Abstract
Pladienolide B is a potent cancer cell growth inhibitor that targets the SF3b1 subunit of the spliceosome. There is considerable interest in the compound as a tool to study SF3b1 function in cancer. However, so far little information is available on the molecular mechanism of SF3b1 eliciting apoptosis in cancer cells. Here, we investigated the molecular mechanism of SF3b1 eliciting apoptosis in human cervical carcinoma cells. We demonstrated that inhibition of SF3b1 by pladienolide B inhibited proliferation of HeLa cells at low nanomolar concentrations in a dose- and time-dependent manner. It also induced G2/M phase arrest and significant rise of apoptotic cells. Moreover, it is indicated that inhibition of SF3b1 by pladienolide B induced Tap73/ΔNp73 expression and consequently down-regulated Bax/Bcl-2 ratio, cytochrome c release and caspase-3 expression. Thus, our results showed that SF3b1 plays a pivotal role in cycle arrest, apoptosis induction, and p73 splicing in human cervical carcinoma cells, suggesting that SF3b1 could be used as a potential candidate for cervical cancer therapy.
Introduction
Cervical cancer is one of the most common cancers and the second leading cause of cancer-related death in women worldwide [Citation1,Citation2], with 274,000 deaths annually [Citation3], which is also a complex disease involving the abnormal expression of many oncogenes and tumour suppressor genes [Citation4]. The discovery of novel therapeutics requires a comprehensive understanding of the disease biology. Recently, growing evidence has demonstrated that changes in alternative splicing patterns play a crucial role in cancer [Citation5–8] and other diseases [Citation9]. Through pre-mRNA splicing, a single pre-mRNA transcript may give rise to multiple different combinations of introns and exons, resulting in increased transcript diversity and the synthesis of alternative proteins [Citation10,Citation11].
Recently, considerable attention has been drawn to a new class of anticancer agents targeting the spliceosome [Citation12,Citation13]. For many eukaryotic introns, splicing is carried out in a series of reactions which are catalysed by the spliceosome, a complex of small nuclear ribonucleoproteins [Citation14,Citation15]. As such, the spliceosome has emerged as an attractive target for anticancer treatment [Citation16]. SF3b1 is a principal player in the spliceosome and a target of inhibitor compounds [Citation17,Citation18]. Several spliceosome modulators have already been identified [Citation19]. One of them is pladienolide B, which directly binds splicing factor 3b1 (SF3b1) in the spliceosome [Citation20], inhibits the splicing process in tumor cells [Citation21–23]. SF3B1 result in alternative splicing events and may constitute drivers and a novel therapeutic target in cancers [Citation24]. Importantly, this compound has been demonstrated to be potent antitumor agents, and minimally toxic to normal cells [Citation25].
Although the binding affinities of pladienolide derivatives to SF3b correlate with their inhibition of cell proliferation [Citation26] and apoptosis [Citation12,Citation27], the precise antitumor mechanism of the compound has not been fully elucidated. In the present study, we present preliminary data which could help to clarify the antitumor mechanism of SF3b1 in HeLa cells. Cells can enter into the death phase through different pathways, including apoptosis, autophagy, cell cycle arrest and necrosis [Citation28]. The outcome of the study revealed that pladienolide B effectively suppresses cervical carcinoma cell growth by the inhibition of cell cycle and induction of apoptosis. Overall, these results suggested that SF3b1 is a very promising target for cervical cancer therapy and the balance of pro-apoptotic protein Tap73 and anti-apoptotic proteins ΔNp73 might be a key point regulating SF3b1-induced apoptosis.
Materials and methods
Cell culture
The human cervical carcinoma Hela cells were purchased from the First Hospital of Lanzhou University. Cells were grown at 37 °C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM, Minghai Biochem, Lanzhou, China) supplemented with 10% (v/v) foetal bovine serum (Minghai Biochem, Lanzhou, China).
Cell survival detection
Cell proliferation was assessed using methyl tetrazolium salt (MTS) assay, and the methodology was previously discussed [Citation29]. After incubation with different concentration of pladienolide B, the number of viable cells grown in a 96-well plate was estimated by using Cell Titer 96 AQueous One Solution Cell Proliferation Assay (Promega, Beijing, China) after 30 min incubation at 37 °C. The absorbance in each well was measured with a microplate spectrophotometer reader (Thermal Lab System, Finland) at 490 nm, then viability of cells in each well was shown as percentage of the control.
Clonogenic assay
Colony-forming ability was compared between untreated and treated with different concentrations of pladienolide B cell cultures as previously described [Citation30]. The cells were seeded into 60 mm in triplicate dishes. After 24 h, the cells were treated with different concentrations of pladienolide B. After incubated at 37 °C for 8–14 days, colonies were then fixed with chilled methanol and stained with 0.4% crystal violet. Colonies containing ≥50 cells were counted, and plating efficiency and surviving fraction were determined.
Cell-cycle assay
Cells were treated with different concentrations pladienolide B. After incubated at 37 °C for 24 h, then harvested and fixed with 70% ethanol in PBS for 30 min. The fixed cells were centrifuged at 1800 g for 5 min and washed three times with PBS, then stained with a solution containing 5 μg/ml Propidium iodide (PI, Sigma, St Louis, MO, USA) in the dark for 30 min at RT, as previously described [Citation30]. The samples were detected with BD Accuri C6 (Becton Dickinson, San Jose, CA, USA). Cell-cycle distribution was analysed with using BD Accuri C6 Plus 1.0.23.1 Installer software with a minimum of 10,000 cells. Each experiment was performed at least three times.
Cell apoptosis assay
Cells were treated with different concentrations pladienolide B. After incubated at 37 °C for 48 h, cells were harvested and washed twice with PBS and diluted with 75 μl binding buffer, then 2.5 μl annexin V and 2.5 μl propidium iodide (Annexin V-FITC Apoptosis Detection Kit I, BD, USA) were added to 75 μl of cell suspension, as previously described [Citation30]. After incubated in the dark for 15 min at RT, the samples were detected with BD Accuri C6 (Becton Dickinson, San Jose, CA, USA) and immediately analysed on BD Accuri C6 Plus 1.0.23.1 Installer software.
Western blot analysis
Cells were treated with different concentrations of pladienolide B. After incubated at 37 °C for 48 h, cells were harvested with trypsin. Protein was extracted from the samples using RIPA lysis buffer (Solarbio, China) added PMSF, as described in our previous work [Citation30]. The protein concentration of the lysates was measured with the Protein BCA Assay Kit (Bio-Rad, USA). Proteins were fractionated by 10% SDS-PAGE and transferred to a methanol activated PVDF membrane (GE Healthcare, Beijing, China). Antibodies against Tap73, ΔNp73 (Imgenex, San Diego, USA), β-actin (Cell Signaling Technology, Beverly, MA, USA) and Bax, Bcl-2, Cytochrome c, pro-caspase 3 (Santa Cruz, CA,USA) were used in western blot analysis according to the manufacturer's instruction. HRP-linked anti-mouse or anti-rabbit IgG antibodies (Zsjqbio, China) were used as secondary antibodies. The results were normalized by β-actin levels.
Immunofluorescence
It was performed according to previous descriptions [Citation29,Citation30]. Cells were treated with different concentrations of pladienolide B. After incubated at 37 °C for 48 h, cells were thoroughly washed in 0.01 M PBS (pH 7.4), permeabilized with 0.5% Triton X-100/PBS for 15 min at RT, blocked for 25 min at RT by the drop wise addition of 5% BSA, primary antibody 4 °C overnight then incubated secondary antibody at 37 °C for 1 h. The above steps were repeated with 0. 01 M PBS (pH 7.4) and washed twice for 5 min each wash, then VECTASHIELD mounting medium with 4',6-diamidino-2-phenylindole (DAPI) (Vector, Laboratories, USA) was added. The samples were detected with a confocal laser microscope (LSM, Carl Zeiss AG, Germany).
Statistical analysis
Data are expressed as means ± SD. Statistical analysis was performed on the means of the data obtained from at least three independent experiments. Student’s t-test program in Microsoft Excel was used to detect statistical significance. p < .05 was considered to indicate statistically significant.
Results
Pladienolide B inhibits human cervical carcinoma cells viability
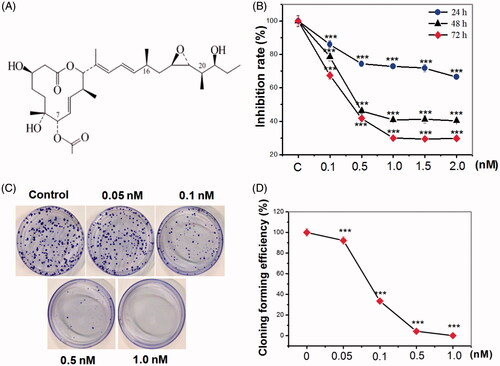
To evaluate the toxicity of pladienolide B itself, HeLa cells were treated with several concentrations of pladienolide B, and MTS assay revealed that pre-treatment of pladienolide B significantly decreased cell viability, and the decrease was concentration- and time-dependent (). Moreover, the colony-forming assay showed that HeLa cells treated with very low concentration of pladienolide B are sensitive ().
Figure 1. Pladienolide B significantly inhibited HeLa cell viability and colony formation. (A) Chemical structure of pladienolide B. (B) MTS assay revealed that the viability affected by pladienolide B. (C) Representative images of the colony formation potential of HeLa cells treated with different concentration of pladienolide B. (D) Quantitative representation of the colony counts was shown. It is indicated that pladienolide B decreased cell and colony growth. All experiments were performed in triplicate. The data are expressed as the mean ± SD.***p < .001 (vs. control group).
Pladienolide B reduces SF3b1 expression in human cervical carcinoma cells
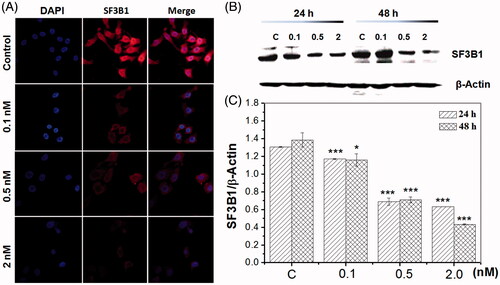
To validate whether pladienolide B regulates the cancer spliceosome, we carried out immunofluorescence and immunoblotting to confirm the expression of SF3b1 following pladienolide B treatment in HeLa cells. As shown in , pladienolide B induced a time- and concentration-dependent decrease in cellular SF3b1 proteins, consistent with the results obtained from the immunofluorescence analysis. Interestingly, significant changes in protein level of SF3b1 in response to pladienolide B were detected, suggesting that pladienolide B down-regulated SF3b1 expression.
Figure 2. Pladienolide B inhibited SF3b1 expression. (A) The effect of pladienolide B on spacial distribution of SF3b1 protein. (B) Representative western blot images. (C) Quantitative analysis showed the effect of 0.1 nM, 0.5 nM, and 2 nM pladienolide B on SF3b1 expression in HeLa cells. All experiments were performed in triplicate. The data are expressed as the mean ± SD. ***p < .001 (vs. control group). *p < .05 (vs. control group).
Pladienolide B induces cell cycle arrest and apoptosis
To elucidate the mechanism of the effect of pladienolide B of HeLa cell growth, the cell cycle progression was analysed using flow cytometry. It was revealed that an obvious increase in the ratio of G2/M phase cells was observed in HeLa cells at 24 h after 0.1, 0.5 and 2.0 nM pladienolide B treatment, which might contribute to the decreased cell viability ().
Figure 3. Inhibition of SF3b1 by pladienolide B inhibited cell cycle arrest, but induced apoptosis in human cervical carcinoma HeLa cells. (A) Cell cycle arrest observation was investigated with flow cytometry. (B) Apoptotic effect of pladienolide B on human cervical carcinoma HeLa cells. (C) Percentages of G2/M phase of cell population was calculated. (D) Percentages of apoptotic and dead rate and of cell population was calculated. All experiments were performed in triplicate. The data are expressed as the mean ± SD. ***p < .001 (vs. control group). **p < .01 (vs. control group).
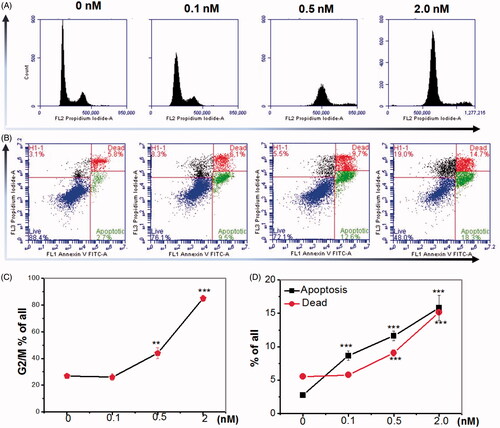
To determine whether the observed reduced viability of HeLa cells pre-treated by pladienolide B was caused by apoptosis. To examine apoptosis, flow cytometry was used. As shown in , the apoptotic cells were highly induced at 24 h after pladienolide B treatment (p > .001).
Pladienolide B up-regulates Tap73 and down-regulates ΔNp73, indicating activation of apoptosis
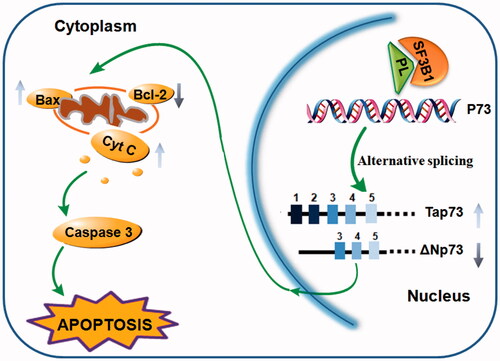
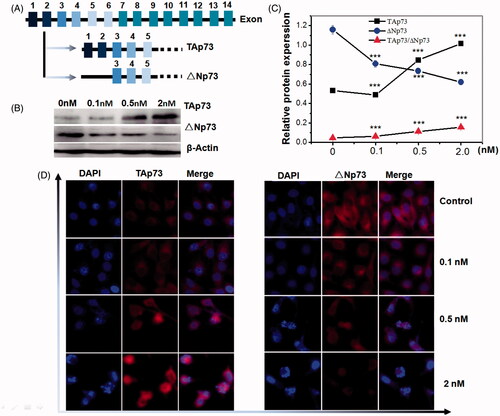
Since p73 has been reported to play a crucial role in inducing apoptosis in a p53-independent manner in cancer [Citation29–31], we examined if p73 is involved in pladienolide B -induced apoptosis in human cervical carcinoma cells. Western blot and immunofluorescence were described in our previous published papers [Citation29,Citation30] and applied to analyse the protein expression, respectively. As shown in , the p73 gene possesses an extrinsic P1 promoter and an intrinsic P2 promoter, resulting in TAp73 and DNp73 isoforms, respectively. We examined the levels of Tap73 and ΔNp73 proteins by western blot analysis, using β-Actin as a control. Levels of Tap73 were strongly increased in HeLa cells at 24 h after different concentration of pladienolide B treatment compared with control group. In contrast, levels of ΔNp73 were slowly decreased. Obviously, pladienolide B induced an increase in the Tap73/ΔNp73 ratio (). To verify the expression of Tap73 and ΔNp73 proteins and examine the subcellular localization of these proteins, we next performed immunofluorescent staining. As shown in , weak fluorescence of Tap73 was detected in the nucleus and cytoplasm, but strong fluorescence of ΔNp73 was detected in the cytoplasm. Consistent with the western blot analysis, Tap73 expression was obviously up-regulated in HeLa cells treated with pladienolide B compared with untreated HeLa cells while ΔNp73 expression was significantly decreased. Thus, inhibition of SF3b1 by pladienolide B up-regulated Tap73 and down-regulated ΔNp73, indicating activation of p53 responsive apoptosis genes.
Figure 4. Inhibition of SF3b1 by pladienolide B increased the protein ratio of Tap73//Np73 in human cervical carcinoma HeLa cells, indicating activation of apoptosis in p53-independent manner. (A) Gene structure and alternative transcripts of human TP73. (B) Representative western blot images. (C) Quantitative analysis of Tap73 and ΔNp73 proteins in HeLa cells by western blot analysis. (D) The localization of Tap73 and ΔNp73 protein in HeLa cells supplemented with Pladienolide B were determined by fluorescent microscopy. All experiments were performed in triplicate. The data are expressed as the mean ± SD. ***p < .001 (vs. control group).
SF3b1 regulates apoptosis via mitochondrial pathway
Bcl-2 family members, cytochrome c, and caspase-3 are crucial mediators of mitochondrial pathway of apoptosis. To determine whether SF3b1 promote apoptosis via mitochondrial pathway in human cervical carcinoma cells, we analysed the expression of these mediators. The detailed methodologies were discussed in our previous published paper [Citation29]. As shown in , inhibition of SF3b1 by pladienolide B induced a sharp increase in the protein level of pro-apoptotic Bax and a sharp decrease in the protein level of anti-apoptotic Bcl-2 (. Additionally, the cytochrome c expression levels were up-regulated at 24 h after pladienolide B treatment (). Furthermore, as can be seen in , regulation of caspase 3 was observed in HeLa cells at 24 h after 0.1, 0.5 and 2.0 nM Pladienolide B by appearance of the 35-kDa product and its cleaved form of 17 and 19-kDa, while the pladienolide B-pre-treated samples showed down-regulated expression of the corresponding caspase 3 and up-regulated expression of its cleavage. These results indicated that inhibition of SF3b1 by pladienolide B induced apoptosis via down-regulating Bax/Bcl-2 ratio, cytochrome c release and caspase-3 levels.
Figure 5. Inhibition of SF3b1 by pladienolide B up-regulates Bax/Bcl-2 ratio, and induces caspase activation in human cervical carcinoma HeLa cells. (A) Representative western blot images. (B, C) Quantitative analysis of the ratio of Bax/Bcl-2, pro-caspase 3 were represented by column graphs. β-Actin was used as a loading control. All experiments were performed in triplicate. The data are expressed as the mean ± SD. ***p < .001(vs. control group). **p < .01(vs. control group).
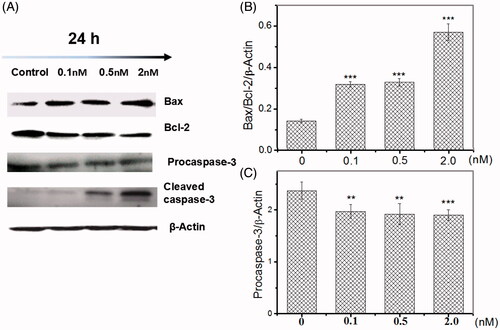
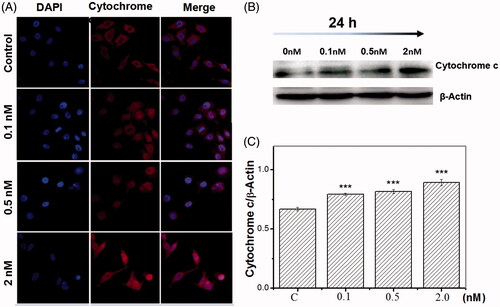
Figure 6. Inhibition of SF3b1 by pladienolide B promotes cytochrome c release in human cervical carcinoma HeLa cells. (A) The localization of cytochrome c protein in HeLa cells was determined by fluorescent microscopy. (B) Representative western blot images. (C) Quantitative analysis of cytochrome c was represented by column graphs. β-Actin was used as a loading control. All experiments were performed in triplicate. The data are expressed as the mean ± SD. ***p < .001 (vs. control group).
Discussion
More than 90% of human genes undergo alternative splicing to generate multiple mRNA variants in a tissue-specific manner [Citation10]. The corresponding protein isoforms have different, even antagonistic properties. Their deregulation has been implicated in various disease states, including cancer [Citation32]. Numerous studies have also demonstrated that overexpression and mutations of key spliceosome components are resulting in aberrant splicing activity and the emergence of hallmark cancer phenotypes [Citation33]. SF3b1 is one of the proteins that makes up the spliceosome and regulates excision of introns from pre-mRNA [Citation17,Citation21,Citation34]. Interestingly, SF3b1 is also the target of pladienolide B, a natural product with antitumour activity both in cancer cell lines and in mouse xenograft models [Citation20,Citation22,Citation35]. There is considerable interest in the compound as a potential chemotherapeutic, as well as a tool to study SF3b1 function in splicing and cancer development [Citation23]. By impairing the binding of the spliceosome to the branch sequence, pladienolide B leads to a splicing dysfunction that results in an accumulation of un-spliced mRNA in the cells and consequently in a reduction of cell viability [Citation23,Citation26]. Nevertheless, its mechanism of action is still not fully understood. This drug could represent a new therapeutic target in the treatment of such diseases. Apoptosis, or programmed cell death, which is a complex, genetically determined process involved in the development and maintenance of homeostasis in multicellular organisms, has proven to be critical for the development and sustained growth of many, and perhaps all cancers, and induction of apoptosis has now been considered as an important method for antitumor therapy [Citation36]. In this study, we investigated the molecular mechanism of SF3b1 eliciting apoptosis in human cervical carcinoma cells.
One striking finding is that SF3b1 knockdown led to activation of p53 pathway [Citation37]. p73 is homologue of the tumour suppressor p53, which functions as a transcriptional factor [Citation38]. The p73 locus encodes two types of transcription factors: full-length pro-apoptotic isoforms (TAp73), and N-terminally truncated anti-apoptotic proteins (ΔNp73) [Citation31]. Christine et al. showed that Tap73 could, at least when overproduced, activate the transcription of p53-responsive genes and inhibit cell growth in a p53-like manner by inducing apoptosis (programmed cell death) [Citation39]. However, ΔNp73 are able to bind to DNA and to form dimers with TAp73, thereby behaving as dominant negative, anti-apoptotic factors [Citation40]. Indeed, many in vitro and in vivo evidences demonstrated that the Tap73 was a pro-apoptotic isoform and ΔNp73 was an anti-apoptotic isoform. Recently, some studies showed that the ratio between Tap73 and ΔNp73 determined the fate of the cell-survive or die [Citation41]. Our previous study showed that carbon beams unregulated the ratio of Tap73/np73, and the combination of the radiation and diallyl disulphide induced higher ratio of Tap73/ΔNp73 in HeLa cells [Citation30]. Interestingly, administration with diallyl disulphide in mouse testis prevented radiation-induced Tap73/ΔNp73 expression and consequently down regulated p53 responsive genes [Citation29]. Although there is ample evident that the p73 is associated with apoptosis, the ratio between Tap73 and ΔNp73 involved in SF3b1-induced apoptosis in human cervical carcinoma cells remains unclear. In the study, our results showed that pladienolide B treatment obviously induced increase in the Tap73/ΔNp73 ratio, indicating that administration with pladienolide B induced apoptosis in p73 dependent manner.
Mitochondrial-mediated apoptosis is induced by p73 expression. Our previous work showed that Tap73/ΔNp73 plays an important role in regulating apoptosis via mitochondrial pathway [Citation29,Citation30]. Apoptosis is regulated by pro-apoptotic and anti-apoptotic effectors that involve a different pathway, such as Bcl-2 and caspase pathways which serve as important regulators of apoptosis pathways [Citation42]. In our previous work, the magnitude of radiation effects in mouse testis evoked by carbon ion irradiation was marked by morphology changes, significant rise in apoptotic cells, up-regulation the ratio of pro-apoptotic Tap73/anti-apoptotic ΔNp73, as well as alterations of crucial mediator of the mitochondrial pathway [Citation29]. To better understand how SF3b1 regulates the mitochondrial apoptosis pathway in human cervical carcinoma cells, the relative levels of pro-apoptosis versus anti-apoptosis Bcl-2 family, cytochrome c release and caspase-3 activation were determined in all treatments group at 24 h after pladienolide B treatment.
Noticeably, our data revealed that pladienolide B employing increased the Bax level and Bax/Bcl-2 ratio. This indicated that the modulation of the levels of anti-apoptotic and pro-apoptotic protein in human cervical carcinoma cells, mediated by pladienolide B probably caused mitochondria-dependent apoptosis. Additionally, we also found that the cytochrome c markedly increased in human cervical carcinoma cells, at 24 h after pladienolide B treatment. Previous research reported that efflux cytochrome c from mitochondria to the cytosol was essential for caspase-3 activation and activated downstream cell death pathway. Moreover, the research from our study found that the activation of caspase-3 was induced by Pladienolide B, and the cleavage of caspase-3 was found in .
Taken together, our results strongly suggest that inhibition of SF3b1 by pladienolide B evokes cycle arrest, apoptosis induction, and regulates the ratio of Tap73/FNp73 in human cervical carcinoma cells, suggesting that SF3b1 could be used as a potential candidate for cervical cancer therapy (. We propose that SF3b1 could be a promising molecular therapeutic target for cervical cancer and the splicing regulation of p73 might be the down stream signalling pathway of SF3b1 in p53-independent manner. However, this would require further investigation using tumor-bearing animal model and clinical trials to understand the effects of the SF3b1.
Disclosure statement
The authors report no conflict of interest in this work.
Additional information
Funding
References
- Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548.
- Chen Z, Zhang M, Qiao Y, et al. MicroRNA-1297 contributes to the progression of human cervical carcinoma through PTEN. Artif Cells Nanomed Biotechnol. 2018;46(sup 2):1120–1126.
- Javadi H, Lotfi AS, Hosseinkhani S, et al. The combinational effect of E6/E7 siRNA and anti-miR-182 on apoptosis induction in HPV16-positive cervical cells. Artif Cells Nanomed Biotechnol. 2018;46(sup2):727–736.
- Cong J, Liu R, Wang X, et al. MiR-634 decreases cell proliferation and induces apoptosis by targeting mTOR signaling pathway in cervical cancer cells. Artif Cells Nanomed Biotechnol. 2016;44:1694–1701.
- Dutertre M, Vagner S, Auboeuf D. Alternative splicing and breast cancer. RNA Biol. 2010;7:403–411.
- Urbanski LM, Leclair N, Anczukow O. Alternative-splicing defects in cancer: splicing regulators and their downstream targets, guiding the way to novel cancer therapeutics. Wiley Interdiscip Rev RNA. 2018;9:e1476.
- Neelamraju Y, Gonzalez-Perez A, Bhat-Nakshatri P, et al. Mutational landscape of RNA-binding proteins in human cancers. RNA Biol. 2018;15:115–129.
- Paschalis A, Sharp A, Welti JC, et al. Alternative splicing in prostate cancer. Nat Rev Clin Oncol. 2018;15:663–675.
- Kumar N, Chugh H, Tomar R, et al. Exploring the interplay between autoimmunity and cancer to find the target therapeutic hotspots. Artif Cells Nanomed Biotechnol. 2018;46:658–668.
- Wang ET, Sandberg R, Luo S, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476.
- De Conti L, Baralle M, Buratti E. Exon and intron definition in pre-mRNA splicing. Wiley Interdiscip Rev RNA. 2013;4:49–60.
- Bonnal S, Vigevani L, Valcarcel J. The spliceosome as a target of novel antitumour drugs. Nat Rev Drug Discov. 2012;11:847–859.
- Matera AG, Wang Z. A day in the life of the spliceosome. Nat Rev Mol Cell Biol. 2014;15:108–121.
- Vosseberg J, Snel B. Domestication of self-splicing introns during eukaryogenesis: the rise of the complex spliceosomal machinery. Biol Direct. 2017;12:30.
- Blencowe BJ. The relationship between alternative splicing and proteomic complexity. Trends Biochem Sci. 2017;42:407–408.
- van Alphen RJ, Wiemer EA, Burger H, et al. The spliceosome as target for anticancer treatment. Br J Cancer. 2009;100:228–232.
- Effenberger KA, Urabe VK, Prichard BE, et al. Interchangeable SF3B1 inhibitors interfere with pre-mRNA splicing at multiple stages. RNA. 2016;22:350–359.
- Kim Guisbert KS, Guisbert E. SF3B1 is a stress-sensitive splicing factor that regulates both HSF1 concentration and activity. PLoS One. 2017;12:e0176382.
- Scotti MM, Swanson MS. RNA mis-splicing in disease. Nat Rev Genet. 2016;17:19–32.
- Yokoi A, Kotake Y, Takahashi K, et al. Biological validation that SF3b is a target of the antitumor macrolide pladienolide. FEBS J. 2011;278:4870–4880.
- Wu G, Fan L, Edmondson MN, et al. Inhibition of SF3B1 by molecules targeting the spliceosome results in massive aberrant exon skipping. RNA. 2018;24:1056–1066.
- Sato M, Muguruma N, Nakagawa T, et al. High antitumor activity of pladienolide B and its derivative in gastric cancer. Cancer Sci. 2014;105:110–116.
- Effenberger KA, Anderson DD, Bray WM, et al. Coherence between cellular responses and in vitro splicing inhibition for the anti-tumor drug pladienolide B and its analogs. J Biol Chem. 2014;289:1938–1947.
- Wu G, Fan L, Edmonson MN, et al. Inhibition of SF3B1 by molecules targeting the spliceosome results in massive aberrant exon skipping. RNA. 2018;24:1056–1066.
- Mizui Y, Sakai T, Iwata M, et al. Pladienolides, new substances from culture of Streptomyces platensis Mer-11107. III. In vitro and in vivo antitumor activities. J Antibiot. 2004;57:188–196.
- Kotake Y, Sagane K, Owa T, et al. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat Chem Biol. 2007;3:570–575.
- Maciejewski JP, Padgett RA. Defects in spliceosomal machinery: a new pathway of leukaemogenesis. Br J Haematol. 2012;158:165–173.
- Khan I, Bahuguna A, Bhardwaj M, et al. Carvacrol nanoemulsion evokes cell cycle arrest, apoptosis induction and autophagy inhibition in doxorubicin resistant-A549 cell line. Artif Cells Nanomed Biotechnol. 2018;46(sup 1):664–675.
- Di CX, Han L, Zhang H, et al. Diallyl disulfide attenuated carbon ion irradiation-induced apoptosis in mouse testis through changing the ratio of Tap73/ΔNp73 via mitochondrial pathway. Sci Rep. 2015;5:16020.
- Di C, Sun C, Li H, et al. Diallyl disulfide enhances carbon ion beams-induced apoptotic cell death in cervical cancer cells through regulating Tap73/DeltaNp73. Cell Cycle. 2015;14:3725–3733.
- Di C, Yang L, Zhang H, et al. Mechanisms, function and clinical applications of DNp73. Cell Cycle. 2013;12:1861–1867.
- Kalsotra A, Cooper TA. Functional consequences of developmentally regulated alternative splicing. Nat Rev Genet. 2011;12:715–729.
- Xargay-Torrent S, Lopez-Guerra M, Rosich L, et al. The splicing modulator sudemycin induces a specific antitumor response and cooperates with ibrutinib in chronic lymphocytic leukemia. Oncotarget. 2015;6:22734–22749.
- Kramer A. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu Rev Biochem. 1996;65:367–409.
- Aouida M, Eid A, Mahfouz MM. CRISPR/Cas9-mediated target validation of the splicing inhibitor Pladienolide B. Biochim Open. 2016;3:72–75.
- Zhang J, Du J, Liu Q, et al. Down-regulation of STAT3 expression using vector-based RNA interference promotes apoptosis in Hepatocarcinoma cells. Artif Cells Nanomed Biotechnol. 2016;44:1201–1205.
- Huang Y, Hale J, Wang Y, et al. SF3B1 deficiency impairs human erythropoiesis via activation of p53 pathway: implications for understanding of ineffective erythropoiesis in MDS. J Hematol Oncol. 2018;11:19.
- Goh AM, Lane DP. How p53 wields the scales of fate: arrest or death?. Transcription. 2012;3:240–244.
- Jost CA, Marin MC, Kaelin WG. Jr., p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature. 1997;389:191–194.
- Muller M, Schilling T, Sayan AE, et al. TAp73/Delta Np73 influences apoptotic response, chemosensitivity and prognosis in hepatocellular carcinoma. Cell Death Differ. 2005;12:1564–1577.
- Lucena-Araujo AR, Kim HT, Thome C, et al. High ΔNp73/TAp73 ratio is associated with poor prognosis in acute promyelocytic leukemia. Blood. 2015;126:2302–2306.
- Sadat Shandiz SA, Shafiee Ardestani M, Shahbazzadeh D, et al. Novel imatinib-loaded silver nanoparticles for enhanced apoptosis of human breast cancer MCF-7 cells. Artif Cells Nanomed Biotechnol. 2017;45(6):1–10.