zhao Z, Yang Z, Hu Y, et al. Multiple functionalization of multiwalled carbon nanotubes with carboxyl and amino groups. Appl Surf Sci. 2013;276:476–481.
- Ding Z, Zhu Y, Branford-White C, et al. Self-assembled transparent conductive composite films of carboxylated multiwalled carbon nanotubes/poly(vinyl alcohol) electrospun nanofiber mats. Mater Lett. 2014;128:310–313.
- Reddy KR, Sin BC, Ryu KS, et al. Conducting polymer functionalized multiwalled carbon nanotubes with noble metal nanoparticles: synthesis, morphological characteristics and electrical properties. Synth Met. 2009;159:595–603.
- Hashida Y, Tanaka H, Zhou S, et al. Photothermal ablation of tumor cells using a single-walled carbon nanotube–peptide composite. J Controlled Release. 2014;173:59–66.
- Veisi H, Eshbala FH, Hemmati S, et al. Selective hydrogen peroxide oxidation of sulfides to sulfones with carboxylated multiwalled carbon nano tubes (MWCNTs-COOH) as heterogeneous and recyclable nanocatalysts under organic solvent-free conditions. RSC Adv. 2015;5:10152–10158.
- Zhang Y, Zhang K, Ma H. Electrochemical DNA biosensor based on silver nanoparticles/poly(3-(3-pyridyl) acrylic acid)/carbon nanotubes modified electrode. Anal Biochem. 2009;387:13–19.
- Fisher JW, Sarkar S, Buchanan CF, et al. Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes after laser irradiation. Cancer Res. 2010;70:9855–9864.
- Shi Y, Ren L, Li D, et al. Optimization conditions for single-walled carbon nanotubes dispersion. J Surf Eng Mater Adv Technol. 2013;3:6.
- Burke A, Ding X, Singh R, et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc Natl Acad Sci USA. 2009;106:12897–12902.
- Ghosh S, Dutta S, Gomes E, et al. Increased heating efficiency and selective thermal ablation of malignant tissue with DNA-encased multiwalled carbon nanotubes. ACS Nano. 2009;3:2667–2673.
- Aljamali NM. Zetasizer technique in biochemistry. Biochem Anal Biochem. 2015;4:1.
- Nouara A, Wu Q, Li Y, et al. Carboxylic acid functionalization prevents the translocation of multiwalled carbon nanotubes at predicted environmentally relevant concentrations into targeted organs of nematode Caenorhabditis elegans. Nanoscale 2013;5:6088–6096.
- Zhang T, Tang M, Kong L, et al. Comparison of cytotoxic and inflammatory responses of pristine and functionalized multiwalled carbon nanotubes in RAW 264.7 mouse macrophages. J Hazard Mater. 2012;219:203–212.
- Fröhlich E, Meindl C, Höfler A, et al. Combination of small size and carboxyl functionalisation causes cytotoxicity of short carbon nanotubes. Nanotoxicology 2012;7:1211–1224.
- Yu J-G, Jiao F-P, Chen X-Q, et al. Irradiation-mediated carbon nanotubes’ use in cancer therapy. J Can Res Ther. 2012;8:348.
- Abdi Goushbolagh N, Abedi Firouzjah R, Ebrahimnejad Gorji K, et al. Estimation of radiation dose-reduction factor for cerium oxide nanoparticles in MRC-5 human lung fibroblastic cells and MCF-7 breast-cancer cells. Artif Cells Nanomed Biotechnol. 2018;46:1–11.
- Song CW, Shakil A, Griffin RJ, et al. Improvement of tumor oxygenation status by mild temperature hyperthermia alone or in combination with carbogen. Semin Oncol. 1997; 24:626–632.
- Eldridge BN, Bernish BW, Fahrenholtz CD, et al. Photothermal therapy of glioblastoma multiforme using multiwalled carbon nanotubes optimized for diffusion in extracellular space. ACS Biomater Sci Eng. 2016;2:963–976.
- Mocan T, Matea CT, Cojocaru I, et al. Photothermal treatment of human pancreatic cancer using PEGylated multiwalled carbon nanotubes induces apoptosis by triggering mitochondrial membrane depolarization mechanism. J Cancer. 2014;5:679.
- Dong X, Sun Z, Wang X, et al. An innovative MWCNTs/DOX/TC nanosystem for chemo-photothermal combination therapy of cancer. Nanomed Nanotechnol Biol Med. 2017;13:2271–2280.
- Khatti Z, Hashemianzadeh SM, Shafiei SA. A molecular study on drug delivery system based on carbon nanotube compared to silicon carbide nanotube for encapsulation of platinum-based anticancer drug. Adv Pharm Bull. 2018;8:163.