Abstract
Purpose
The function of lncRNAs in cancer stem cells (CSCs) remains to be elucidated. The present study aimed to investigate the regulating role of a novel lncRNA, hypoxia-inducible factor-2α (HIF-2α) promoter upstream transcript (HIF2PUT), in osteosarcoma stem cells.
Methods
The expression of lncRNA HIF2PUT and HIF-2α in osteosarcoma stem cell lines and tissues was monitored by real-time PCR and western blot. The proliferation ability was examined by MTT assay when HIF2PUT overexpression or knockdown. The self-renewing capabilities of the cells were assessed by spheroid formation assay. The migration and invasion of cells were monitored by wound-healing assay and transwell cell assay, respectively. The correlation of HIF2PUT and HIF-2α expression was determined in osteosarcoma cancer tissues.
Results
LncRNA HIF2PUT and HIF-2α were downregulated in osteosarcoma cell lines. HIF2PUT exhibited a significant decline in proliferation capacity. Wound healing and transwell assays showed that lncRNA overexpression inhibited osteosarcoma stem cell migration and invasion. HIF2PUT inhibited sphere formation in osteosarcoma stem cells. Increased HIF2PUT expression inhibited the enrichment of CD133 in osteosarcoma stem cells. There was a strong positive correlation between relative HIF2PUT level and relative HIF-2α level in the 30 paired osteosarcoma cancer tissues.
Conclusions
Overexpression of lncRNA HIF2PUT significantly attenuated the proliferation, migration and invasion of osteosarcoma stem cells. Furthermore, we demonstrated that lncRNA overexpression inhibited the sphere-formation of osteosarcoma stem cells by downregulating HIF-2α. These findings suggest that lncRNA HIF2PUT may act as a tumour suppressor in osteosarcoma. LncRNA HIF2PUT/HIF-2α may be a novel therapeutic target in the treatment of osteosarcoma.
Introduction
Osteosarcoma is a kind of solid tumour with high malignancy. It often occurs in the epiphyses of long tubular bones such as proximal tibia and distal femur. The incidence of osteosarcoma accounted for about 0.2% of human malignant solid tumours [Citation1]. In all primary malignant bone tumour, its incidence is first. In recent years, due to the continuous development of new adjuvant chemotherapy technology and the continuous innovation of various surgical methods, the 5-year survival rate of patients with osteosarcoma without metastasis can reach about 70% [Citation2]. However, the mortality and metastasis rates of osteosarcoma are still very high. So far, the specific mechanism of osteosarcoma is still unclear. Therefore, it is particularly important to strengthen the study on the molecular mechanism of osteosarcoma.
LncRNA is a kind of functional RNA which is longer than 200 nts, lacking in open reading frames and the ability to code for proteins [Citation3]. Many recent studies have demonstrated that aberrant lncRNAs expression can be potentially used as biomarkers for diagnosis and prognosis of pancreatic cancer based on their tissue-specific expression manner [Citation4]. Moreover, lncRNAs play an irreplaceable role in the initiation and progression of osteosarcoma, such as maintaining cell growth, evasion of apoptosis, promotion of invasion and metastasis, stemness maintenance and EMT [Citation5]. However, mechanisms of lncRNAs in the regulation of osteosarcoma cancer stem cells remain unknown. Therefore, investigating the underlying mechanisms of lncRNAs in cancer stem cells would be crucial for indicating the exact molecular mechanisms of osteosarcoma.
LncRNA HIF2PUT (HIF-2α promoter upstream transcript), which is located at 2p21, on the antisense side of the promoter upstream region of a cancer-associated mRNA, hypoxia-inducible factor-2α (HIF-2α) was identified [Citation6]. HIF-2α has previously been demonstrated to be associated with stem cell-like properties in stem cells, and in the cancer stem cells of numerous types of cancer [Citation7–9]. Here, we investigated whether HIF2PUT (HIF-2α promoter upstream transcript) was related to osteosarcoma stem cells and the possible mechanism. We found that lncRNA HIF2PUT could regulate of proliferation and migration of osteosarcoma stem cells through regulating HIF-2α. Our results could provide a novel therapeutic strategy for osteosarcoma.
Materials and methods
Cell culture
Human osteosarcoma cell lines U2OS and MG-63 and normal human osteoplastic cell line hFOB were obtained from ATCC (American Type Culture Collection, Manassas, VA, USA). These cells cultured in F12K medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 5% FBS (Invitrogen, Carlsbad, CA, USA), 100 U/mL penicillin and 100 pg/mL streptomycin (Sigma, St. Louis, MO, USA), at 37 °C in a humidified incubator supplemented with 5% CO2 and 95% air.
Clinical tissue samples
Osteosarcoma cancer tissues and normal adjacent normal tissues were collected from 30 patients undergoing resection or biopsy of osteosarcoma in the The Second Part of First Hospital of Jilin University. None underwent preoperative radiotherapy or chemotherapy. Clinical and pathological information was obtained from patients’ medical records and pathological reports. All the tissues were snap-frozen in liquid nitrogen for further analysis. Patients were included in the study after written informed consent approved by the ethics committee.
Cell transfection
The pcDNA3.1 plasmid and pcDNA-HIF2PUT plasmid were transfected using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. The lncRNA-HIF2PUT siRNAs had the following sequences: HIF2PUT siRNA1, sense 5′-CCUGCCACAUGCCUUAUCUTT-3′, and antisense 5′-AGAUAAGGCAUGUGGCAGGTT-3′; and HIF2PUT siRNA2, sense 5′-GUCUAUAUCUCUCCCUUUATT-3′, and antisense 5′-UAAAGGGAGAGAUAUAGACTT-3′. The HIF2PUT siRNA was transfected according to the X-tremeGENE Transfection reagent kit (Roche Diagnostics, Basel, Switzerland).
Reverse transcription and quantitative real-time PCR
Total RNA was extracted from cells or tissues samples according to the manufacturer’s instructions of the Trizol kit (Invitrogen, CA, USA). RNA was reverse transcribed into cDNA using TaqMan microRNA reverse transcription kit (TaKaRa, Dalian, China). qRT-PCR was performed with SYBR Premix Ex Taq II PCR kit (TaKaRa, Dalian, China). The primers for HIF2PUT are: forward, 5′-CGGAGGTGTTCTATGAGCTGG-3′ and reverse, 5′-AGCTTGTGTGTTCGCAGGAA-3′; the primers for HIF-2α mRNA are: forward, 5′-TGGGATCTAACAGGAACAGC-3′ and reverse, 5′-CTAAATAGCCAGACAAGGGT-3′.
MTT assay
Cultured cells were grown in 96-well plates at the number of 5000 cells/plate. After transfected, the 3–(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide (MTT, Sigma, St. Louis, MO) assay was performed at 24-, 48-, 72-, 96-, 120- and 144-h incubation. The absorbance at 450 nm was measured after incubation with 16 μL of MTT for 4 h. The experiments were repeated three times independently.
Spheroid formation assay
The self-renewing capabilities of the cells were assessed using ultra-low attachment surface 96-well culture dishes (Corning, Inc., Corning, NY, USA) at a density of 5000 cells/well in DMEM/F12 supplemented with B27 supplement (Invitrogen), 10 ng/mL human epidermal growth factor (Sigma, St. Louis, MO, USA), 10 ng/mL human basic fibroblast growth factor (Sigma, St. Louis, MO, USA) at 37 °C in a humidified atmosphere of 95% air and 5% CO2. After one week later, Phase-contrast images were captured seven days later using an Olympus fluorescence microscope (Olympus, Tokyo, Japan).
Wound-healing assay
Cell migration was assessed using wound-healing assays. The cells were seeded in 6-well plates and grown to full confluence in complete media. The monolayer was scratched with a 10 μL pipette tip and then washed twice with serum-free DMEM to remove the detached cells. The cells were incubated for additional 24 h in serum-free DMEM. The wounded areas were observed and imaged by microscopy. The experiments were repeated three times independently.
Transwell cell assay
Cell invasion was evaluated by a modified Matrigel Boyden chamber assay using Bio-Coat Matrigel invasion chambers (Merk Millipore, Darmstadt, Germany). Cells in serum-free medium were seeded on Matrigel-coated filters, and 5% FBS was added to the lower chambers as a chemoattractant. After incubated for 24 h, membranes were washed with PBS. The migration cells in the lower surface were fixed with 4% paraformaldehyde for 30 min, and subsequently stained with 0.2% Triton X-100 for 15 min and 0.1% crystal violet for 5 min. The experiments were repeated three times independently.
Western blot analysis
Cells were treated as described previously. The cells were harvested and lysed on ice for 30 min in buffer. After centrifugation, the concentrations of protein were determined and separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Then, protein was transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was incubated into rabbit anti-ALDH1, CD44 and CD133 (1:1500; Abcam, Cambridge, USA) and mouse anti-β-actin (1:6000; Cell Signaling Technology) at 4 °C overnight. Then, the membrane was treated with chemiluminescence reagents (Santa Cruz) as per the manufacturer’s instructions and analysed by Image J software.
Statistical analysis
All statistical analysis was carried out using the SPSS 22.0 software (SPSS, Inc., Chicago, IL, USA). The data were presented as the mean ± standard deviation. The significant differences were analysed using Student’s t-test or χ2 analysis for difference between two groups, and one-way ANOVA followed by Kruskal–Wallis H-test for multiple comparison. A p values less than .05 was considered statistically significant.
Results
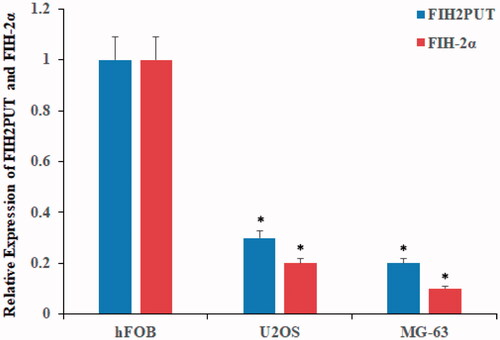
LncRNA HIF2PUT was downregulated in osteosarcoma cancer stem cell lines
We first examined the differential expression of lncRNA HIF2PUT in normal human osteoblastic cell line (hFOB) and human osteosarcoma stem cell lines (U2OS and MG-63). Compared with normal osteoblastic cell line, the lncRNA HIF2PUT was significant downregulation in osteosarcoma stem cell lines (, p < .05). These results indicated that lncRNA HIF2PUT was downregulated in osteosarcoma cell lines.
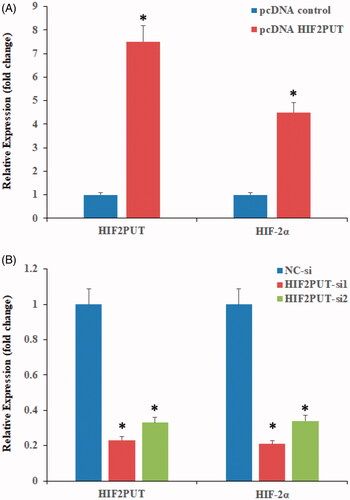
HIF-2α was positively regulated by lncRNA HIF2PUT
HIF2PUT and HIF-2α expression levels were analysed by qPCR in two osteosarcoma stem cell lines. In the overexpression experiment, lncRNA HIF2PUT expression levels were markedly upregulated, and HIF-2α mRNA expression levels were also moderately elevated (). In the knockdown expression, transfection of the cells with HIF2PUT siRNA resulted in a significant downregulation of HIF2PUT expression levels, and HIF-2α mRNA expression levels were also markedly decreased ().
Figure 2. Overexpression or knockdown of lncRNA HIF2PUT co-regulates mRNA expression levels of HIF-2α. (A) Expression levels of HIF2PUT and HIF-2α were significantly elevated in the pcDNA-HIF2PUT group, compared with the negative control in transfected U2OS stem cells (*P < .05). (B) Expression levels of HIF2PUT and HIF-2α were significantly reduced in the HIF2PUT siRNA groups, as compared with the negative control (NC) group in transfected U2OS stem cells (*p < .05).
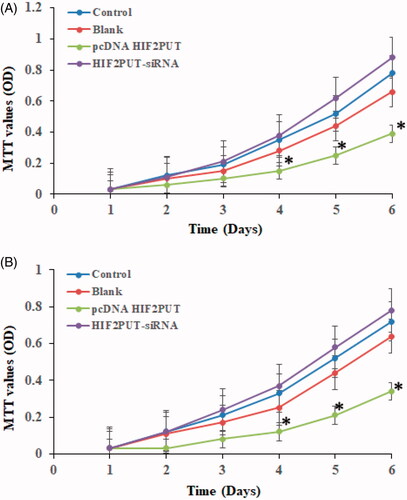
LncRNA HIF2PUT negatively regulates cell proliferation
HIF2PUT cDNA plasmid, HIF2PUT siRNA, and a control lentiviral empty vector were stably transfected into human osteosarcoma cancer stem cell lines (MG-63 and U2OS). HIF2PUT overexpression significant inhibited cells proliferation, while HIF2PUT knockdown has significant enhanced cells proliferation (, p < .05). No statistical difference was found between the empty vector group and control group.
Figure 3. LncRNA HIF2PUT inhibited cell proliferation in both U2OS (A) and MG-63 (B) stem cell lines. MTT assay of control, blank, pcDNA HIF2PUT and HIF2PUT-siRNA groups. The viable cell number was evaluated as the absorbance at 490 nm with a reference wavelength of 630 nm. Values of optical density (OD) were expressed as means ± SD. (*p < .05, compared with the control and blank groups).
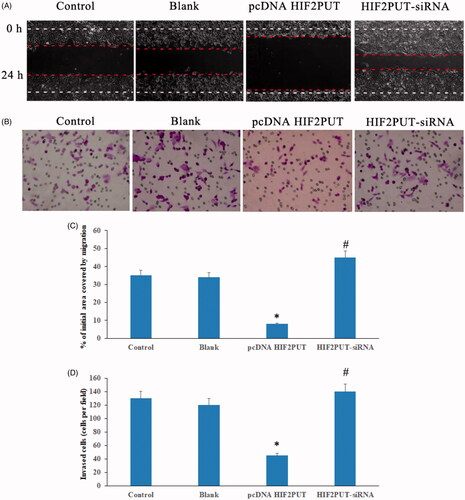
LncRNA HIF2PUT negatively regulates osteosarcoma stem cell migration and invasion
Wound-healing assay showed that lncRNA overexpression inhibited osteosarcoma stem cell migration, while HIF2PUT knockdown promoted osteosarcoma stem cell migration (). Transwell assay suggested lncRNA HIF2PUT overexpression inhibited osteosarcoma stem cell invasion, lncRNA HIF2PUT knockdown promoted osteosarcoma stem cell invasion (). Similar results were obtained in MG-63 stem cell lines (data not shown), which suggested that HIF2PUT played an important role in reducing the osteosarcoma migration and invasion in vitro.
Figure 4. LncRNA HIF2PUT inhibited osteosarcoma stem cell migration and invasion. (A) Wound-healing assay suggested lncRNA HIF2PUT overexpression inhibited osteosarcoma stem cell migration, lncRNA HIF2PUT knockdown promoted osteosarcoma stem cell migration. (B) Transwell assay suggested lncRNA HIF2PUT overexpression inhibited osteosarcoma stem cell invasion, lncRNA HIF2PUT knockdown promoted osteosarcoma stem cell invasion. (C) and (D) Data represented mean ± SD from three independent experiments (*p < .05, compared with the control and blank groups; #p < .05, compared with the pcDNA HIF2PUT group).
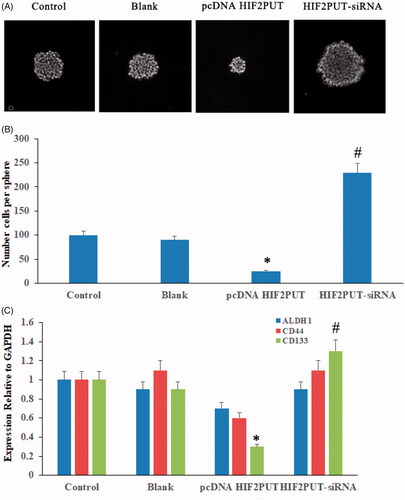
HIF2PUT is critical for the maintenance of osteosarcoma stem cell properties
The sphere-formation assays in HIF2PUT overexpression, knockdown cells, blank and control cells and found that HIF2PUT inhibited sphere formation in osteosarcoma stem cells (). We also examined the effects of HIF2PUT on the expression of stem factors and markers (ALDH1, CD44, and CD133). Increased HIF2PUT expression inhibited the enrichment of CD133 in osteosarcoma stem cells ().
Figure 5. LncRNA HIF2PUT had an inhibited effect for the maintenance of cancer stem cell properties. (A) Bright-field microscopy images showed the typical morphological features of small aggregates and spheres after overexpression or knockdown of HIF2PUT. (B) Quantification of the total number of cells per spheres. (C) Quantification of the expression of stem markers (ALDH1, CD44 and CD133) in control, blank, pcDNA HIF2PUT and HIF2PUT-siRNA groups. (*p < .05, compared with the control and blank groups; #p < .05, compared with the pcDNA HIF2PUT group).
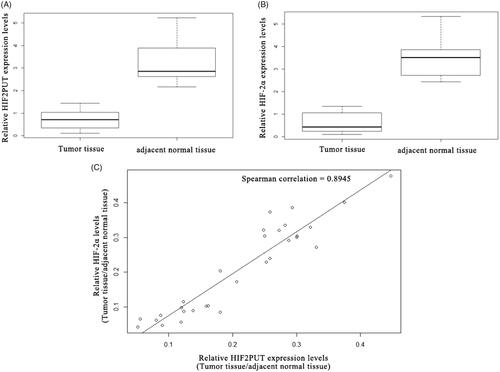
HIF2PUT is positively correlated with HIF-2α in osteosarcoma cancer tissues
HIF2PUT and HIF-2α expression levels were assessed in a group of 30 osteosarcoma patients. The expression levels of HIF2PUT and HIF-2α were significantly down-regulated in the osteosarcoma tissue samples, compared with the normal adjacent normal tissues (, p < .05). There was a strong positive correlation between relative HIF2PUT level and relative HIF-2α level in the 30 paired osteosarcoma cancer tissues ().
Figure 6. LncRNA HIF2PUT expression correlated with HIF-2α levels in human osteosarcoma tissues. (A) The relative lncRNA HIF2PUT expression levels in 30 osteosarcoma tissues compared with adjacent normal tissues were determined via qRT-PCR. GAPDH was used as an internal control. HIF2PUT tended to show lower expression in tumours than in adjacent normal tissues. (B) The relative HIF-2α expression levels in 30 osteosarcoma tissues compared with adjacent normal tissues were determined via qRT-PCR. GAPDH was used as an internal control. HIF-2α tended to show lower expression in tumours than in adjacent normal tissues. (C) The correlation scatterplot (Spearman test) of HIF2PUT and HIF-2α expression in osteosarcoma tissues compared with adjacent normal tissues. Positive correlation was observed between HIF2PUT and HIF-2α expression.
Discussion
Osteosarcoma is the most common human primary malignant bone tumour and mainly characterized by localized pain and early metastasis. Although it can be treated by surgery and chemotherapy, the clinical prognosis remains poor. In recent years, lncRNAs are reported to have important epigenetic regulatory roles in diverse biologic cellular processes. Studies have indicated that lncRNAs play important regulatory roles in tumorigenesis and metastasis [Citation10,Citation11]. Our study demonstrated that lncRNA HIF2PUT may play an anti-oncogene role in the development of osteosarcoma.
Tumour stem cells are a small part of tumour cells with the potential of self-renewal and multi-differentiation in tumour tissues [Citation12]. Previous studies have found that tumour stem cells are the main factors leading to tumour recurrence and metastasis [Citation13]. Numerous efforts have been made to dissect the molecular mechanisms involved in the regulation of osteosarcoma stem cells with the intent to identify novel therapeutic strategies to improve the poor prognosis of osteosarcoma [Citation14]. Although lncRNAs have been implicated in several pathologies, including cancer, and other studies have confirmed their function as critical regulators of gene expression in embryonic stem cells, the previous understanding of their role in cancer stem cells have been limited. Thus, our findings also provide important insights into the relationship between lncRNA HIF2PUT and osteosarcoma stem cells.
HIF-2 gene, also known as EPAS1 gene, has not only been proved to be an important factor related to tumour evolution in a variety of tumours. In normal stem cells and tumour stem cells, HIF-2 is also closely related to the regulation of stem-like properties [Citation6,Citation15–16]. HIF2PUT, previously named as TCONS_00004241, is located on chromosome 2p21, on the antisense side of the promoter upstream region of a cancer-associated mRNA, hypoxia-inducible factor-2α (HIF-2α) [Citation17,Citation18]. Previous study showed that HIF2PUT was significantly correlated with HIF-2α in colorectal cancer tissues [Citation16,Citation19]. In present study, HIF2PUT was down-expression in the osteosarcoma stem cells. Overexpression of HIF2PUT was significantly inhibited osteosarcoma stem cells proliferation, migration and self-renewal by regulating HIF-2α expression.
Compared with non-cancerous bone tissues, HIF-2α mRNA and HIF2PUT expressions were both significantly down-regulated in osteosarcoma tissues. In addition, HIF2PUT expression levels were positively correlated with HIF-2α. Moreover, after down-regulated of HIF2PUT expression, the expression of stem cell related gene OCT4 and SOX2 were significantly decreased and tumour stem cell marker CD44 were also significantly decreased [Citation20], which suggested that HIF2PUT function may be related to the nature of tumour stem cells. In the present study, overexpression of HIF2PUT markedly inhibited cell proliferation and migration, decreased the percentage of CD133 expressing cells, and impaired the osteosarcoma self-renewal of the osteosarcoma MG63 and U2OS cells.
The present study, we found a significant elevation of CD133 and HIF-2α in spheres generated from osteosarcoma stem cells, which is different from reports that used other cell lines to culture spheres. For instance, previous reports found that expression levels of CD44 was remarkably increased in spheres generated in glioma stem cell lines. The CD44 was sufficient to induce hypoxic signalling at perivascular oxygen tensions, and blocking CD44 cleavage decreased HIF-2α stabilization in CD44 expressing cells [Citation9]. In addition, we discovered that HIF2PUT knockdown induced more effective blocked expression levels of CD133 than CD44.
Altogether, to the best of our knowledge, this study is the first to report that HIF2PUT suppresses proliferation, migration and self-renewal of osteosarcoma cells by regulating HIF-2α. Previous studies have indicated that the role of HIF-2α may vary from cancer gene to tumour suppressor in numerous types of cancer [Citation6]. Our results suggested that down-expression of HIF-2α was involved in osteosarcoma carcinogenesis. Further studies have to conform the molecular mechanism of HIF-2α regulating the cancer stem cell growth, especially of the interaction of stem cell markers.
In conclusion, our results showed that HIF2PUT was down-regulated in human osteosarcoma tissues and cell lines. Furthermore, HIF2PUT could inhibit proliferation and migration of osteosarcoma cells, and inhibit self-renewal of osteosarcoma stem cells as well through up-regulating of HIF-2α, suggesting HIF2PUT/HIF-2α as a novel therapeutic target for osteosarcoma treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13.
- Wu PK, Chen WM, Chen CF, et al. Primary osteogenic sarcoma with pulmonary metastasis: clinical results and prognostic factors in 91 patients, Jpn J Clin Oncol. 2009;39:514–522.
- Vadaie N, Morris KV. Long antisense non-coding RNAs and the epigenetic regulation of gene expression. Biomol Concepts. 2013;4:411–415.
- Zhao X, Ping W, Jing I, et al. Gas5 exerts tumor-suppressive functions in human glioma cells by targeting miR-222. Mol Ther. 2015;23:1899.
- Ma C, Shi X, Zhu Q, et al. The growth arrest-specific transcript 5 (GAS5): a pivotal tumor suppressor long noncoding RNA in human cancers. Tumor Biol. 2016;37:1437.
- Wang Y, Yao J, Meng H, et al. A novel long non-coding RNA, hypoxia-inducible factor-2α promoter upstream transcript, functions as an inhibitor of osteosarcoma stem cells in vitro. Mol Med Rep. 2015;11:2534–2540.
- Yan Y, Liu F, Han L, et al. HIF-2α promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways. J Exp Clin Cancer Res. 2018;37:256.
- Sun JC, He F, Yi W, et al. High expression of HIF-2α and its anti-radiotherapy effect in lung cancer stem cells. Genet Mol Res. 2015;14:18110–18120.
- Johansson E, Grassi ES, Pantazopoulou V, et al. CD44 interacts with HIF-2α to modulate the hypoxic phenotype of perinecrotic and perivascular glioma cells. Cell Rep. 2017;20:1641–1653.
- Yan L, Wu X, Yin X, et al. LncRNA CCAT2 promoted osteosarcoma cell proliferation and invasion. J Cell Mol Med. 2018;22:2592–2599.
- Feng Y, Liu X, Li X, et al. LncRNA TUG1 is upregulated and promotes cell proliferation in osteosarcoma. Open Medicine. 2016;11:163–167.
- Zhou Y, Xia L, Wang H, et al. Cancer stem cells in progression of colorectal cancer. Oncotarget. 2018;70:33403–33415.
- Brown H, Tellez-Gabriel M, Heymann D. Cancer stem cells in osteosarcoma: stem cells and osteosarcoma. Cancer Lett. 2017;386:189–195.
- Qu H, Xue Y, Lian W, et al. Melatonin inhibits osteosarcoma stem cells by suppressing SOX9-mediated signaling. Life Sci. 2018;207:253–264.
- Yao J, Li J, Geng P, et al. Knockdown of a HIF-2alpha promoter upstream long noncoding RNA impairs colorectal cancer stem cell properties in vitro through HIF-2alpha downregulation. Onco Targets Ther. 2015;8:3467–3474.
- Li W, He X, Xue R, et al. Combined over-expression of the hypoxia-inducible factor 2α gene and its long non-coding RNA predicts unfavorable prognosis of patients with osteosarcoma. Pathol Res Pract. 2016;212:861–866.
- Preker P, Almvig K, Christensen MS, et al. PROMoter uPstream Transcripts share characteristics with mRNAs and are produced upstream of all three major types of mammalian promoters. Nucleic Acids Res. 2011;39:7179–7193.
- Taft RJ, Kaplan CD, Simons C, et al. Evolution, biogenesis and function of promoter-associated RNAs. Cell Cycle. 2009;8:2332–2338.
- Yao J, Li J, Geng P, et al. Knockdown of a HIF-2α promoter upstream long noncoding RNA impairs colorectal cancer stem cell properties in vitro through HIF-2α downregulation. Onco Targets Ther. 2015;8:3467–3474.
- Li ZH, Dou PC, Liu T, et al. Application of long noncoding RNAs in osteosarcoma: biomarkers and therapeutic targets. Cell Physiol Biochem. 2017;42:1407–1419.