Abstract
Breast cancer is a common malignant tumor with a high incidence of recurrence and metastasis. It has been reported that propofol has certain anti-breast cancer effects, but the intrinsic molecular mechanism remains unclear. This study investigated the effect of propofol on breast cancer MCF-7 cells and its possible regulatory mechanisms. MCF-7 cells were treated by propofol, and then the effects of propofol on cell growth and epithelial-mesenchymal transition (EMT) were studied. We subsequently testified whether miR-21 was a downstream effector of propofol. As a result, propofol repressed the proliferation and migration of MCF-7 cells, but significantly induced apoptosis. Meanwhile, miR-21 expression and EMT were inhibited by propofol stimulation. The effects of propofol on MCF-7 cells proliferation, apoptosis and EMT were all attenuated when miR-21 was overexpressed. Besides this, the activation of PI3K/AKT and Wnt3a/β-catenin pathways was reduced by propofol stimulation in a miR-21-depedent manner. In conclusion, propofol can inhibit the proliferation and EMT of MCF-7 cells by down-regulating miR-21 expression. Moreover, miR-21 can further regulate PI3K/AKT and Wnt/β-catenin pathways.
Introduction
Breast cancer is a common malignant tumor around the world. Genetic susceptibility and combined risk factors are main reasons for inducing breast cancer [Citation1]. Cancer cell proliferation and epithelial-mesenchymal transition (EMT) are important factors of cancer recurrence. Currently, the treatment of breast cancer is mainly surgical resection, but it also brings many side-effects, such as infection and complications caused by seroma [Citation2,Citation3].
Propofol is a common intravenous anesthetic [Citation4], usually used to induce and maintain anesthesia, as well as the procedural and intensive care sedation in children. It has been widely established that propofol exerts suppressing functions towards diverse human cancers, like leukemia, pancreatic cancer, liver cancer, colon cancer, breast cancer [Citation5] and so forth. For the selected examples, propofol inhibits the growth and invasion of pancreatic cancer PANC-1 cells in both dose- and time-dependent manner [Citation6]. Likewise, propofol inhibits the proliferation, migration and invasion, but promotes apoptosis of hepatocarcinoma cells through down-regulating miR-374a in hepatocarcinoma cell lines [Citation7]. Besides this, the anti-tumor activity of propofol was also found in breast cancer cells. As reported by Li et al., propofol plays a significant role in impeding the invasion and migration of breast cancer cells [Citation8]. However, the intrinsic molecular mechanism of propofol’s anti-breast cancer effects remains unclear.
microRNAs (miRNAs) are a sort of RNAs with non-protein coding capacity that have been extensively studied in recent years [Citation9]. They play biological roles by regulating gene expression, protein translation, and the stability of target RNAs [Citation10]. Increasing evidence has recently identified the oncogenic effect of miR-21 in various cancers [Citation11]. miR-21 is capable of promoting cell proliferation through PTEN-dependent PI3K/Akt signaling pathway in cancer cells [Citation12]. In terms of breast cancer, Chanyshev et al. confirmed that miR-21 is involved in regulating ACAT1 expression and thus led to the increase in MCF-7 cells proliferation and the decrease of late apoptosis [Citation13].
In this article, we studied the effects of propofol on MCF-7 cells, and the relationship between propofol and miR-21, in order to reveal a possible molecular mechanism involved in propofol’s anti-tumor activity.
Materials and methods
Cell culture and treatment
MCF-7 cells (ATCC, Manassas, VA) were cultured in RPMI-1640 medium (Gibco/BRL, Grand Island, NY) containing 10% calf serum (Hyclone, Logan, UT) at 37 °C with 5% CO2.
To induce EMT, cells were induced with 10 ng/mL transforming growth factor (TGFβ1) (Abcam, Cambridge, MA) for 72 h. Different concentrations (0–10 μg/mL) of propofol (Diprivan, AstraZeneca, Macclesfield, UK) were used to treat cells for 48 h.
CCK-8 assay
The viability of cells in sterile 96-well cell plates with 5000 cells/well was estimated by Cell Count Kit-8 (CCK-8, Dojindo Molecular Technologies, Gaithersburg, MD). The cells were stimulated with propofol for 48 h, after which 10 μL CCK-8 solution was added to the medium for additional 1 h incubation. Absorbance at 450 nm was detected by a Microplate Reader (Bio-Rad, Hercules, CA).
Proliferation assay
After transfection and stimulation with propofol (6 μg/mL), bromodeoxyuridine (BrdU) from BrdU Cell Proliferation ELISA Kit (Abcam, Cambridge, UK) at a concentration of 1 mg/mL was added, and the plates were cultured for 3 h. According to the manufacturer’s instructions, cells were fixed and incubated with primary antibody, secondary antibody and tetra-methylbenzidine peroxidase substrate successively. After addition of stop solution, absorbance at 450 nm was detected by a Microplate Reader (Bio-Rad, Hercules, CA). At least 1000 cells were counted in each condition, and each condition contained at least five replicates.
Apoptosis assay
After transfection and stimulation with propofol (6 μg/mL), the cells were dished with phosphate buffered saline (PBS), cells were collected, suspended in binding buffer with 50 μg/mL RNase A (Sigma, St. Louis, MO) and stained by using FITC Annexin V/Dead Cell Apoptosis Kit (Invitrogen, Caslsbad, CA). Flow cytometry analysis was performed by FACS can (Beckman Coulter, Fullerton, CA). These data were analyzed by FlowJo software (Tree Star, San Carlos, CA).
Transfection
miR-21 mimic (sense, 5′-UAGCUUAUCAGACUGAUGUUGA-3′; antisense, 5′-AACAUCAGUCUGAUAAGCUAUU-3′) and the negative control (NC) were synthesized by GenePharma (Shanghai, China). The cells were transfected by using Lipofectamine 3000 reagent (Invitrogen) system and followed exactly in accordance with the instructions.
qRT-PCR
Total RNA was isolated using Trizol reagent (Invitrogen) following the instructions. Isolated RNAs (500 ng) were converted to cDNA by the Taqman MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), cDNA was served as templates for real-time PCR using the Taqman Universal Master Mix II (Applied Biosystems). Relative expression of miR-21 was calculated by 2−ΔΔCt method [Citation14]. U6 served as the internal control.
Western blot
The total protein was isolated using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) with inhibitors (Roche, Basel, Switzerland). The protein concentration was quantified using the BCATM Protein Assay Kit (Pierce, Appleton, WI). The protein sample was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membrane (Sigma, St. Louis, MO). PVDF membranes were blocked by 5% non-fat milk prior to incubation with primary antibody at 4 °C overnight. The primary antibodies specific for p53 (ab131442), p21 (ab109520), cyclinD1 (ab16663), Mcl-1 (ab32087), Bcl-1 (ab86108), E-cadherin (ab40772), N-cadherin (ab18203), Vimentin (ab92547), Snail (ab53519), cleaved caspase-3 (ab13847), cleaved caspase-9 (ab2324), β-actin (ab8227), Wnt3a (ab28472), β-catenin (ab32572), t-PI3K (ab180967), p-PI3K (ab182651), t-AKT (ab53519), and p-AKT (ab38449) were all obtained from Abcam, Cambridge, UK. After rising, a secondary antibody (goat anti-rabbit, ab205718) harboring a horseradish peroxidase marker was used to incubate membranes at room temperature for 1 h. The PVDF membrane with blots and antibody was transferred to the Bio-Rad ChemiDocTM XRS system. 200 μL immobilon Western chemiluminescent HRP Substrate (Millipore, MA) was dropped to cover the surface of the membrane. Signal and bands intensities were visualized by Image Lab™ Software (Bio-Rad).
Statistical analysis
All experiments were repeated three times. The results of multiple experiments were expressed as mean ± SD. Statistical analyses were performed using SPSS 19.0 software (Chicago, IL). The p values were calculated using one-way analysis of variance (ANOVA) or Student t-test. These data obey normal distribution, and normality was tested by Kolmogorov–Smirnov one sample test. A p value of <.05 is considered to indicate a statistically significant result.
Results
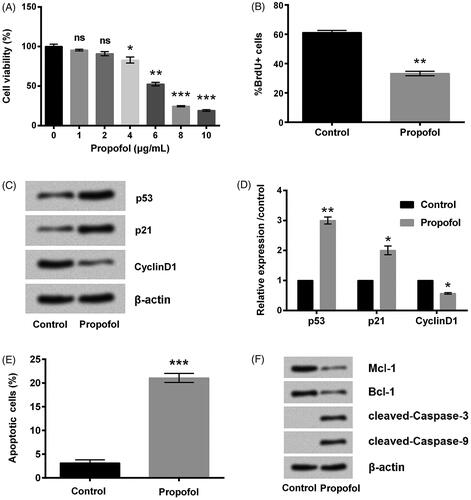
Propofol inhibits MCF-7 cells proliferation and promotes apoptosis
MCF-7 cells were suffered from different doses of propofol, and then the viability of cells was tested to select the optimum dose of propofol. As shown in , the viability of cell was clearly declined by propofol and the decrease was in a dose-dependent way. Considering that cell viability was inhibited in a half by 6 μg/mL propofol, 6 μg/mL was selected as an optimal concentration for use in the following experiments. Then, BrdU assay was used for evaluating cell proliferation. Data in demonstrated that treating cells with propofol dramatically lowered cell proliferation when compared with non-treating group (p < .01). The suppressive effects of propofol on cell proliferation were confirmed by the up-regulated p53 and p21 (p < .01 and p < .05), as well as the down-regulated CyclinD1 (p < .05, ). These results indicated propofol could inhibit MCF-7 cells proliferation. Additionally, treating cells with propofol led to a significant cell apoptosis (p < .001, ), which accompanied by the down-regulation of Mcl-1 and Bcl-1 as well as the cleavage of caspase-3/9 ().
Figure 1. Propofol inhibits breast cancer cell proliferation and promotes apoptosis. (A) The cells were stimulated with 0–10 μg/mL propofol for 48 h, cell viability was assessed by Cell Counting Kit-8 assay. The cells were stimulated with 6 μg/mL propofol for 48 h. (B) Percentage of BrdU positive cells by BrdU incorporation assay. (C, D) The expression of cell survival-related proteins by Western blot analysis. (E) Apoptotic cells rate was detected by flow cytometer. (F) The expression of apoptosis-related proteins by Western blot analysis. Data represented as mean ± SD. n = 3 per group. ns: no significance; *p < .05; **p < .01; ***p < .001 compared to control group.
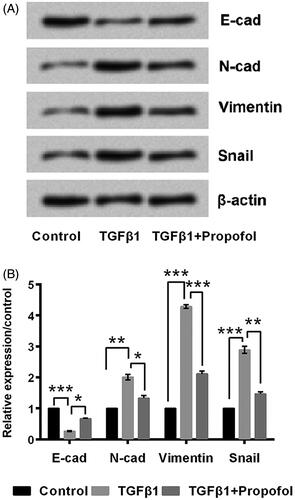
Propofol inhibits EMT
In order to detect the effect of propofol on EMT progress, MCF-7 cells were treated with TGFβ1 to induce EMT. The expression of E-cadherin was significantly down-regulated (p < .001), while N-cadherin, Vimentin, and Snail were significantly up-regulated (p < .01, p < .001, and p < .001, )) by TGFβ1 stimulation. More interestingly, these alteration induced by TGFβ1 were all reversed by addition of propofol (). Above all, it indicates that propofol can inhibit EMT effectively.
Figure 2. Propofol inhibits cell EMT. The cells were stimulated with 6 μg/mL propofol for 48 h. EMT was induced with 10 ng/mL TGFβ1 for 72 h. (A, B) Expression of EMT-related proteins by Western blot analysis. Data represented as mean ± SD. n = 3 per group. *p < .05; **p < .01; ***p < .001 compared to control group.
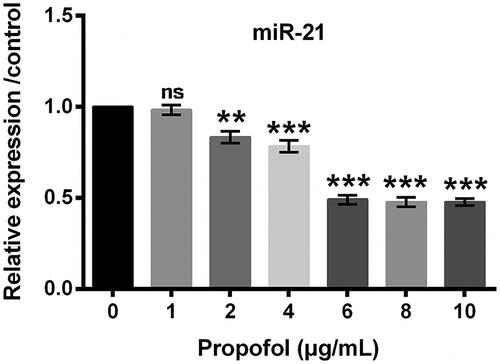
Propofol down-regulates miR-21 expression
Next, miR-21 expression following propofol treatment was tested. qRT-PCR data showed that the expression of miR-21 was lower in propofol groups, and the suppressive function of propofol on miR-21 expression might be via a dose-dependent way ().
Propofol inhibits cell proliferation, promotes apoptosis, and inhibits EMT by down-regulation of miR-21 expression
In order to detect whether the suppressive function of propofol was associated with miR-21, the mimic and NC for miR-21 was transduced into MCF-7 cells. qRT-PCR results showed that miR-21 expression was much higher in mimic group than that in NC group (p < .01, ). Thereupon, the importance of miR-21 on MCF-7 cells treated by propofol was examined. The results in showed that cell viability decrease induced by propofol was remarkably increased by transfection with miR-21 mimic (p < .05). Same trends were also observed in , as BrdU positive cells rate was significantly increased by miR-21 mimic transfection, even in propofol-treated MCF-7 cells (p < .05). Additionally, the result showed that mimic transfection resulted in the down-regulation of p53 and p21, and the up-regulation of CyclinD1 (p < .05, ). Unsurprisingly, propofol-induced apoptosis was impeded by miR-21 overexpression, as shown in ). These results indicated that propofol affected MCF-7 cells through regulating miR-21 expression.
Figure 4. Propofol inhibits EMT by down-regulation of miR-21 expression. The cells were stimulated with 6 μg/mL propofol for 48 h. EMT was induced with 10 ng/mL TGFβ1 for 72 h. (A) The expression of miR-21 by qRT-PCR analysis. (B) Cell viability was assessed by Cell Counting Kit-8 assay. (C) Percentage of BrdU positive cells by BrdU incorporation assay. (D, E) The expression of cell-survival-relate proteins by Western blot analysis. (F) Apoptotic cells rate was detected by flow cytometer. (G) The expression of apoptosis-related proteins by Western blot analysis. (H, I) Expression of EMT-related proteins by Western blot analysis. Data represented as mean ± sd. n = 3 per group. *p < .05; **p < .01; ***p < .001 compared to the indicated group.
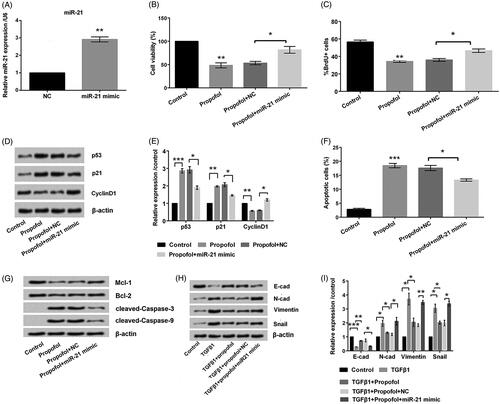
Western blot results in showed that propofol altered the dysregulation of EMT indicators was flattened by miR-21. As compared to TGFβ1 + Propofol group, E-cadherin was down-regulated and N-cadherin, Vimentin and Snail were up-regulated in TGFβ1 + Propofol + miR-21 mimic group. Above all, these data clearly suggested that propofol inhibited cell proliferation, promoted apoptosis, and inhibited EMT of MCF-7 cells by down-regulation of miR-21 expression.
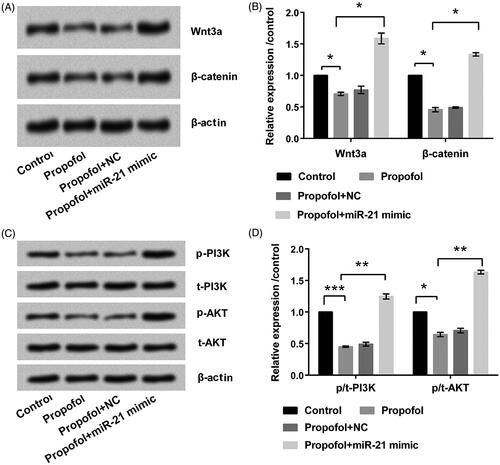
Propofol inhibits Wnt/β-catenin and PI3K/AKT signaling pathways by down-regulating miR-21
The expression of key components of Wn3a/β-catenin and PI3K/AKT pathways was detected by Wsestern blot to investigate mechanism in the propofol-associated regulations. Expression levels of Wnt3a, β-catenin, p-PI3K, and p-AKT were down-regulated in propofol group compared to control group (p < .05 or p < .001, )). But the result induced by propofol was reversed by transfecting miR-21mimic. These data imply that propofol inhibits Wnt/β-catenin and PI3K/AKT signaling pathways by down-regulating expression of miR-21.
Figure 5. Propofol inhibits Wnt/β-catenin and PI3K/AKT signaling pathways by down-regulating miR-21. The cells were stimulated with 6 μg/mL propofol for 48 h. Expression of (A, B) Wnt/β-catenin and (C, D) PI3K/AKT signaling pathways-related proteins by Western blot analysis. Data represented as mean ± SD. n = 3 per group. *p < .05; **p < .01; ***p < .001 compared to control group.
Discussion
Breast cancer is a common hormone-dependent tumor in women, accounting for about 23% of female malignant tumors. The incidence of breast cancer increase year by year with a growth trend of 3.1% [Citation15]. Currently, surgical resection is one of the main methods for treating tumors, but the postoperation is still unsatisfactory due to the easy recurrence and metastasis [Citation16]. Cell migration, proliferation, invasion and revascularization are important features of cancer cells. They are all complex processes regulated by a variety of genes and proteins. Similar to many other tumors, more than 90% of patients with breast cancer die of metastasis [Citation17]. Searching a new way to inhibit proliferation and metastasis of breast cancer will improve survival rate of patients with this deadliest cancer.
Propofol is a commonly used intravenous anesthetic in clinic. Previous studies on propofol have focused on evaluating its clinical effects and accompanying side-effects [Citation18–20]. But beyond that, the ever-expanding effect of propofol has been studied recently. Studies have shown that propofol is capable of suppressing metastasis of cancer cells [Citation21,Citation22], suggesting that propofol has anti-cancer effect [Citation23,Citation24]. Paravertebral injection of propofol decreases the metastasis of breast cancer [Citation9]. Propofol inhibited growth of breast cancer cells by inhibiting miR-24 overexpression [Citation25]. Herein, we also identified that propofol could inhibit the proliferation and EMT by regulating miR-21.
The p53 protein encoded by the p53 gene is a transcriptional factor that controls the initiation of the cell cycle [Citation26]. Many signals about cell health are sent to the p53 protein. The protein determines whether to start cell division. If the cell is damaged and cannot be repaired, the p53 protein will participate in the initiation process, causing the cell to die in apoptosis [Citation27]. Cells with p53 deficiency do not have this control and continue to divide even under adverse conditions. p21 is downstream gene of p53, induced by p53 selectively, as a mediator of p53-initiated cell cycle arrest [Citation1]. In this study, the expression levels of p53, p21, and CyclinD1 were detected to assess MCF-7 cells proliferation. As a result, we found that p53 and p21 expression levels were up-regulated, whereas CyclinD1 level was down-regulated by propofol stimulation, inferring its anti-proliferating effects via regulating p53, p21 and CyclinD1. In addition, the expression of Bcl-2 and Mcl-1 was down-regulated, and cleaved-caspase-3 and -9 was cleaved. Thus, it seemed that propofol promoted breast cancer cells apoptosis via a mitochondria-dependent pathway.
EMT refers to the transformation of epithelial to mesenchymal cells. EMT regulation is a complex network involved TGFβ1 family, Wnts and other signaling pathways [Citation28]. A decrease in the level of E-cadherin leads to decreased adhesion of the cells, giving the cells the ability to be easily invaded and metastasized. N-cadherin is a class of calcium-dependent transmembrane glycoproteins. Vimentin is a type of fibrin, also known as a marker of mesenchymal cells. Snail is a regulatory extracellular matrix that inhibits cell cycle arrest by inhibiting cell-cell tight junction-associated protein expression [Citation28]. In this experiment, after adding of propofol, E-cadherin expression was up-regulated, N-cadherin, Vimentin and Snail were down-regulated, suggesting propofol inhibited MCF-7 cells migration via regulating these EMT-related factors.
It has been pointed out that miR-21 knockdown suppressed growth, invasiveness, and metastatic properties of breast cancer cells by PIK3R1 [Citation29]. Consistently, the promoting effects of miR-21 mimic on MCF-7 cells growth were found elsewhere [Citation25]. According to our study, MCF-7 cells proliferation and viability were promoted, while apoptosis was inhibited by miR-21 mimic. Our findings were consistence with previous studies described above, indicating the oncogenic role of miR-21 in breast cancer. Besides, we found propofol can regulate expression of miR-21 on MCF-7 cells, and the inhibitory effects of propofol on MCF-7 cells survival and EMT were attenuated when miR-21 was overexpressed. Thus, we inferred that propofol inhibits MCF-7 cells proliferation and EMT by down-regulating expression miR-21.
The PI3K/AKT signaling pathway is one of the signal transduction pathways that determines cell fate. Its main functions include inducing stem cell differentiation and metastasis, promoting cell proliferation, inhibiting apoptosis, regulating tissue inflammation, and modulation of tumor growth and invasion. Like PI3K/AKT, Wnt signaling pathway is also important in regulating cell differentiation, proliferation, apoptosis, and migration [Citation30,Citation31]. In this study, these two signaling pathways were found to be suppressed by propofol. Moreover, the effects of propofol on pathways were reversed when miR-21 was overexpressed, revealing the promoting effects of miR-21 on the activation of PI3K/AKT and Wnt/β-catenin signaling pathways. This phenomenon was in line with a previous study [Citation32], in which these two signaling pathways can be blocked by miR-21 silence. Taken together, these results indicated propofol suppressed PI3K/AKT and Wnt/β-catenin signaling pathways via regulating miR-21.
In conclusion, propofol inhibited MCF-7 cells proliferation and EMT might be via down-regulating miR-21, which further regulated PI3K/AKT and Wnt/β-catenin signaling pathways. This study provided a novel regulatory mechanism for propofol in MCF-7 cells. Propofol might be an innovative therapeutic target for breast cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Manikandan P, Ramyachitra D. Bacterial foraging optimization -genetic algorithm for multiple sequence alignment with multi-objectives. Scient Rep. 2017;7:8833.
- Bodai BI, Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015;19:48–79.
- von Bayern AMP, Heathcote RJP, Rutz C, et al. The role of experience in problem solving and innovative tool use in crows. Curr Biol. 2009;19:1965–1968.
- Barquera S, Pedroza-Tobias A, Medina C, et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46:328–338.
- Tsuchiya M, Asada A, Arita K, et al. Induction and mechanism of apoptotic cell death by propofol in HL-60 cells. Acta Anaesthesiol Scand. 2002;46:1068–1074.
- Liu Z, Zhang J, Hong G, et al. Propofol inhibits growth and invasion of pancreatic cancer cells through regulation of the miR-21/Slug signaling pathway. Am J Transl Res. 2016;8:4120.
- Marques-Rocha JL, Samblas M, Milagro FI, et al. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 2015;29:3595–3611.
- Li Q, Zhang L, Han Y, et al. Propofol reduces MMPs expression by inhibiting NF-κB activity in human MDA-MB-231 cells. Biomed Pharmacother. 2012;66:52–56.
- Collins AR, Lyon CJ, Xia X, et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circulat Res. 2009;104:e42–e54.
- Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483.
- Pfeffer SR, Yang CH, Pfeffer LM. The role of miR-21 in cancer. Drug Dev Res. 2015;76:270–277.
- Liu X, Abraham JM, Cheng Y, et al. Synthetic circular RNA functions as a miR-21 sponge to suppress gastric carcinoma cell proliferation. Mol Therapy Nucl Acids. 2018;13:312–321.
- Liu G, Wang B, Zhang J, et al. Total panax notoginsenosides prevent atherosclerosis in apolipoprotein E-knockout mice: role of downregulation of CD40 and MMP-9 expression. J Ethnopharmacol. 2009;126:350–354.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408.
- Shan S, Lv Q, Zhao Y, et al. Wnt/β-catenin pathway is required for epithelial to mesenchymal transition in CXCL12 over expressed breast cancer cells. Int J Clin Exp Pathol. 2015;8:12357–12367.
- Pateras I, Giaginis C, Tsigris C, et al. NF-kappaB signaling at the crossroads of inflammation and atherogenesis: searching for new therapeutic links. Expert Opin Therapeut Targets. 2014;18:1089–1101.
- Liang YJ, Wang QY, Zhou CX, et al. MiR-124 targets Slug to regulate epithelial–mesenchymal transition and metastasis of breast cancer. Carcinogenesis. 2013;34:713–722.
- Bodnar J. A review of agents for palliative sedation/continuous deep sedation: pharmacology and practical applications. Hospice J. 2017;31:16–37.
- Finsterer J, Frank M. Propofol is mitochondrion-toxic and may unmask a mitochondrial disorder. J Child Neurol. 2016;31:1489–1494.
- Peng K, Liu HY, Wu SR, et al. Does propofol anesthesia lead to less postoperative pain compared with inhalational anesthesia? A systematic review and meta-analysis. Anesthes Analgesia. 2016;123:846.
- Cui WY, Liu Y, Zhu YQ, et al. Propofol induces endoplasmic reticulum (ER) stress and apoptosis in lung cancer cell H460. Tumour Biol. 2014;35:5213–5217.
- Wang P, Chen J, Mu LH, et al. Propofol inhibits invasion and enhances paclitaxel- induced apoptosis in ovarian cancer cells through the suppression of the transcription factor slug. Eur Rev Med Pharmacol Sci. 2013;17:1722–1729.
- Zhang D, Zhou XH, Zhang J, et al. Propofol promotes cell apoptosis via inhibiting HOTAIR mediated mTOR pathway in cervical cancer. Biochem Biophys Res Commun. 2015;468:561–567.
- Xu YJ, Li SY, Cheng Q, et al. Effects of anaesthesia on proliferation, invasion and apoptosis of LoVo colon cancer cells in vitro. Anaesthesia. 2016;71:147–154.
- Takahashi M, Masuyama J, Ikeda U, et al. Suppressive role of endogenous endothelial monocyte chemoattractant protein-1 on monocyte transendothelial migration in vitro. Arterioscler Thromb Vasc Biol. 1995;15:629–636.
- Gigante A, Giannakakis K, Di Mario F, et al. BMI, nephroangiosclerosis and glomerulonephritis: is there any meeting point? Nephrology (Carlton, Vic). 2018;23:991–996.
- Gaete H, Alvarez M, Lobos G, et al. Assessment of oxidative stress and bioaccumulation of the metals Cu, Fe, Zn, Pb, Cd in the polychaete Perinereis gualpensis from estuaries of central Chile. Ecotoxicol Environ Safety. 2017;145:653–658.
- Roever L, O'Connell JL, Chagas AC. Arterial stiffness in preschool children. Eur J Prevent Cardiol. 2017;24:1891–1894.
- Bao MH, Li GY, Huang XS, et al. Long non-coding RNA LINC00657 acting as miR-590-3p sponge to facilitate low concentration oxidized low-density lipoprotein-induced angiogenesis. Mol Pharmacol. 2018;93:368–375.
- Johnson ML, Rajamannan N. Diseases of Wnt signaling. Rev Endocr Metab Disord. 2006;7:41–49.
- Polakis P. ASTE and NEPCON present scholarships. (News Briefs). (American Society of Test Engineers) (Brief Article). Cold Spring Harbor Perspect Biol. 2012;4:1–10.
- Zhou B, Wang D, Sun G, et al. Effect of miR-21 on apoptosis in lung cancer cell through inhibiting the PI3K/Akt/NF-kappaB signaling pathway in vitro and in vivo. Cell Physiol Biochem. 2018;46:999–1008.