Abstract
Lung adenocarcinoma is one of the leading causes of cancer-related death worldwide. Low expression of Interleukin-33 (IL-33) was reported to be associated with the progression of pulmonary adenocarcinoma. However, the IL-33-mediated immunoregulation in pulmonary adenocarcinoma remains unclear. In this study, we found that IL-33 treatment evidently repressed tumour growth, induced CD4+ T cells infiltration and IL-17 expression in pulmonary adenocarcinoma. Notably, IL-33 treatment increased the number of Dendritic Cells (DCs) in pulmonary adenocarcinoma. More importantly, IL-33 induced maturation and regulated the function of DCs by increasing expression of DCs mature markers (CD40 and CD80, CD86) DCs-function-related gene including antigen presentation genes (HLA-DMA, HLA-DMB and CD74) and cytokines (IL-1β, IL-6 and TNF). Mechanistic studies demonstrated that IL-33 treatment induced DCs maturation by upregulating CYLD expression in DCs. In addition, CYLD played an important role in DCs-induced T cell proliferation and IL-17 secretion. In conclusion, our study demonstrated that IL-33 mediated immunoregulation in pulmonary adenocarcinoma by improving DC-induced T cell proliferation by upregulating CYLD expression.
Introduction
Pulmonary adenocarcinoma is one of lung cancer with 30% of tumour-related deaths worldwide [Citation1]. Despite the improvements of lung cancer treatment, including radiotherapy, chemotherapy, EGFR-TKI therapy, the patients with pulmonary adenocarcinoma have poor prognosis outcomes and with the 5-year survival rate only ∼10% [Citation2]. Therefore, it is necessary to look into the novel efficacious therapeutic avenues by revealing the molecular mechanisms underlying pulmonary adenocarcinoma.
Interleukin-33 (IL-33) is a multifunctional cytokine, constitutively expressed by various cells including epithelium and stromal cells, endothelial cells and fibroblasts [Citation3]. IL-33 was reported to be involved in the inflammatory response including allergic lung inflammation, acute colitis, Septic Arthritis, rheumatoid arthritis and hypoxia-induced pulmonary hypertension by regulating the activation of Th cells, Dendritic Cells (DCs), NK cells and CD8+ cells [Citation4–7]. Accumulating studies showed that IL-33 was dysregulated in various cancers and was involved in the progression of the tumour by regulating tumour microenvironment [Citation8]. For example, Xiao et al. demonstrated IL-33 as a critical mediator of myeloid-derived suppressor cells in breast cancer [Citation8]. Fang et al. showed that IL-33 regulated colon cancer progression by recruiting macrophages [Citation9]. Overexpressed IL-33 was also reported to promote antitumour immunity by activating NK cells, CD8+ T cells and ILC2 cells in melanoma and Lewis lung carcinoma [Citation10–12]. IL-33 is also reported to regulate the antitumour activity of DCs and eosinophils [Citation13,Citation14]. Recently, low expression of IL-33 was observed to be associated with poor prognosis of pulmonary adenocarcinoma [Citation15,Citation16]. However, the immunoregulation of IL-33 in pulmonary adenocarcinoma remains unclear.
In this study, we demonstrated the important role of CYLD in IL-33-induced DC activation, which plays an important role on antitumour immunity by inducing T cell proliferation and IL-17 secretion, implying the important clinical implications of IL-33 in pulmonary adenocarcinoma treatment.
Methods
Cells culture and treatment
The L1C2 (Line 1 alveolar cell carcinoma) adenocarcinoma cell line was cultured in RPMI 1640 medium enriched with 10% fetal calf serum (FCS) (Gibco, Invitrogen).
DCs were generated from lungs tissues as previously described [Citation17]. In brief, the lung was digested with Collagenase type 2 and filtered using 40 µm cell strainer (Corning). Dendritic cells were collected using CD11c microbeads (Miltenyi Biotec) following FACS sorting with FACS Aria II (BD Biosciences).
The siRNAs of CYLD were purchased from GenePharma (Shanghai, China), DCs were transfected with siRNAs using the Lipo 2000 (Invitrogen).
CD4+ T cells were isolated from splenocytes using CFSE labelling kits (Molecular Probes, Oregon, USA) as previously described [Citation17]. For co-culture experiment, T Cells were co-cultured with DCs at 7 °C for 72 h in 2 ml of RPMI 1640 with 10% FBS.
Mice and treatment
C57BL/6 mice were purchased from SLAC (Shanghai, China). All animal experiments in this study were approved by Institutional Animal Care and Use Committee of the Fujian Provincial Hospital of Fujian Medical University. For the pulmonary adenocarcinoma mouse model, L1C2 cells were injected into C57BL/6 mice by tail vein. For IL-33 treatment, C57BL/6 mice were injected (i.p.) 1 μg rIL-33 daily.
Flow cytometry analysis
The cells were collected and stained with immunofluorescence surface antibody as previously described [Citation2] The antibodies were purchased from BD Pharmingen and used as follows: FITC-conjugated anti-CD45, PE-conjugated anti-CD4, A647-conjugated anti-CD11c, PE-conjugated anti-CD24 and BV711-conjugated anti-MHCII. The cells were detected by 3-color FACSCalibur cytometer (BD Biosciences, Mountain View, CA) and was analyzed using FlowJo10 software (BD Biosciences).
qPCR analysis
Total RNA was isolated using TRIZOL (Invitrogen, California, USA). 1 μg RNA was used for cDNA using All-in-One First-Strand cDNA Synthesis SuperMix (TransGen). The mRNA expression of IL-17, CYLD, HLA-DMA, HLA-DMB and CD74, IL-1β, IL-6 and TNF were detected by real-time PCR. (Applied Biosystems, Foster City, CA, USA). The expression levels of mRNA were normalized by the GAPDH.
Enzyme-Linked immunosorbent assay
The concentration of IL-17 was detected by ELISA (BD Biosciences) as previously described [Citation18].
Statistical analysis
The Student’s t-test (2 groups) and one-way ANOVA (≥3 groups) were used to compare various experimental groups. A p-value <.05 was considered significant.
Results
Exogenous IL-33 inhibits the growth of pulmonary adenocarcinoma
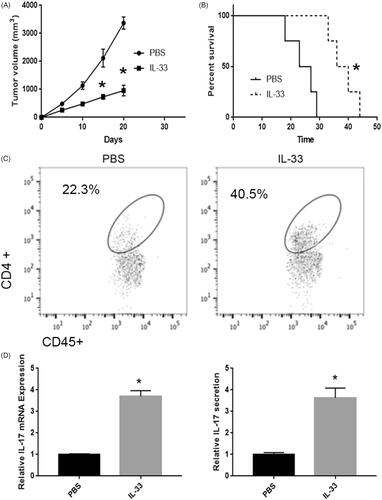
Low expression levels of IL-33 was detected and reported to be associated with poor prognosis of pulmonary adenocarcinoma in several recent studies [Citation15,Citation16]. However, the potential roles and molecular mechanism of IL-33 in pulmonary adenocarcinoma are barely understood. To investigate the role of IL-33 on the progression of pulmonary adenocarcinoma, we established lung adenocarcinoma mice model and then i.p. injected with rIL-33 daily. As shown in 1 μg IL-33 evidently repressed tumour growth. And the survival rate was also increased by IL-33 injection (). Moreover, the flow cytometry results showed that rIL-33 treatment remarkably increased the CD4+ T cells infiltration (). In addition, the qPCR and ELISA analysis showed that IL-33 evidently increased the expression and secretion of IL-17 in pulmonary adenocarcinoma (). Collectively, these results indicated IL-33 repressed the growth by regulating immune microenvironment.
Figure 1. rIL-33 involved tumour progression in lung adenocarcinoma mice. (A) rIL-33 treatment inhibited tumour growth in lung adenocarcinoma mice. vs PBS group. (B) Mice were sacrificed when tumour volume reached ∼3 cm3. *p < .01 vs PBS group. (C) rIL-33 treatment increased CD4+ T cell infiltration in lung adenocarcinoma mice. (D) rIL-33 treatment increased IL-17 expression. (E) rIL-33 treatment increased IL-17 secretion in lung adenocarcinoma mice. *p < .01 vs PBS group.
IL33 affects the infiltration, maturation and function of DCs
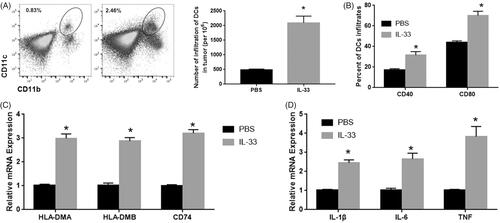
DCs plays an important role in the immune micro-environments of various tumours including lung cancer [Citation19]. Previous studies showed that IL-33 regulates T cell immunity through DCs [Citation20,Citation21]. To determine the role of DCs in lung adenocarcinoma, the number of DCs was detected by flow cytometry. The results showed that IL-33 significantly induced the DCs infiltration in pulmonary adenocarcinoma (). IL-33 also induced upregulation of CD40 and CD80 among these DC infiltrates (), significantly increased expression of CD40 and CD80 were also observed in IL-33 treated group. To reveal the role of IL-33 on DCs function, qPCR was used to detect expression levels of the DCs-related gene including antigen presentation genes (HLA-DMA, HLA-DMB and CD74) and cytokines (IL-1β, IL-6 and TNF), and conducted verification (). We found that these genes were repressed by IL-33 injection in tumour-induced DCs [Citation18]. In addition, expression levels of the chemokines CCL18 and CCL17 were increased by IL-33 injection. The data indicate that exogenous IL-33 may induce DC infiltration, activation and maturation within the tumour microenvironment, thereby promoting antitumour T cell immunity.
Figure 2. IL33 affects the infiltration, maturation and function of DCs. (A) The number of DCs was detected by flow cytometry. (B) CD40 and CD80 in these DC infiltrates. (C) The mRNA expression of antigen presentation genes (HLA-DMA, HLA-DMB and CD74). (D) The mRNA expression of cytokines (IL-1β, IL-6 and TNF). *p < .01 vs PBS group.
IL-33 promotes the antitumour activity of DCs by regulating CYLD expression
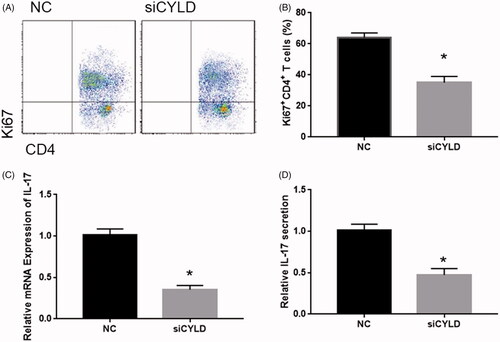
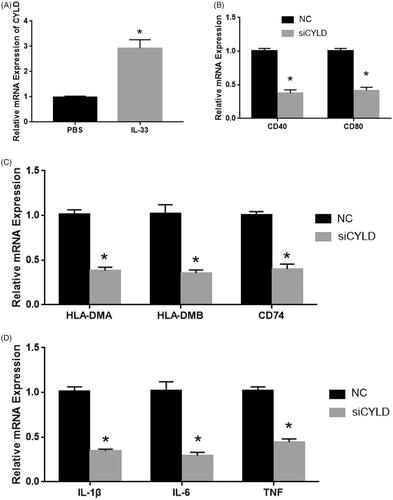
Previous studies showed that CYLD plays an important role in the immune cell, including DCs. To determine the mechanism of IL-33 in DC activity, we detected the expression levels of CYLD in IL-33 treatment mice. As shown in , the CYLD expression was significantly upregulated in IL-33 treatment mice. And the CYLD expression was significantly upregulated in DCs from IL-33 treatment mice. Next, we detected the role of CYLD on DCs mature and function. As shown in , the siRNA of CYLD significantly repressed the mRNA expression of CYLD. Silenced CYLD evidently reduced the cell surface marker (CD40 and CD80) and DCs-related gene expression ().
Figure 3. IL-33 promotes the antitumour activity of DCs by regulating CYLD expression. (A) CYLD expression was detected by qPCR. (B) The mRNA expression of CD40 and CD80. (C) The mRNA expression of antigen presentation genes (HLA-DMA, HLA-DMB and CD74). (D) The mRNA expression of cytokines (IL-1β, IL-6 and TNF). *p < .01 vs NC group.
IL-33 promotes the proliferation of CYLD -activated DC-treated CD4+ T cells
Next, to determine the role of CYLD on DCs stimulated T cells, we co-cultured CYLD-silenced DCs with T cells. We found that DCs-induced T cell proliferation was significantly repressed in CYLD-silenced DCs at 48 and 72 h (). In addition, the CD4+ T cell population was markedly depleted at 72 h (). qPCR and ELISA analysis showed the decreased expression and secretion of IL-17 in CYLD-silenced DCs treated T cell (). Taken together, these results clearly demonstrate that CYLD plays an important role in DCs-induced T cells [Citation17].
Discussion
IL-33, a multifunctional cytokine from IL-1 family, was reported to be involved in the immune response in allergic lung inflammation, acute colitis, septic arthritis, rheumatoid arthritis and hypoxia-induced pulmonary hypertension [Citation4–7]. Recent studies showed that IL-33 was dysregulated in various cancers including pulmonary adenocarcinoma [Citation16]. However, the potential molecular mechanism of IL-33 underlying the progression of pulmonary adenocarcinoma remains limited. In this study, our work revealed the important role of IL-33 in DCs-induced T cell proliferation, implying an antitumour efficacy of IL-33 by regulating immune microenvironment in pulmonary adenocarcinoma.
Tumour microenvironment plays a critical role in the progression of the tumour [Citation22]. Studies showed that the dysregulated immune response contributed to the immune escape in tumour tissue [Citation23]. The dysfunction of various immune cells was reported to be involved in the tumour microenvironment. For example, naive CD4+ T cells were abundant and associated with the poor prognosis for breast cancer [Citation24]. The Tregs are increased in tumour tissue and they played their immunosuppressive role by suppressing the anti-tumour function of effector T cells and natural killer cells [Citation25]. The tumour-associated macrophage was reported to induce a Treg/Th17 cell imbalance epithelial ovarian cancer [Citation26]. DCs, a class of professional antigen presenting cells (APC), pays an important role in the activation and recruitment of immune cells [Citation27]. Recently, dysfunction of DCs was observed in the tumour, which was involved in the tumour induced immune escape. For example, Zhang et al. showed that dendritic cells improved T cell responses in murine Lewis lung carcinoma [Citation28]. DCs were also reported to inducted antitumour Th9 cells differentiation [Citation29]. The antitumour effects of DCs that were observed, exhibited a potential therapy for the tumour. Garzon-Muvdi et al. showed that DCs improved the immunotherapy mediated by anti-PD-1 in glioblastoma [Citation30]. DCs were also reported to play an important role in effector T cell trafficking and adoptive T cell therapy [Citation31]. IL-33-activated DCs plays an important role on Th9 and Th2-mediated antitumour function [Citation29,Citation32]. In this study, we found that IL-33 induced DCs infiltration, maturation and function. Consistent with this result, IL-33 also reported to induce DCs maturation, which further produces inflammatory cytokines including IL-1β and IL-6 [Citation29,Citation32,Citation33].
The cylindromatosis (CYLD), a deubiquitination enzyme, was initially identified as a tumour suppressor in skin cancer [Citation34], colon carcinoma [Citation35], chronic lymphocytic leukaemia [Citation36] and acute lymphoblastic leukaemia [Citation37] hepatocellular carcinoma [Citation38]. Moreover, the CYLD was involved in inflammation in colitis [Citation39] and acute lung injury [Citation40]. Recent studies showed that CYLD was involved in immune regulatory by regulating the function of immune cells. For example, Tang et al. showed that Transgenic CYLD prevented the differentiation of Treg cells and Th17 cells, and increased differentiation of Th1 cells [Citation39]. Wex et al. showed that CYLD in macrophages was involved in antibacterial immune responses [Citation41,Citation42]. CYLD was also reported to regulate DCs function [Citation43]. In this study, IL-33 treatment induced DCs maturation by upregulating CYLD expression in DCs. In addition, CYLD played an important role in DCs-induced T cell proliferation and IL-17 secretion.
In summary, our studies showed that IL-33 induced CYLD-activated DCs which subsequently induced T cell proliferation and IL-17 secretion in pulmonary adenocarcinoma, providing novel insights in IL-33/DCs therapy for pulmonary adenocarcinoma.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References:
- Yong KJ, Basseres DS, Welner RS, et al. Targeted BMI1 inhibition impairs tumour growth in lung adenocarcinomas with low CEBPα expression. Sci Transl Med. 2016;8:350ra104.
- Caronni N, Simoncello F, Stafetta F, et al. Downregulation of membrane trafficking proteins and lactate conditioning determine loss of dendritic cell function in lung cancer. Cancer Res. 2018;78:1685–1699.
- Eissmann MF, Dijkstra C, Wouters MA, et al. Interleukin 33 signaling restrains sporadic colon cancer in an interferon-gamma-dependent manner. Cancer Immunol Res. 2018;6:409–421.
- Lopetuso LR, De Salvo C, Pastorelli L, et al. Il-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proc Natl Acad Sci USA. 2018;115:E9362–e9370.
- Staurengo-Ferrari L, Trevelin SC, Fattori V, et al. Interleukin-33 receptor (st2) deficiency improves the outcome of Staphylococcus aureus-induced septic arthritis. Front in Immunol. 2018;9:962.
- Takatori H, Makita S, Ito T, et al. Regulatory mechanisms of IL-33-ST2-mediated allergic inflammation. Front in Immunol. 2018;9:2004.
- Xiong Y, Cui X, Li W, et al. BLT1 signaling in epithelial cells mediates allergic sensitization via promotion of IL-33 production. Allergy 2019;74:495–506.
- Xiao P, Wan X, Cui B, et al. Interleukin 33 in tumour microenvironment is crucial for the accumulation and function of myeloid-derived suppressor cells. Oncoimmunology 2016;5:e1063772.
- Fang M, Li Y, Huang K, et al. IL33 promotes colon cancer cell stemness via jnk activation and macrophage recruitment. Cancer Res. 2017;77:2735–2745.
- Gao K, Li X, Zhang L, et al. Transgenic expression of IL-33 activates CD8+ T cells and NK cells and inhibits tumour growth and metastasis in mice. Cancer Lett. 2013;335:463–471.
- Gao X, Wang X, Yang Q, et al. Tumoural expression of IL-33 inhibits tumour growth and modifies the tumour microenvironment through CD8+ T and NK cells. JI. 2015;194:438–445.
- Kim J, Kim W, Moon UJ, et al. Intratumourally establishing type 2 innate lymphoid cells blocks tumour growth. JI. 2016;196:2410–2423.
- Lucarini V, Ziccheddu G, Macchia I, et al. IL-33 restricts tumour growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 2017;6:e1317420.
- Dominguez D, Ye C, Geng Z, et al. Exogenous IL-33 restores dendritic cell activation and maturation in established cancer. J Immunol. 2017;198:1365–1375.
- Hsu YL, Hung JY, Lee YL, et al. Identification of novel gene expression signature in lung adenocarcinoma by using next-generation sequencing data and bioinformatics analysis. Oncotarget 2017;8:104831–104854.
- Yang M, Feng Y, Yue C, et al. Lower expression level of IL-33 is associated with poor prognosis of pulmonary adenocarcinoma. PLoS One. 2018;13:e0193428.
- Jang JS, Lee JH, Jung NC, et al. Rsad2 is necessary for mouse dendritic cell maturation via the IRF7-mediated signaling pathway. Cell Death Dis. 2018;9:823.
- Li R, Fang F, Jiang M, et al. STAT3 and NF-κB are simultaneously suppressed in dendritic cells in lung cancer. Sci Rep. 2017;7:45395.
- Bergeron A, El-Hage F, Kambouchner M, et al. Characterisation of dendritic cell subsets in lung cancer micro-environments. The Euro Resp J. 2006;28:1170–1177.
- Liu N, Jiang Y, Chen J, et al. IL-33 drives the antitumour effects of dendritic cells via the induction of TC9 cells. Cell Mol Immunol. 2018. https://www.nature.com/articles/s41423-018-0166-0
- Carmi Y, Spitzer MH, Linde IL, et al. Allogeneic igg combined with dendritic cell stimuli induce antitumour t-cell immunity. Nature 2015;521:99–104.
- Gurusamy D, Clever D, Eil R, et al. Novel “elements” of immune suppression within the tumour microenvironment. Cancer Immunol Res. 2017;5:426–433.
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumour microenvironment. Nat Immunol. 2013;14:1014–1022.
- Su S, Liao J, Liu J, et al. Blocking the recruitment of naive CD4+ T cells reverses immunosuppression in breast cancer. Cell Res. 2017;27:461–482.
- Nishikawa H, Sakaguchi S. Regulatory T cells in tumour immunity. Int J Cancer. 2010;127:759–767.
- Zhou J, Li X, Wu X, et al. Exosomes released from tumour-associated macrophages transfer miRNAs that induce a treg/th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol Res. 2018;6:1578–1592.
- Tran Janco JM, Lamichhane P, Karyampudi L, et al. Tumour-infiltrating dendritic cells in cancer pathogenesis. JI. 2015;194:2985–2991.
- Zhang Y, Hu X, Hu Y, et al. Anti-CD40-induced inflammatory E-cadherin + dendritic cells enhance T cell responses and antitumour immunity in murine Lewis lung carcinoma. J Exp Clin Cancer Res. 2015;34:11.
- Chen J, Zhao Y, Jiang Y, et al. Interleukin-33 contributes to the induction of th9 cells and antitumour efficacy by dectin-1-activated dendritic cells. Front Immunol. 2018;9:1787.
- Garzon-Muvdi T, Theodros D, Luksik AS, et al. Dendritic cell activation enhances anti-PD-1 mediated immunotherapy against glioblastoma. Oncotarget 2018;9:20681–20697.
- Spranger S, Dai D, Horton B, et al. Tumour-residing batf3 dendritic cells are required for effector t cell trafficking and adoptive t cell therapy. Cancer Cell. 2017;31:711–723.
- Arae K, Morita H, Unno H, et al. Chitin promotes antigen-specific th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1β production by DCs. Sci Rep. 2018;8:11721.
- Park SH, Kim MS, Lim HX, et al. IL-33-matured dendritic cells promote th17 cell responses via IL-1β and IL-6. Cytokine 2017;99:106–113.
- Alameda JP, Fernandez-Acenero MJ, Moreno-Maldonado R, et al. CYLD regulates keratinocyte differentiation and skin cancer progression in humans. Cell Death & Disease 2011;2:e208.
- Hellerbrand C, Bumes E, Bataille F, et al. Reduced expression of CYLD in human colon and hepatocellular carcinomas. Carcinogenesis 2007;28:21–27.
- Liu P, Xu B, Shen W, et al. Dysregulation of TNFα-induced necroptotic signaling in chronic lymphocytic leukemia: suppression of CYLD gene by LEF1. Leukemia 2012;26:1293–1300.
- Yang Y, Ran J, Sun L, et al. CYLD regulates noscapine activity in acute lymphoblastic leukemia via a microtubule-dependent mechanism. Theranostics 2015;5:656–666.
- Pannem RR, Dorn C, Ahlqvist K, et al. CYLD controls c-myc expression through the jnk-dependent signaling pathway in hepatocellular carcinoma. Carcinogenesis 2014;35:461–468.
- Tang Y, Reissig S, Glasmacher E, et al. Alternative splice forms of CYLD mediate ubiquitination of SMAD7 to prevent TGFB signaling and promote colitis. Gastroenterology 2019;156:692–707.
- Lim JH, Stirling B, Derry J, et al. Tumour suppressor CYLD regulates acute lung injury in lethal streptococcus pneumoniae infections. Immunity 2007;27:349–360.
- Wex K, Schmid U, Just S, et al. Receptor-interacting protein kinase-2 inhibition by CYLD impairs antibacterial immune responses in macrophages. Front in Immunol. 2015;6:650.
- Legarda D, Justus SJ, Ang RL, et al. CYLD proteolysis protects macrophages from TNF-mediated auto-necroptosis induced by LPS and licensed by type I IFN. Cell Reports. 2016;15:2449–2461.
- Srokowski CC, Masri J, Hovelmeyer N, et al. Naturally occurring short splice variant of CYLD positively regulates dendritic cell function. Blood 2009;113:5891–5895.