Abstract
Bacteria play a pivotal role in the pathological initiation and progression of pulpitis. Lipopolysaccharide (LPS) is recognized as a major component of the outer wall of Gram-negative bacteria. Saxagliptin, a potent inhibitor of dipeptidyl peptidase-4 (DPP-4), has been licensed for the treatment of type 2 diabetes. In this study, we aimed to evaluate the protective effects of saxagliptin against LPS-induced intracellular insults in human dental pulp cells (HDPCs). We found that DPP-4 is expressed in HDPCs. Interestingly, the expression of DPP-4 was increased in response to LPS treatment. We also found that saxagliptin ameliorated LPS-induced production of ROS and reduction of glutathione (GSH). Additionally, saxagliptin prevented LPS-induced mitochondrial dysfunction by increasing the levels of mitochondrial membrane potential (MMP) and the production of adenosine triphosphate (ATP). Importantly, saxagliptin ameliorated LPS-induced reduction of cell viability and lactate dehydrogenase (LDH) release. Our results indicate that saxagliptin significantly inhibited LPS-induced expression and secretions of tumour necrosis factor alpha (TNF-α), interleukin (IL)-1β and IL-6 in HDPCs. Mechanistically, we found that saxagliptin inhibited the phosphorylation of p38 and the activation of NF-κB. Our findings suggest that saxagliptin might have a potential therapeutic capacity for the treatment of pulpitis through mitigating inflammatory signalling in dental pulp cells.
Introduction
Pulpitis (tooth pulp inflammation) is one of the most common oral diseases affecting millions of people worldwide. Oral bacterial infection has been extensively associated with the pathogenesis of pulpitis [Citation1]. A variety of components and products of bacteria have been shown to invade the dentin and root canal, including lipopolysaccharide (LPS) [Citation2]. LPS is also known as endotoxin, which has been identified as the major component of the outer membrane of Gram-negative bacteria [Citation3]. LPS acts as an important inducer of pulpitis and plays a pivotal role in many intracellular responses to pulpal infection [Citation4]. LPS can induce the expression and secretions of various proinflammatory cytokines and chemokines, including tumour necrosis factor alpha (TNF-α), interleukin (IL)-1β and IL-8 in human dental pulp cells (HDPCs) [Citation5] through activating the expression of toll-like receptor 4 (TLR4) [Citation6]. Excessive production of these cytokines facilitates the pathological progression of pulpitis [Citation7]. Also, it has been reported that LPS treatment reduced cell survival rate of HDPCs [Citation8]. LPS exposure leads to the initiation of many intracellular signalling pathways. For example, activation of the mitogen-activated protein kinase (MAPK) p38 plays an important role in mediating the production of pro-inflammatory cytokines in response to LPS exposure [Citation9]. Importantly, LPS stimulation induces the activation of the NF-κB signalling pathway, which has acted as a central regulator in inflammation reactions [Citation10]. Blockage of LPS-induced inflammation signalling and cellular insults has become an important strategy for the treatment of pulpitis.
Saxagliptin, a potent and selective dipeptidyl peptidase-4 (DPP-4) inhibitor, has been licensed for the treatment of type 2 diabetes by reducing blood glucose through increasing the levels of the incretin hormones glucagonlike peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) [Citation11,Citation12]. Previous studies have demonstrated that saxagliptin possesses multiple pharmacological abilities besides its anti-hyperglycaemia capacity. For example, it has been recently reported that administration of saxagliptin is able to improve endothelial dysfunction in early diabetes before macrovascular complications appear [Citation13]. Blockage of DPP-4 using saxagliptin ameliorates angiotensin II (Ang II)-induced cardiac diastolic dysfunction, fibrosis and inflammation through causing unique shifts in CD11c-expressing leukocytes and CD8+ lymphocytes [Citation14]. Importantly, saxagliptin has displayed anti-inflammatory capacities in various types of tissues and cells. Inhibition of DPP-4 using saxagliptin delays the progression of diabetic nephropathy by preventing activation of the NLRP3 inflammasome and reducing the production of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6 and IL-18 [Citation15]. Poorly controlled diabetes mellitus has been associated with inflammatory and structural components of dental pulp [Citation16]. In this study, we aimed to investigate whether saxagliptin could protect HDPCs against LPS-induced insults.
Materials and methods
Cell culture and treatment
HDPCs were obtained from ATCC, Manassas, VA. Cells were cultured in DMEM/α-MEM supplemented with 10% FBS in 5% CO2 incubator at 37 °C. Cells were treated with 50, 100, 150 ng/ml LPS for 24 h to determine the expression of DPP-4. Additionally, cells were stimulated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 2, 24 or 48 h based on different experimental conditions.
Real time PCR analysis
Total intracellular RNA was isolated from HDPCs using Trizol reagent (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. An amount of 1 μg isolated RNA was used to create first-strand cDNA with oligo-dT priming using a rapid iscript reverse transcription (RT) Kit (Bio-Rad, Hercules, CA). An aliquot of 2 μl synthesized cDNA was used for quantitative real time PCR analyses on an ABI Prism 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA) with a SYBR Green mater mix kit (Applied Biosystems). Relative expression of the target gene was normalized relative to the level of the control (GAPDH) using 2−ΔΔCt [Citation17].
Western blot analysis
Proteins were extracted from HDPCs with cell lysis buffer (Cell Signaling Technology, Danvers, MA) supplemented with protease cocktail inhibitors. Protein concentration was determined by the BCA method. An amount of 20 μg proteins was loaded and run on a 10% sodium dodecyl sulphate-polyacrylamide (SDS-PAGE) gel. Separated proteins were transferred to PVDF membranes. Non-specific sites were blocked with 5% non-fat milk for 2 h at room temperature (RT). The membranes were then incubated with primary antibodies overnight at 4 °C. After washing three times with TBST, membranes were probed with secondary antibody coupled to horseradish peroxidase (HRP) (Santa Cruz Biotechnology, Inc., Dallas, TX). Blots were visualized with an enhanced chemiluminescent detection kit (Sigma-Aldrich, St. Louis, MO). β-actin was used as a positive control.
Enzyme-linked immunosorbent assay (ELISA)
HDPCs were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 48 h. Secretions of TNF-α, IL-1β and IL-8 in the culture medium were determined by ELISA kits from R&D Systems Inc., Minneapolis, MN according to manufacturer’s instructions.
Reactive oxygen species (ROS) assay
Intracellular reactive oxygen species (ROS) in HDPCs was evaluated using 2′,7′-dichlorofluorescin diacetate dye (DCFH-DA). Cells were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 24 h. Briefly, cells were loaded with 10 μM and incubated at 37 °C for 30 min. After three washes, fluorescence was detected using a fluorescent microscope [Citation18]. The fluorescent density of images was quantified using Image J software.
Determination of reduced glutathione (GSH)
Intracellular reduced glutathione (GSH) in HDPCs was determined using a fluorometric assay. Briefly, cells were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 24 h. After that, cells were collected and centrifuged at 500×g for 5 min. Precipitation was resuspended in ice cold 5% meta-phosphoric acid (MPA), followed by a brief sonication. Samples were subjected to another centrifugation at 14000×g for 5 min. Supernatant was collected in order to mix with OPAME (Sigma-Aldrich) in methanol and borate buffer. After incubation for 15 min at RT, fluorescent signals were read at 350 nm excitation and 420 nm emission.
TMRM staining
Levels of mitochondrial transmembrane potential in HDPCs were assessed by TMRM staining. HDPCs cells were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 48 h, followed by being loaded with 5 μM TMRM and incubated for 30 min at 37 °C. Fluorescent signals were captured using a fluorescence microscope (Leica, Germany).
MTT assay
Cell viability of HDPCs was assayed using the MTT method. HDPCs were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 48 h. After that, cells were loaded with 1 mg/ml MTT for 4 h in serum-free medium. The reaction product was dissolved by dimethyl sulphoxide (DMSO). Absorbance recorded at 560 nm with a microplate spectrophotometer was used to index cell viability.
Lactate dehydrogenase (LDH) release
Leakage of LDH into the culture medium was assessed with a cytotoxicity detection kit (Roche Applied Science, Penzberg, Germany). HDPCs were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 48 h. An aliquot of 50 µl of sample medium was mixed with 50 µl reaction reagents and incubated at room temperature (RT) for 30 min. Reaction was then stopped with 50 μl stop buffer. Absorbance was determined at 490 nm and used to represent the leakage of LDH.
Measurement of adenosine triphosphate (ATP)
Intracellular levels of adenosine triphosphate (ATP) were assayed using an ATP Bioluminescence assay kit (Thermo Fisher Scientific, Waltham, MA). HDPCs were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 48 h. Cells were then lysed with a lysis buffer, followed by a brief centrifugation at 10,000×g for 10 min at 4 °C. Equal volume (100 μl) of supernatant and luciferin/luciferase reagent was mixed together to catalyse the light production from ATP and luciferin. Light output was immediately measured using a microplate luminometer [Citation19].
NF-κB promoter assay
Transcriptional activity of NF-κB was assessed using a NF-κB promoter assay. NF-κB binding site-containing luciferase vector (Thermo Fisher Scientific) and a firefly luciferase promoter were transfected into cells using Lipofectamine 2000 reagents (Invitrogen, Carlsbad, CA). Twenty-four-hour post-transfection, cells were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 24 h. Cells were then lysed and the dual activity of renila and firefly luciferase was measured using the dual-luciferase reporter kit (Promega, Madison, WI).
Statistical analysis
Experimental data are presented as the mean ± standard deviation (SD). Comparisons were analysed using analysis of variance (ANOVA). p Values less than .05 were considered statistically significant.
Results
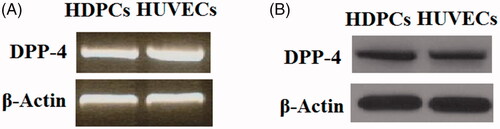
First, we examined whether DPP-4 is expressed in HDPCs. A previous study has shown that DPP-4 is expressed in human umbilical vein endothelial cells (HUVECs) [Citation20], which were used as a positive control in this study. Both reverse transcription PCR (RT-PCR) analysis () and western blot analysis () results indicate that DPP-4 could be detected in HDPCs at both the gene level and protein level, respectively.
Figure 1. DPP-4 is expressed in human dental pulp cells. Human umbilical vein endothelial cells (HUVECs) were used as a positive control. (A). Reverse transcription PCR (RT-PCR) analysis of DPP-4; (B). Western blot analysis of DPP-4.
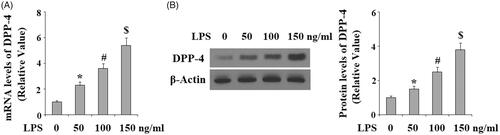
LPS has been reported to be a critical risk factor mediating bacteria-induced insults in HDPCs. Hence, we evaluated the effects of LPS in DPP-4 expression. HDPCs were treated with LPS at the concentrations of 50, 100 and 150 ng/ml for 24 h. Both real time PCR analysis () and western blot analysis () results indicated that the expression of DPP-4 was increased in response to LPS treatment in a dose-dependent manner at both the mRNA levels and protein levels, suggesting that DPP-4 might play a role in mediating LPS-induced damage in HDPCs.
Figure 2. Lipopolysaccharide (LPS) treatment increased the expression of DPP-4 in human dental pulp cells. Human dental pulp cells were treated with LPS at the concentrations of 50, 100, 150 ng/ml for 24 h. (A). mRNA levels of DPP-4 were determined by real time PCR analysis; (B). Protein levels of DPP-4 were determined by western blot analysis (*, #, $ p < .01 vs. previous column group).
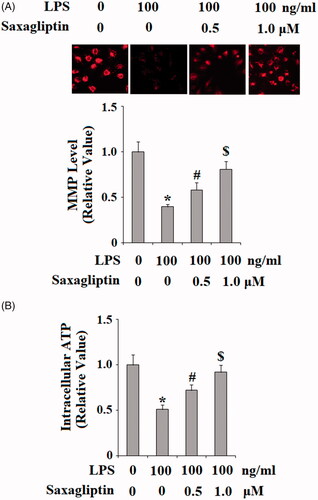
HDPCs were then treated with 100 ng/ml LPS in the presence or absence of saxagliptin, a potent DPP-4 inhibitor; at the concentrations of 500 nM and 1 μM for 24 h. Patterns of oxidative stress were examined in HDPCs by measuring intracellular levels of ROS and GSH. DCFH-DA staining results in indicate that LPS exposure induced a significant increase in ROS production, which was prevented by saxagliptin in a dose-dependent manner. Reduced GSH is an important antioxidant in various tissues and cells type. Here, we found that LPS exposure significantly reduced intracellular level of reduced GSH. However, the presence of saxagliptin restored the intracellular levels of GSH. Mitochondrial function was then assessed by measuring mitochondrial membrane potential (MMP) and intracellular ATP. TMRM staining in shows that LPS exposure significantly reduced the level of MMP, which was ameliorated by the presence of saxagliptin in a dose-dependent manner. Similarly, results in demonstrate that saxagliptin attenuated LPS-induced ATP depletion. These findings indicate that saxagliptin plays a pivotal role in mitigating LPS-induced oxidative stress and mitochondrial dysfunction in HDPCs.
Figure 3. Saxagliptin ameliorated LPS-induced oxidative stress in human dental pulp cells. Human dental pulp cells were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 24 h. (A). Production of reactive oxygen species (ROS); (B). Intracellular levels of GSH (*, #, $ p < .01 vs. previous column group).
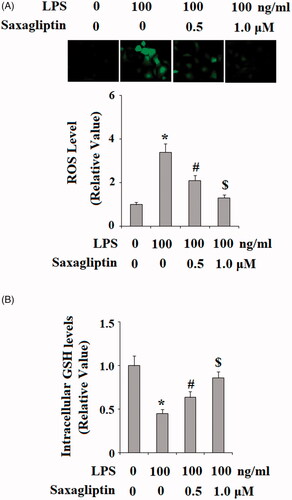
Figure 4. Saxagliptin ameliorated LPS-induced mitochondrial dysfunction in human dental pulp cells. Human dental pulp cells were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 48 h. (A). MMP was determined by TMRM; (B). Intracellular ATP (*, #, $ p < .01 vs. previous column group).
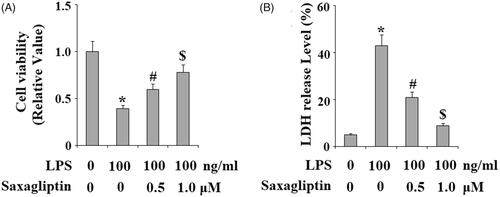
We then evaluated the effects of saxagliptin in LPS-induced cell death. Cell viability of HDPCs was determined using the MTT assay. Results in display that treatment with saxagliptin ameliorated LPS-induced reduction of cell viability in HDPCs. Consistently, LDH assay demonstrates that HDPCs exhibited obviously augmented LDH release into the medium in response to LPS treatment; however, the presence of saxagliptin reduced LDH release in a dose-dependent manner.
Figure 5. Saxagliptin prevented LPS-induced cell death of human dental pulp cells. Human dental pulp cells were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 48 h. (A). Cell viability was determined by MTT assay; (B). LDH release (*, #, $ p < .01 vs. previous column group).
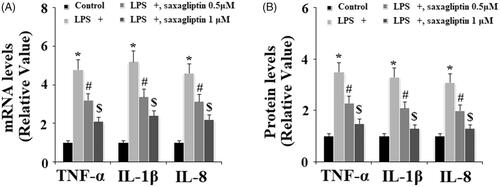
Excessive production of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-8 plays crucial roles in LPS-induced inflammation in LPS. The expression of TNF-α, IL-1β and IL-8 at the mRNA level and the secretion of TNF-α, IL-1β and IL-8 at the protein level into cell culture medium were determined by real time PCR and ELISA methods, respectively. Results indicate that the presence of saxagliptin inhibited the expression and secretion of TNF-α, IL-1β and IL-8 at the mRNA levels () and the protein levels (), respectively.
Figure 6. Saxagliptin ameliorated LPS-induced pro-inflammatory cytokines in human dental pulp cells. Human dental pulp cells were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 48 h. (A). TNF-α, IL-1β, IL-8 at the mRNA levels were determined by real time PCR analysis; (B). TNF-α, IL-1β, IL-8 at the protein levels were determined by western blot analysis (*, #, $ p < .01 vs. previous column group).
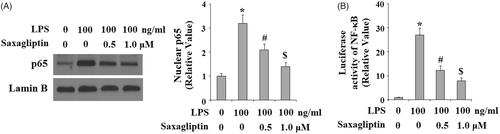
A plenty of intracellular signalling pathways are reported to participate in the pathological progression of pulpitis, including MAPK p38/NF-κB signalling pathway. Here, we found that LPS exposure significantly increased phosphorylation of p38, which was inhibited by saxagliptin in a dose-dependent manner (. Importantly, LPS exposure induced nuclear translocation of NF-κB p65, which was prevented by saxagliptin (). Transcriptional activity of NF-κB was evaluated with NF-κB luciferase activity analysis. Results in indicate that LPS treatment remarkably increased NF-κB luciferase activity, which was obviously mitigated by saxagliptin treatment, implying a potential involvement of the NF-κB signalling pathway.
Figure 7. Saxagliptin inhibited LPS-induced activation of p38 in human dental pulp cells. Human dental pulp cells were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 2 h. Phosphorylated and total levels of p38 were determined by western blot analysis (*, #, $ p < .01 vs. previous column group).
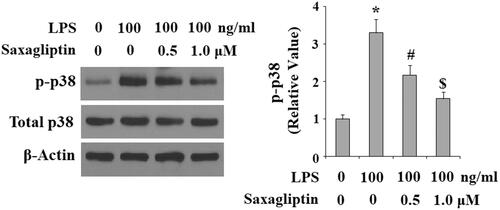
Figure 8. Saxagliptin inhibited LPS-induced activation of NF-κB. Human dental pulp cells were treated with 100 ng/ml LPS in the presence or absence of saxagliptin (500 nM, 1 μM) for 24 h. (A). Nuclear translocation of p65; (B). Luciferase activity of NF-κB (*, #, $ p < .01 vs. previous column group).
Discussion
Bacterial penetration has been reported as an important risk factor of pulp inflammation, necrosis and periapical pathosis [Citation21]. LPS, the main component of the outer membrane of Gram-negative bacteria, plays a key role in bacteria-induced dental pulp inflammation and pulpitis [Citation22]. Saxagliptin is an important DPP-4 inhibitor and an anti-diabetic agent approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of type 2 diabetes [Citation23]. The effects of saxagliptin in pulpitis and HDPCs have not been reported before. In this study, we report, for the first time, that treatment with saxagliptin protected HDPCs against LPS-induced insults. First, we found that DPP-4 is expressed in HDPCs and the expression of which is upregulated in response to LPS treatment. Second, our results indicate that saxagliptin treatment ameliorated LPS induced oxidative stress and mitochondrial dysfunction. Third, our findings demonstrate that saxagliptin treatment attenuated LPS-induced cell death of HDPCs. Fourth, we show that saxagliptin treatment reduced LPS-induced production of TNF-α, IL-1β and IL-8. Mechanistically, we found that saxagliptin treatment inhibited the activation of p38 and NF-κB signalling pathway.
Recent studies have reported that saxagliptin possesses pleiotropic effects beyond its anti-diabetic influence in several tissues and cell types, including a diversity of anti-inflammatory capacities [Citation24]. Saxagliptin treatment resulted in reduced CD40 expression in inflammatory monocytes and macrophages, which are implicated in pathological development of atherosclerosis [Citation25]. Inhibition of DPP-4 with saxagliptin is able to reduce blood pressure and attenuate inflammatory reaction in hypertensive rats [Citation26]. Excessive production of pro-inflammatory cytokines including TNF-α, IL-1β and IL-8 plays a pivotal role in the pathogenesis of inflammatory conditions [Citation27]. LPS exposure leads to increased expression of several inflammatory cytokines, including TNF-α, IL-1β and IL-8 in HDPCs [Citation28]. Inflammatory effects of LPS are mediated by several intracellular signalling pathways, including the MAPK p38 and the NF-κB signalling pathway [Citation29]. In this study, our results demonstrated that saxagliptin reduced the expressions and secretions of TNF-α, IL-1β and IL-8 induced by LPS in HDPCs through inhibiting the p38/NF-κB activation. The inhibitory effects of saxagliptin on these pathways indicate that saxagliptin possesses a potent ability to suppress inflammatory mediators and cytokines. The transcriptional factor NF-κB has been recognized as a key mediator of inflammation and the immune response [Citation30]. Oxidative stress- induced activation of p38 plays a pivotal role in modulating NF-κB transcriptional activity through promoting nuclear translocation of NF-κB p65 [Citation31]. Activation of p38/NF-κB has been shown to mediate dental pulp inflammation in HDPCs. Blockage of the NF-κB signalling pathway has been considered as an important therapeutic strategy for the treatment of pulpitis [Citation32]. Consistently, a recent study demonstrated that saxagliptin limits renal hypertrophy, transforming growth factor β-related tubulointerstitial fibrosis and NF-κB p65-mediated macrophage infiltration [Citation33]. As NF-κB regulates a wide spectrum of inflammation reactions in various diseases [Citation34], the inhibitory effect of saxagliptin in NF-κB activation implicates that it might possess a wide range of anti-inflammatory effects in diverse diseases.
Taken together, our findings indicate that saxagliptin exerts important protective actions in HDPCs against LPS-induced inflammation and damage. We concluded that saxagliptin might become a potential candidate as a pulp capping agent in vital pulp therapy.
Disclosure statement
Authors have declared that they had none conflict of interest need to be disclosed.
References
- Rechenberg DK, Galicia JC, Peters OA. Biological markers for pulpal inflammation: a systematic review. PLoS One. 2016;11:e0167289.
- Hahn CL, Liewehr FR. Relationships between caries bacteria, host responses, and clinical signs and symptoms of pulpitis. J Endod. 2007;33:213–219.
- Hicks G, Jia Z. Structural basis for the lipopolysaccharide export activity of the bacterial lipopolysaccharide transport system. Int J Mol Sci. 2018;19:2680.
- Chung MK, Lee J, Duraes G, et al. Lipopolysaccharide-induced pulpitis up-regulates TRPV1 in trigeminal ganglia. J Dent Res. 2011;90:1103–1107.
- Jung JY, Woo SM, Kim WJ, et al. Simvastatin inhibits the expression of inflammatory cytokines and cell adhesion molecules induced by LPS in human dental pulp cells. Int Endod J. 2017;50:377–386.
- Borio A, Holgado A, Garate JA, et al. Disaccharide-based anionic amphiphiles as potent inhibitors of lipopolysaccharide-induced inflammation. Chem Med Chem. 2018;13:2317–2331.
- Hirsch V, Wolgin M, Mitronin AV, et al. Inflammatory cytokines in normal and irreversibly inflamed pulps: a systematic review. Arch Oral Biol. 2017;82:38–46.
- Liu L, Huang R, Yang R, et al. OCT4B1 regulates the cellular stress response of human dental pulp cells with inflammation. Biomed Res Int. 2017;2017:2756891.
- He W, Qu T, Yu Q, et al. LPS induces IL-8 expression through TLR4, MyD88, NF-kappaB and MAPK pathways in human dental pulp stem cells. Int Endod J. 2013;46:128–136.
- He W, Wang Z, Zhou Z, et al. Lipopolysaccharide enhances Wnt5a expression through toll-like receptor 4, myeloid differentiating factor 88, phosphatidylinositol 3-OH kinase/AKT and nuclear factor kappa B pathways in human dental pulp stem cells. J Endod. 2014;40:69–75.
- Men P, Li XT, Tang HL, et al. Efficacy and safety of saxagliptin in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2018;13:e0197321.
- Billiones R. Saxagliptin in type 2 diabetes. Drugs Today. 2010;46:101–108.
- Dore FJ, Domingues CC, Ahmadi N, et al. The synergistic effects of saxagliptin and metformin on CD34+ endothelial progenitor cells in early type 2 diabetes patients: a randomized clinical trial. Cardiovasc Diabetol. 2018;17:65.
- Brown SM, Smith CE, Meuth AI, et al. Dipeptidyl peptidase-4 inhibition with saxagliptin ameliorates angiotensin ii-induced cardiac diastolic dysfunction in male mice. Endocrinology. 2017;158:3592–3604.
- Birnbaum Y, Bajaj M, Qian J, et al. Dipeptidyl peptidase-4 inhibition by Saxagliptin prevents inflammation and renal injury by targeting the Nlrp3/ASC inflammasome. BMJ Open Diab Res Care. 2016;4:e000227.
- Catanzaro O, Dziubecki D, Lauria LC, et al. Diabetes and its effects on dental pulp. J Oral Sci. 2006;48:195–199.
- Jafarlou M, Shanehbandi D, Dehghan P, et al. Enhancement of chemosensitivity by simultaneously silencing of Mcl-1 and Survivin genes using small interfering RNA in human myelomonocytic leukaemia. Artif Cells Nanomed Biotechnol. 2018;46:1792–1798.
- Fatima F, Pathak N, Verma SR, et al. Toxicity and immunomodulatory efficacy of biosynthesized silver myconanosomes on pathogenic microbes and macrophage cells. Artif Cells Nanomed Biotechnol. 2018;46:1637–1645.
- Sheng B, Gong K, Niu Y, et al. Inhibition of gamma-secretase activity reduces Abeta production, reduces oxidative stress, increases mitochondrial activity and leads to reduced vulnerability to apoptosis: implications for the treatment of Alzheimer’s disease. Free Radic Biol Med. 2009;46:1362–1375.
- De Nigris V, Prattichizzo F, Mancuso E, et al. Teneligliptin enhances the beneficial effects of GLP-1 in endothelial cells exposed to hyperglycemic conditions. Oncotarget. 2017;9:8898–8910.
- Hernández Vigueras S, Donoso Zúñiga M, Jané-Salas E, et al. Viruses in pulp and periapical inflammation: a review. Odontology. 2016;104:184–191.
- Zhang L, Bai L, Ren Q, et al. Protective effects of SIRT6 against lipopolysaccharide (LPS) are mediated by deacetylation of Ku70. Mol Immunol. 2018;101:312–318.
- Nephan G, Coskun ZM, Bolkent S. Dipeptidyl peptidase-4 inhibition prevents cell death via extrinsic and intrinsic apoptotic pathways in rat pancreas with insulin resistance. Cell Biochem Funct. 2018;36:212–220.
- Singh TP, Vangaveti VN, Malabu UH. Dipeptidyl peptidase-4 inhibitors and their potential role in the management of atherosclerosis–A review. Diabetes Metab Syndr. 2015;9:223–229.
- Mason RP, Jacob RF, Kubant R, et al. Effect of enhanced glycemic control with saxagliptin on endothelial nitric oxide release and CD40 levels in obese rats. J Atheroscler Thromb. 2011;18:774–783.
- Mason RP, Jacob RF, Kubant R, et al. Dipeptidyl peptidase-4 inhibition with saxagliptin enhanced nitric oxide release and reduced blood pressure and sICAM-1 levels in hypertensive rats. J Cardiovasc Pharmacol. 2012;60:467–473.
- Tang J, Luo K, Li Y, et al. Capsaicin attenuates LPS-induced inflammatory cytokine production by upregulation of LXRa. Int Immunopharmacol. 2015;28:264–269.
- Kim DS, Shin MR, Kim YS, et al. Anti-inflammatory effects of glutamine on LPS-stimulated human dental pulp cells correlate with activation of MKP-1 and attenuation of the MAPK and NF-κB pathways. Int Endod J. 2015;48:220–228.
- Lee SI, Min KS, Bae WJ, et al. Role of SIRT1 in heat stress – and lipopolysaccharide-induced immune and defense gene expression in human dental pulp cells. J Endod. 2011;37:1525–1530.
- Chen J, Stark LA. Crosstalk between NF-κB and nucleoli in the regulation of cellular homeostasis. Cells. 2018;7:157.
- Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86.
- Zhao Y, Wang CL, Li RM, et al. Wnt5a promotes inflammatory responses via nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways in human dental pulp cells. J Biol Chem. 2014;289:21028–21039.
- Gangadharan Komala M, Gross S, Zaky A, et al. Saxagliptin reduces renal tubulointerstitial inflammation, hypertrophy and fibrosis in diabetes. Nephrology (Carlton). 2016;21:423–431.
- Giridharan S, Srinivasan M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J Inflamm Res. 2018;11:407–419.
