Abstract
Osteoblastic bone formation is important for maintaining the balance of bone turnover. However, the underlying mechanisms are still needed to be elucidated. Histamine H1 type receptor (H1R) is a major subtype of histamine membrane receptors family, which has displayed diverse biological functions in various tissues and cells. In the current study, we have identified a novel physiological function of H1R in regulating osteoblastic differentiation and mineralization of the MC3T3-E1 cells. We found that H1R is expressed in the MC3T3-E1 cells. Interestingly, H1R is up-regulated in the process of differentiation and mineralization of the MC3T3-E1cells induced by osteogenic medium (OM). Blockage of H1R using its specific antagonist Loratadine prevented differentiation and mineralization of the MC3T3-E1 cells by reducing ALP activity, bone matrix deposition, and the expressions of osteogenic marker genes including ALP, OCN, Osx, and type I collagen as well as the transcriptional factor RUNX-2, which is a central regulator of osteoblastogenesis. In contrast, we found that activation of H1R with Histamine exerts opposite actions by increasing the expressions of RUNX-2. Finally, we found that the effects of H1R in osteoblastic differentiation and mineralization are mediated by the AMPK/eNOS signaling. Based on these findings, we concluded that H1R might be an important therapeutic target for the treatment of skeletal disorders.
Introduction
Osteoporosis, one of the most common skeletal disorders characterized by loss of bone mineral density and impairment of bone strength, threatens the health of millions of ageing people all over the world [Citation1]. The pathological mechanisms of osteoporosis are complicated and a diversity of risk factors have been linked with the pathogenesis of this disease. An imbalance in bone homeostasis including dysregulated osteoblastic bone formation has been identified in patients with osteoporosis [Citation2]. The differentiation of mesenchymal stem cells and pre-osteoblasts to osteoblast and subsequent mineralization and maturation of osteoblast play a crucial role in bone formation [Citation3]. Osteoblastogenesis is a complex process and the underlying mechanisms are still unknown [Citation4]. Several signaling cascades have been found to mediate the differentiation, mineralization, and maturation of osteoblasts. Inducible factors initiated the phosphorylation of AMP-activated protein kinase (AMPK), which then activates eNOS and promotes nitric oxide (NO) generation in pre-osteoblasts, leading to the activation of runt-related transcription factor 2 (RUNX-2) [Citation5]. RUNX-2 acts as a central regulator of osteoblastogenesis [Citation6]. Activation of RUNX-2 is able to induce the differentiation matrix deposition and mineralization of osteoblastic cells, enhance osteoblast activity, and stimulate bone formation through driving the expression of alkaline phosphatase (ALP), osteocalcin (OCN), and type 1 collagen (Col-I), and non-collagenous proteins [Citation7]. Promoting osteoblastogenesis and increasing osteoblast activity using potential therapeutic agents have been considered as important therapeutic strategies for the treatment of osteoporosis [Citation8].
Histamine H1 type receptor (H1R), a typical member of the histamine membrane receptors family, mediates the effects of Histamine in allergic and inflammatory responses [Citation9]. Histamine is a low-molecular-weight amine, derived from a variety of cells and tissues, including the central nervous system and peripheral system. Pleiotropic physiological functions of Histamine have been reported in previous studies [Citation10]. Histamine regulates the biological activities of cell proliferation, polarization, differentiation, and migration through activating its membrane receptors, including H1R [Citation11]. Aberrant expression and activation of H1R have been involved in the pathophysiological processes of many diseases. Blockage of H1R using its specific antagonists has proved to be a successful approach to treating hypersensitivity allergic disease [Citation12]. A recent study reports that the H1R specific antagonist Loratadine exerts a protective action against oxidized LDL-induced endothelial inflammation by reducing ROS generation production of vascular adhesion molecules and pro-inflammatory cytokines [Citation13]. However, whether H1R has an influence on the development of osteoblastogenesis and the pathological progression hasn’t been reported before. In the current study, we examined the physiological function of H1R in differentiation and mineralization of pre-osteoblast MC3T3-E1 cells and explored the underlying molecular mechanisms.
Material and methods
Cell culture and treatment
MC3T3‐E1 cells (subclone 4) was purchased from ATCC, VA, USA. Cells were cultured in α‐minimum essential medium (α‐MEM) supplemented with 10% FBS and 1% penicillin-streptomycin. To induce osteoblastic differentiation, MC3T3‐E1 cells were stimulated with osteogenic differentiation medium (OM): α-MEM, 4 mM β-glycerophosphate, and 25 μg/ml ascorbic acid for various time durations (0, 3, 7, 14 days) [Citation14]. Cells were cultured in OM in the presence or absence of Loratadine (50 µM) or Histamine (0.5 µM) for 14 days.
Quantitative real-time polymerase chain reaction (real time PCR)
Total intracellular RNA was extracted from MC3T3-E1 cells with Trizol reagent (Invitrogen, USA). RNA was precipitated using isopropanol by centrifugation at 12,000 × g for 10 min at 4 °C. Concentration and purity of isolated RNA were evaluated by NanoDrop Spectrophotometers. 1 μg purified RNA was used for a reverse transcription PCR with OneStep RT-PCR Kit (Qiagen, USA) containing Oligo dTs, dNTPs, RNase-free water, and reverse transcriptase to produce cDNA. Synthesized cDNA was then applied for a real-time PCR using SYBR green master mix (Applied Biosystems, USA) on 7500 Real-Time PCR System with the following procedures: 1 cycle (95 °C for 10 min, 56 °C for 1 min, 72 °C for 30 s) and 44 cycles (95 °C for 30 s, 56 °C for 1 min, 72 °C for 30 s).
Western blot analysis
After necessary stimulation and treatment, MC3T3-E1 cells were harvested and lysed with cell lysis buffer containing 1 mM PMSF and phosphatase and protease inhibitor cocktails. Samples were centrifuged at 12,000 × g for 15 min at 4 °C. The supernatant was collected and the concentration was measured using a BCA protein assay kit from Beyotime Biotechnology, China. Equal amounts of sample from each group were fractioned using 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels. fractioned samples were transferred to polyvinylidene fluoride (PVDF) membranes and blocked with 5% skim milk for 2 h at room temperature (RT). After 3 washes with TBST, membranes were sequentially incubated with primary antibodies at 4 °C overnight and horseradish peroxidase (HRP)-conjugated secondary antibody for 2 h at RT. The immunoreactivity was examined using an enhanced chemiluminescent substrate [Citation15].
ALP activity
MC3T3-E1 cells were seeded in 96-well plates at a density of 2.5 × 104 cells/well. 12 h later, normal medium was replaced by the osteogenic medium (OM) in the presence or absence of Loratadine (50 µM) or Histamine (0.5 µM) for 14 days. To measure ALP activity, 0.1 M NaHCO3-Na2CO3 buffer (pH 10.0) containing 0.1% Triton X-100, 2 mM MgSO4, and 6 mM p-nitrophenol inorganic phosphate (PNPP) was added and incubated for 30 min at 37 °C. The OD value at 405 nm and 550 nm were recorded and the ratio (A405/A550) was used to calculate the relative ALP activity [Citation16].
Mineralization analysis
MC3T3-E1 cells were seeded in 96-well plates at a density of 2.5 × 104 cells/well. 12 h later, normal medium was replaced by the osteogenic medium (OM) in the presence or absence of Loratadine (50 µM) or Histamine (0.5 µM) for 14 days. Mineralization was assessed using the Alizarin red staining assay. Briefly, MC3T3-E1 cells were fixed with 70% ethanol for 10 min, followed by incubation with the Alizarin red solution (40 mM, pH 4.2) for 15 min at RT. After three gentle washes with PBS, staining was captured with a fluorescent microscope [Citation17].
Measurement of nitric oxide (NO) production
MC3T3-E1 cells were incubated with Histamine (0.5 µM) for 1 h, 3 h, and 6 h. After washing twice with PBS, cells were loaded with DAF FM DA at a final concentration of 10 µM for 30 min in phenol-red free DMEM [Citation18]. After three washes with PBS, green fluorescent signals were visualized using a fluorescent microscope. Integrated optical density (IOD) was calculated to index the NO level.
Statistical analysis
Experimental data were expressed as the mean ± SD. Statistical significance was analyzed by the use of ANOVA, followed by Tukey’s multiple comparison tests. A value <.05 was considered as significant.
Results
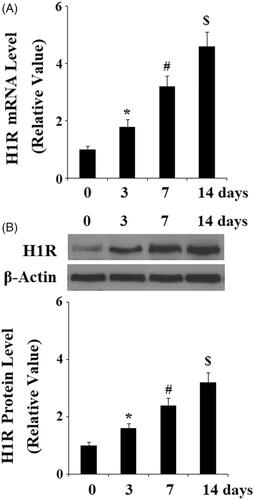
Firstly, we investigated the expression patterns of H1R in pre-osteoblast MC3T3-E1 cells. B16-F10 cells were used as an important positive control [Citation19]. RT-PCR and western blot results in indicate that H1R could be detected in both gene level and protein level in pre-osteoblast MC3T3-E1 cells. Notably, our results () demonstrate that H1R expression was significantly increased in response to culturing with osteogenic medium (OM) at both the gene and protein level in a time-dependent manner from 3 to 14 days. This finding implicates that H1R might participate in the osteoblastic differentiation process of MC3T3-E1 cells.
Figure 1. Histamine H1 receptor (H1R) is expressed in pre-osteoblast MC3T3-E1 cells. (A) RT-PCR results revealed that H1R is expressed in MC3T3-E1 cells at the gene level; (B) Western blot analysis revealed that H1R is expressed in MC3T3-E1 cells at the protein level. B16-F10 cells were used as an important positive control.

Figure 2. Expression of Histamine H1 receptor (H1R) was increased during osteoblast differentiation process of MC3T3-E1 cells. Pre-osteoblast MC3T3-E1 cells were cultured with osteogenic medium (OM) for various time durations (0, 3, 7 and 14 days). (A) Real-time PCR analysis of H1R; (B) Western blot analysis of H1R (*, #, $, P < .01 vs. previous column group).
We then set out to evaluate whether H1R is involved in osteoblastic differentiation by blocking H1R with its specific antagonist Loratadine. MC3T3-E1 cells that were cultured with osteogenic medium (OM) in the presence or absence of Loratadine at 50 µM for 14 days. Then the biomarkers of osteoblast differentiation were assessed. Alkaline phosphatase (ALP) is an enzyme, playing a key role in the process of osteoblast differentiation. ALP activity is increased in the early-stage osteoblast differentiation. Here, we found that the presence of Loratadine significantly abolished OM-induced increase in ALP activity (). Importantly, alizarin red assay indicates that Loratadine prevented osteogenic medium (OM)- induced mineralization of MC3T3-E1 cells (). Consistently, the presence of Loratadine reduced the expressions of osteogenic markers, including ALP, OCN, Osx, and Col-I (). The transcriptional factor RUNX-2 is a central regulator of osteoblast differentiation and mineralization by activating the transcription of osteogenic marker genes. OM- induced expression of RUNX-2 was inhibited by Loratadine at both the gene level () and the protein level ().
Figure 3. Antagonism of Histamine H1 receptor (H1R) using Loratadine prevented the differentiation and mineralization of MC3T3-E1 cells. MC3T3-E1 cells were cultured with osteogenic medium (OM) in the presence or absence of Loratadine at 50 µM for 14 days. (A) Loratadine reduced OM-induced increase in ALP activity; (B) Alizarin Red staining assay revealed that Loratadine prevented OM-induced mineralization of MC3T3-E1 cells; (C) Real-time PCR analysis demonstrated that Loratadine reduced the gene expression of ALP, OCN, Osx, and Col-I (*, #, P < .01 vs. previous column group).
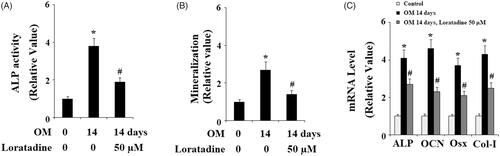
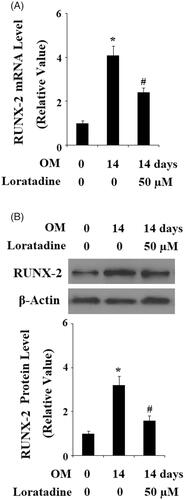
Figure 4. Antagonism of Histamine H1 receptor (H1R) using Loratadine reduced the expression of RUNX-2 during osteoblast differentiation process of MC3T3-E1 cells. Pre-osteoblast MC3T3-E1 cells were treated with osteogenic medium (OM) in the presence or absence of Loratadine (50 µM). (A) Real-time PCR analysis of RUNX-2; (B) Western blot analysis of RUNX-2 (*, #, P < .01 vs. previous column group).
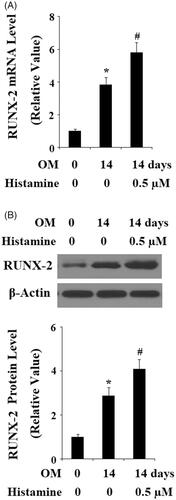
To further confirm the possible involvement of H1R in osteoblast differentiation and mineralization, Histamine was applied when pre-osteoblast MC3T3-E1 cells were stimulated with OM. As expected, we found that the presence of Histamine enhanced the increase of ALP activity (). Alizarin red staining in demonstrates that Histamine promoted OM-induced mineralization of MC3T3-E1 cells. Similarly, the presence of Histamine also promoted the increase in the expressions of ALP, OCN, Osx, and Col-I () as well as the transcriptional factor RUNX-2 (). These findings suggested that activation of H1R could promote osteoblast differentiation and mineralization. In contrast, antagonism of H1R prevented osteoblast differentiation and mineralization.
Figure 5. Histamine promoted the differentiation and mineralization of MC3T3-E1 cells. MC3T3-E1 cells were cultured with osteogenic medium (OM) in the presence or absence of Histamine at 0.5 µM for 14 days. (A). Histamine promoted OM-induced increase in ALP activity; (B). Alizarin Red staining assay revealed that Histamine promoted OM-induced mineralization of MC3T3-E1 cells; (C). Real-time PCR analysis demonstrated that Histamine promoted the gene expression of ALP, OCN, Osx, and Col-I (*, #, P < .01 vs. previous column group).
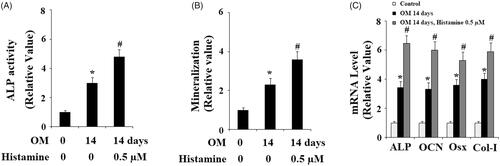
Figure 6. Histamine promoted the expression of RUNX-2 during osteoblast differentiation process of MC3T3-E1 cells. Pre-osteoblast MC3T3-E1 cells were treated with osteogenic medium (OM) in the presence or absence of Histamine at 0.5 µM. (A). Real time PCR analysis of RUNX-2; (B). Western blot analysis of RUNX-2 (*, #, P < .01 vs. previous column group).
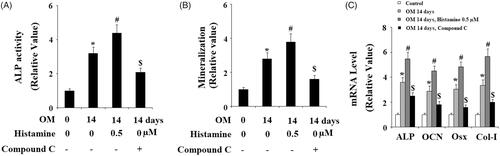
Activation of eNOS by its phosphorylation and subsequent generation of NO, mediated by AMP-activated protein kinase (AMPK) are essential for osteogenic differentiation [Citation20]. Hence, we examined whether activation of H1R had an impact on the eNOS/NO signaling. Results in indicate that treatment with Histamine (0.5 µM) significantly increased the level of p-eNOS in a time-dependent manner. However, the total level of eNOS remained consistent. Correspondingly, DAF-FM DA staining assay revealed that Histamine significantly increased the production NO in MC3T3-E1 cells (). Also, we found that Histamine treatment increased the level of p-AMPK, but not the total AMPK (). To further confirm the involvement of AMPK, its specific inhibitor compound C was used. MC3T3-E1 cells were cultured in OM in the absence or presence of Histamine (0.5 μM) or compound C for 14 days. We found that compound C treatment abolished the effects of Histamine in ALP activity (), matrix mineralization (), and expressions of osteoblastic differentiation marker genes (). These findings implied that the stimulatory effects of H1R activation in osteogenic differentiation and mineralization are mediated by AMPK.
Figure 7. Activation of H1R by Histamine promoted the phosphorylation of eNOS and the production of nitric oxide (NO). (A) Pre-osteoblast MC3T3-E1 cells were treated with Histamine (0.5 µM) for 15 min, 30 min, and 1 h, phosphorylated and total levels of eNOS were determined by western blot analysis; (B) Pre-osteoblast MC3T3-E1 cells were treated with Histamine (0.5 µM) for 1 h, 3 h, and 6 h, production of NO was determined by DAF FM DA; (C) Pre-osteoblast MC3T3-E1 cells were treated with Histamine (0.5 μM) for 15 min, 30 min, and 1 h, phosphorylated and total levels of AMPK were determined by western blot analysis (*, #, $, P < .01 vs. previous column group).
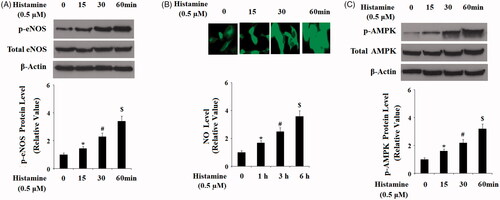
Figure 8. The effects of H1R activation by Histamine on the differentiation and mineralization of MC3T3-E1 cells were suppressed by the specific AMPK inhibitor compound C. MC3T3-E1 cells were maintained in osteogenic medium in the absence or presence of Histamine (0.5 μM) or compound C for 14 days. (A) ALP activity; (B) Quantification of Alizarin Red S staining; (C) Gene expression of ALP, OCN, Osx, and Col-I (*, #, $, P < .01 vs. previous column group).
Discussion
Dysregulated bone remodeling has been involved in several skeletal disorders, including osteoporosis [Citation21]. Normal bone turnover homeostasis is maintained by the balance of bone formation and bone resorption [Citation22]. Osteoblastic bone formation plays a crucial role in normal bone function. Amount of osteoblasts could be increased by differentiating from mesenchymal stem cells (MSCs) and pre-osteoblasts, which is accompanied by the formation of bone matrix proteins and bone mineralization [Citation23]. MC3T3-E1 cells are a pre-osteoblast cell line and possess the ability to differentiate into osteoblasts, which have been widely used for an ideal model for the differentiation, matrix deposition, and mineralization of osteoblastic cells [Citation24]. Boosting osteogenic differentiation has been considered as a critical therapeutic approach for the treatment of skeletal diseases. In the current study, we aimed to investigate whether H1R affects osteoblastic differentiation and mineralization. Firstly, we found that H1R is expressed in pre-osteoblast MC3T3-E1 cells and the expression of which was increased during the differentiation and mineralization process of MC3T3-E1 cells induced by osteoblastogenic factors. Secondly, we found that antagonism of H1R using its specific antagonist Loratadine abolished OM-induced osteoblastogenesis by reducing ALP activity, bone matrix deposition, and the expressions of osteogenic marker genes, such as ALP, OCN, Osx, and Col-I. Importantly, we found that the presence of Loratadine inhibited the expression of the transcriptional factor RUNX-2, a central player in osteoblastogenesis. In contrast, activation of H1R using Histamine had an opposite effect. Mechanistically, we found that the eNOS/AMPK signaling might be involved in the effects of H1R in osteogenic formation.
H1R is one of the subtypes of histamine receptors, which belong to the G-protein-coupled receptor family. H1R is expressed in a diversity of tissues and cells and exerts various physiological functions in many chronic disorders [Citation25]. However, to the best of our knowledge, this is the first time to report the roles of H1R in osteoblastogenesis. Consistently, a recent study has demonstrated that antagonism of H2R, another member of the histamine receptors, with its specific antagonist famotidine inhibits osteogenic differentiation in posterior longitudinal ligament (OPLL)- derived mesenchymal stem cells (MSCs) by reducing the expressions of RUNX2, OCN, and bone morphogenetic protein 2 (BMP2) [Citation26]. RUNX-2 is a multifunctional transcription factor playing a critical role in regulating osteoblast differentiation and chondrocyte maturation by driving the downstream gene expressions, including type I collagen [Citation27]. RUNX-2 has become an important therapeutic target for the treatment of several skeletal diseases [Citation28]. The phosphorylation of eNOS induced by activation of the kinase AMPK and the subsequent production of NO has been shown to play pivotal roles in osteoblast differentiation and function [Citation29]. In the current study, activation of H1R using Histamine was reported to obviously increase eNOS phosphorylation and NO generation in osteoblastic MC3T3-E1 cells. Importantly, the effects of Histamine and H1R in osteoblastic differentiation and mineralization were abolished by the AMPK inhibitor compound C, suggesting the involvement of AMPK. Indeed, histamine has been reported to induce eNOS activation in endothelial cells through a LKB1-AMPK dependent pathway [Citation30]. AMPK plays a wide range of roles in bone physiology [Citation31]. The effects of H1R in AMPK activation suggest that it might have a wide range of biological roles in bone function.
Taken together, we have identified a novel function of H1R in regulating osteoblastogenesis. Our results suggest that H1R might become a potential therapeutic target for bone-loss related diseases.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Akkawi I, Zmerly H. Osteoporosis: current concepts. Joints. 2018;6:122–127.
- Weitzmann MN, Ofotokun I. Physiological and pathophysiological bone turnover - role of the immune system. Nat Rev Endocrinol. 2016;12:518–532.
- Marie PJ. Osteoblast dysfunctions in bone diseases: from cellular and molecular mechanisms to therapeutic strategies. Cell Mol Life Sci. 2015;72:1347–1361.
- van Wijnen AJ, van de Peppel J, van Leeuwen JP, et al. MicroRNA functions in osteogenesis and dysfunctions in osteoporosis. Curr Osteoporos Rep. 2013;11:72–82.
- Wang C, Lin K, Chang J, et al. Osteogenesis and angiogenesis induced by porous β-CaSiO(3)/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials. 2013;34:64–77.
- Zhong X, Xiu LL, Wei GH, et al. Bezafibrate enhances proliferation and differentiation of osteoblastic MC3T3-E1 cells via AMPK and eNOS activation. Acta Pharmacol Sin. 2011;32:591–600.
- Komori T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol. 2018;149:313–323.
- (a) Marie PJ, Kassem M. Osteoblasts in osteoporosis: past emerging and future anabolic targets. Eur J Endocrinol. 2011;165:1–10.; (b) Canalis E. New treatment modalities in osteoporosis. Endocr Pract. 2010;16:855–863.
- Leurs R, Church MK, Taglialatela M. H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects. Clin Exp Allergy. 2002;32:489–498.
- Akdis CA, Jutel M, Akdis M. Regulatory effects of histamine and histamine receptor expression in human allergic immune responses. Chem Immunol Allergy. 2008;94:67–82.
- Veglia E, Pini A, Moggio A, et al. Histamine type 1-receptor activation by low dose of histamine undermines human glomerular slit diaphragm integrity. Pharmacol Res. 2016;114:27–38.
- Okamoto T, Iwata S, Ohnuma K, et al. Histamine H1-receptor antagonists with immunomodulating activities: potential use for modulating T helper type 1 (Th1)/Th2 cytokine imbalance and inflammatory responses in allergic diseases. Clin Exp Immunol. 2009;157:27–34.
- Zhou Y, Gao C, Wang H, et al. Histamine H1 type receptor antagonist loratadine ameliorates oxidized LDL induced endothelial dysfunction. Biomed Pharmacother. 2018;106:1448–1453.
- Koç A, Elçin AE, Elçin YM. Ectopic osteogenic tissue formation by MC3T3-E1 cell-laden chitosan/hydroxyapatite composite scaffold. Artif Cells Nanomed Biotechnol. 2016;44:1440–1447.
- Sheng B, Wang X, Su B, et al. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem. 2012;120:419–429.
- Bhattarai G, Lee YH, Lee NH, et al. PPARγ delivered by Ch-GNPs onto titanium surfaces inhibits implant-induced inflammation and induces bone mineralization of MC-3T3E1 osteoblast-like cells. Clin Oral Impl Res. 2013;24:1101–1109.
- Kim JE, Takanche JS, Kim JS, et al. Phelligridin D-loaded oral nanotube titanium implant enhances osseointegration and prevents osteolysis in rat mandible. Artif Cells Nanomed Biotechnol. 2018;12:1–11.
- Sheng B, Gong K, Niu Y, et al. Inhibition of gamma-secretase activity reduces Abeta production, reduces oxidative stress, increases mitochondrial activity and leads to reduced vulnerability to apoptosis: Implications for the treatment of Alzheimer’s disease. Free Radic Biol Med. 2009;46:1362–1375.
- Pos Z, Wiener Z, Pocza P, et al. Histamine suppresses fibulin-5 and insulin-like growth factor-II receptor expression in melanoma. Cancer Res. 2008;68:1997–2005.
- Barbagallo I, Vanella A, Peterson SJ, et al. Overexpression of heme oxygenase-1 increases human osteoblast stem cell differentiation. J Bone Miner Metab. 2010;28:276–288.
- Li Z, Müller R, Ruffoni D. Bone remodeling and mechanobiology around implants: Insights from small animal imageing. J Orthop Res. 2018;36:584–593.
- Weitzmann MN. Bone and the immune System. Toxicol Pathol. 2017;45:911–924.
- Kim HM, Kim DH, Han HJ, et al. Ginsenoside Re promotes osteoblast differentiation in mouse osteoblast precursor MC3T3-E1 cells and a Zebrafish model. Molecules 2016;22:E42.
- Kodama H-a, Amagai Y, Sudo H, et al. Establishment of a clonal osteogenic cell line from newborn mouse calvaria. Jpn J Oral Biol. 1981;23:899–901.
- Vena GA, Cassano N, Buquicchio R, et al. Antiinflammatory effects of H1-antihistamines: clinical and immunological relevance. CPD. 2008;14:2902–2911.
- Liu X, Kumagai G, Wada K, et al. Suppression of osteogenic differentiation in mesenchymal stem cells from patients with ossification of the posterior longitudinal ligament by a histamine-2-receptor antagonist. Eur J Pharmacol. 2017;810:156–162.
- Valenti MT, Dalle Carbonare L, Mottes M. Osteogenic differentiation in healthy and pathological conditions. IJMS. 2016;18:41.
- Komori T. Roles of Runx2 in skeletal development. Adv Exp Med Biol. 2017;962:83–93.
- Fan X, Rahnert JA, Murphy TC, et al. Response to mechanical strain in an immortalized pre-osteoblast cell is dependent on ERK1/2. J Cell Physiol. 2006;207:454–460.
- Thors B, Halldórsson H, Thorgeirsson G. eNOS activation mediated by AMPK after stimulation of endothelial cells with histamine or thrombin is dependent on LKB1. Biochim Biophys Acta. 2011;1813:322–331.
- Jeyabalan J, Shah M, Viollet B, et al. AMP-activated protein kinase pathway and bone metabolism. J Endocrinol. 2012;212:277–290.
