 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Zinc oxide nanoparticles (NPs) have emerged as a novel elicitor for enhanced biosynthesis of secondary metabolites in in vitro plant cell cultures. The current study was aimed to explore elicitation abilities of ZnO-NPs for enhanced accumulation of lignans and neolignans in cell cultures of Linum usitatissimum. We optimized concentration of zinc oxide NPs before carrying out a full-fledged experiment. Subsequently, an optimum dose of 100 mg/l was introduced into the culture medium on day 0, days 0 and 15, and finally days 0 and 25. We observed that repeated elicitation stimulated various parameters and physiological responses in Linum usitatissimum cell cultures than one-time elicitation. Repeated elicitation of cell cultures on day 0 and 15 resulted in highest fresh weight (412.16 g/l) and lignans production (secoisolariciresinol diglucoside 284.12 mg/l: lariciresinol diglucoside 86.97 mg/l). Contrarily, repeated elicitation on day 0 and 25 resulted in highest DW (13.53 g/l), total phenolic production (537.44 mg/l), total flavonoid production (123.83 mg/l) and neolignans production (dehydrodiconiferyl alcohol glucoside 493.28 mg/l: guaiacylglycerol-β-coniferyl alcohol ether glucoside 307.69 mg/l). Enhancement in plant growth and secondary metabolites accumulation was several fold higher than controls. Furthermore, a linear relationship existed between total phenolic and flavonoid contents which in turn was correlated with higher antioxidant activities.
Introduction
Linum usitatissimum, a novel plant belonging to the family linaceae has a rich history regarding industrial applications—linen fiber production and linseed oil extraction—however, currently it is increasingly exploited as a great source of some health-promoting phenolic metabolites known as lignans and neolignans [Citation1]. These metabolites are naturally occurring polyphenols, which have potential therapeutic applications mainly against different types of cancer, some microbial infections and inflammation as well. Major lignans are secoisolariciresinol diglucoside (SDG) and lariciresinol diglucoside (LDG), whereas dehydrodiconiferyl alcohol glucoside (DCG) and guaiacylglycerol-β-coniferyl alcohol ether glucoside (GGCG) are well-known neolignans [Citation2]. Both lignans and neolignans share similar structural conformation, but they can be easily characterized by difference in (a minor but significant) bonding pattern. Generally, lignans consist of phenylpropane dimers that are bonded through the carbon atoms (C8/C8∼) of their side chains, whereas in neolignans, these dimers are connected through bond other than C8/C8∼ [Citation3].
A variety of established techniques and methods exist for commercial production of phytomedicines. Among these, biotechnology-based plant cell cultures are superior and modern methods, employed for extraction and isolation of phytomedicinal products. However, certain modifications and adaptations to such a system on the whole or some operational conditions are essential for enhanced accumulation of plant-derived secondary metabolites because production potential of such systems is intrinsically low, therefore they need stimulation [Citation4]. For this purpose, elements of diverse nature are used as stimulatory agents, which elicit enhanced biosynthesis of secondary metabolites in in vitro plant cell cultures. These agents include elements from both biotic and abiotic sources [Citation5]. Nanoparticles (NPs) are emerging abiotic elicitors used for obtaining improved yields of plant-derived medicinal compounds, such as, recently, we have reported effective scheme for elicitation of Linum usitatissimum derived cell suspension cultures through addition of chemogenic Ag-NPs [Citation6]. Generally, NPs have distinct physicochemical properties that enable them to interact with plant metabolic systems in a conducive way, rarely observed while using source/candidate material in raw form [Citation7,Citation8]. Furthermore, zinc as a micronutrient play a significant role in plant growth and metabolism. Phyto-hormonal regulation especially auxins, carbohydrate metabolism, protein synthesis [Citation9] and stress alleviating responses have been associated with adequate supply of zinc [Citation10,Citation11]. In addition, a great number of reports have described positive aspects of ZnO-NPs regarding their impacts on plant growth, physiology and secondary metabolism, details of which are provided in the discussion section. Thus, the current study was also aimed along the same lines to enhance accumulation of polyphenolic secondary metabolites (lignans and neolignans) in cell suspension cultures of Linum usitatissimum using biogenic ZnO-NPs as an abiotic elicitor.
Materials and methods
Inoculum preparation
The inoculum was prepared according to our previous protocol [Citation6]. Both callus and cell suspension cultures were grown on MS medium as described previously [Citation2]. Firstly, aseptic stem explants excised from Linum usitatissimum’s seed germinated plantlets were inoculated on MS medium containing 1 mg/l NAA as a PGR and agar as a soldifying agent, for callogenesis (). Secondly, callus obtained was immersed in liquid MS medium comprising same chemical constituents to that of callus inducing medium, but without agar (). These cultures were maintained in controlled conditions on a shaker for 15 days and then used for establishment of cell suspension cultures.
Characteristics of ZnO-NPs
ZnO-NPs were biologically synthesized using root extracts of Linum usitatissimum and described by our research group earlier [Citation12]. Adventitious roots extract of Linum usitatissimum (10 g/100 ml) and zinc nitrate hexahydrate (0.1 mM) were mixed in same proportions and then heated at 60 °C for 3 h under continuous stirring. Synthesis of ZnO-NPs was detected by precipitation of yellow particulates and later validated by UV-visible spectroscopy. Particles size, shape and texture were analyzed using XRD and TEM techniques, respectively. XRD results confirmed that NPs were hexagonal in shape with crystalline appearance, whereas the average size was up to 35 nm as examined by TEM. The physical stability of ZnO-NPs was determined using UV-visible spectroscopy before its application. For this purpose, absorbance value of 2.5 and peak value between 230 and 800 nm were used as standard parameters.
Optimization of ZnO-NPs concentration
Current experiment spanned for a total of 40 days. At first, an optimum dose of ZnO-NPs was determined for elicitation of cell suspension cultures. Consequently, a dose of biogenic ZnO-NPs, i.e. 1, 5, 10, 20, 30, 40, 50, 75, 100, 200 and 500 µg/l, respectively, was introduced to cell suspension cultures on day zero. All experimental elicitation steps were carried out in liquid MS medium containing 10 ml inoculum.
ZnO-NPs treatments
During the optimization experimental part, we recorded that 100 µg/l ZnO-NPs resulted in maximum fresh and dry biomass accumulation on day 25 after the initial day of culturing, therefore, we carried on this dose of ZnO-NPs for further experimentation. ZnO-NPs were introduced periodically into cell suspension cultures on three occasions, firstly on day 0, secondly on day 0 and 15, and finally day 0 and 25 (). Cells from culture medium were harvested at periodical breaks of 5 days until day 40 of the cell suspension culture, after each elicitation step. Control treatment did not receive any dose of ZnO-NPs at any stage.
Biomass determination
Cells harvested from culture medium were gently squeezed using several layers of filter papers to extract excess water. Fresh weight was recorded instantly; using electronic weighing balance, afterward cells were dried at 50 °C in a heating incubator until 24 h, and then dry weight was recorded. Both fresh and dry biomass were expressed in g/l.
Phytochemical analysis
Methanolic extracts of dried and powdered cells were used for phytochemical analysis; 50 mg samples were suspended in 250 µl methanol, then vortexed for 3 min and sonicated for 30 min, respectively. Next, samples were subjected to centrifugation at 13,000 rpm for 5 min. The supernatant obtained was collected carefully and kept for phytochemical analysis. Total flavonoid content (TFC) was evaluated using aluminium chloride colorimetric method as described by Ul-Haq et al. [Citation13]. Total flavonoid production (TFP) was mathematically calculated according to the method of Zahir et al. [Citation2] and expressed as mg/l. Total phenolic content (TPC) was evaluated using the Folin-Ciocalteu (FC) method as reported by Singleton et al. [Citation14]. Total phenolic production (TPP) was also mathematically calculated according to the method described earlier [Citation2] and expressed as mg/l.
Free radical scavenging assay
1,1-Diphenyl-2-picrylhydrazyl (DPPH) was used as a source of free radicals to assess the scavenging capacity of samples collected from cell suspension cultures, according to the method of [Citation15]. Methanol based cellular extracts and DPPH solution were mixed and kept in dark for 30 min. FRSA was evaluated using spectrophotometer at 517 nm and calculations based on the comparison of absorbance values was used to obtain% FRSA of samples [Citation2].
HPLC analysis
Accumulation of polyphenols (lignans and neolignans) in cell suspension cultures was quantified using HPLC following method of Corbin et al. [Citation16]. Production of polyphenols was calculated from HPLC data as follows:
Statistical analysis
Experimental design was based on triplicates, repeated twice and were conducted in controlled conditions. ANOVA test was applied to experimental data using Origin Pro software (8.5). All values are presented as means ± SD. Origin Pro 8.5 was used for the generation of all graphs.
Results and discussion
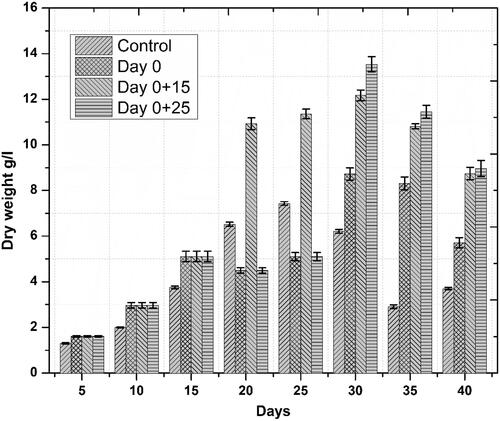
Physiological responses are the first key changes, which occur when plants are exposed to elements of diverse nature. NPs trigger plant physiological response in both positive and negative ways [Citation17,Citation18], primarily depending on the concentration of NPs and plant species [Citation19,Citation20]. Generally, application of NPs in lower concentrations has been found promoting plant growth and secondary metabolites production [Citation21]. We observed the positive impacts of ZnO-NPs on biomass accumulation in Linum usitatissimum derived cell suspension cultures, as we obtained maximum fresh weight, 412.16 g/l—on day 35 after initial culturing when cell suspension cultures were repeatedly elicited on day 0 and 15—() and dry weight, 13.53 g/l—on day 30 after initial culturing when cell suspension cultures were repeatedly elicited on day 0 and 25—(), respectively. Our observations are in accordance with the current perspective on using ZnO-NPs as a potential nanofertilizer, which is gaining wide acceptance because of the increasing reports on their positive impacts on plant growth [Citation22,Citation23]. These reports described the growth promoting effects of ZnO-NPs as a result of effective physiological changes including regulation of plant pigments directly associated with biomass buildup such as chlorophyll and carotenoids [Citation24,Citation25]; increase in nutrients uptake efficiency [Citation26], upregulation of antioxidant metabolism [Citation27] and most important, the involvement of zinc as an essential micronutrient in the metabolism of different plant biomolecules [Citation28].
Figure 2. Temporal effects of repeated elicitation with biogenic ZnO-NPs on fresh biomass of cell suspension cultures of Linum usitatissimum. Values are mean of triplicates ± SD.
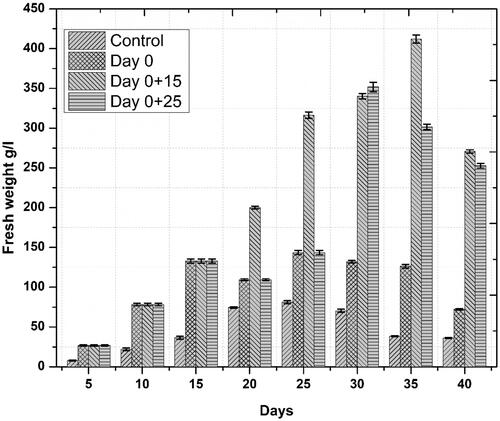
Figure 3. Temporal effects of repeated elicitation with biogenic ZnO-NPs dry biomass of cell suspension cultures of Linum usitatissimum. Values are mean of triplicates ± SD.
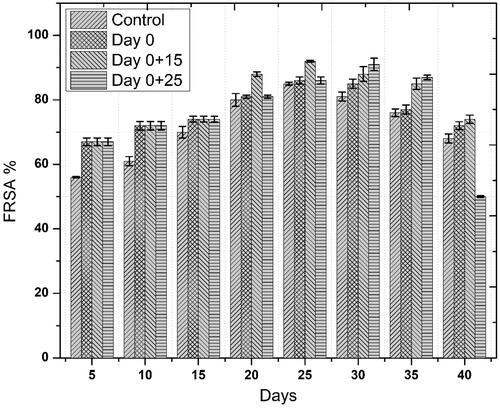
Accumulation of phenolics and flavonoids in response to novel substances as a primary defense line is the principal indicator of active plant secondary metabolism Zahir et al. [Citation29]). Linum usitatissimum contains a wide range of phenolics and some flavonoids. In the current study, similar trends were observed for both phenolics and flavonoids accumulation, we noted that one-time elicitation had not impacted phenolic and flavonoid contents but repeated addition of ZnO-NPs into culture medium, especially at a later stage of culture—log phase—significantly enhanced both total phenolic and flavonoid content/production, respectively, as compared to control treatments. Optimum TPC (39.71 mg GAE/g) and TPP (537.44 mg/l) occurred on day 30 after initial culturing when cell suspension cultures were repeatedly elicited on day 0 and 25 ( and ). This increase in phenolics production was 15.8 fold as compared to control. Optimum TFC and TFP also occurred during the same period. Highest TFC and TFP were recorded to be 9.15 mg QUE/g and 123.83 mg/l, ( and ), respectively. This increase in flavonoids production was 8.8 fold as compared to control. These results are in agreement with our previous finding [Citation6], wherein similar trends in total phenolic and flavonoid contents were observed. Increased polyphenol accumulation is indicated by higher antioxidant activities [Citation30]. A positive correlation between FRSA and phenolic content was recorded in the current study. Maximum FRS activity observed was up to 90% in repeatedly elicited cultures (. Generally, repeated elicitation resulted in enhanced FRS activity at the last stages of culture as compared to control. Our results are supported by the findings of García-López et al. [Citation31] and Zafar et al. [Citation32] who successfully enhanced phenolics and flavonoids, and also observed maximum antioxidant activities as a result of applying ZnO-NPs during germination of Capsicum annum and stem explants response of Brassica nigra, respectively. Significant changes in all the growth parameters as compared to control were reported in these studies.
Figure 4. Temporal effects of repeated elicitation with biogenic ZnO-NPs on TPC in cell suspension cultures of Linum usitatissimum. Values are mean of triplicates ± SD.
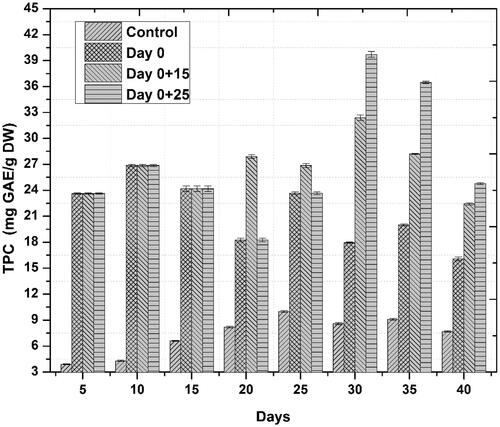
Figure 5. Temporal effects of repeated elicitation with biogenic ZnO-NPs on TPP in cell suspension cultures of Linum usitatissimum. Values are mean of triplicates ± SD.
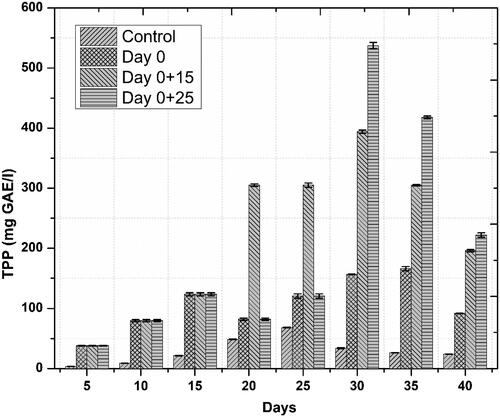
Figure 6. Temporal effects of repeated elicitation with biogenic ZnO-NPs on TFC in cell suspension cultures of Linum usitatissimum. Values are mean of triplicates ± SD.
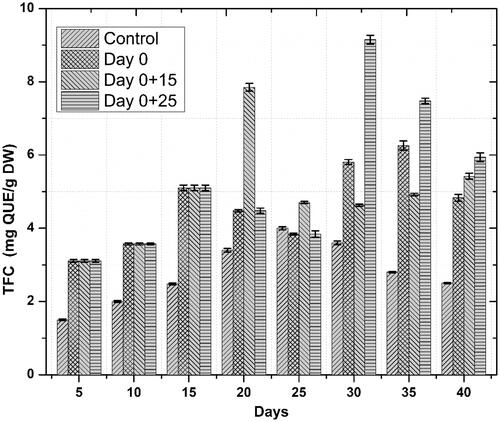
Figure 7. Temporal effects of repeated elicitation with biogenic ZnO-NPs on total flavonoid production in cell suspension cultures of Linum usitatissimum. Values are mean of triplicates ± SD.
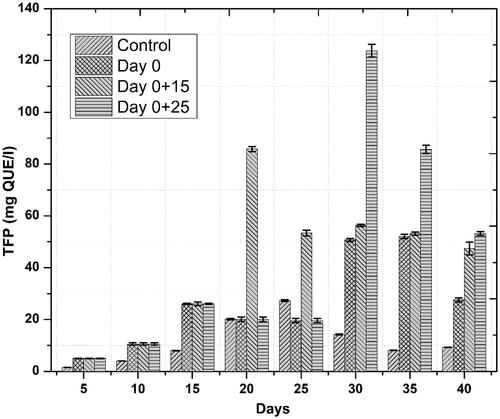
Figure 8. Temporal effects of repeated elicitation with biogenic ZnO-NPs on free radical scavenging assay of cell suspension cultures of Linum usitatissimum. Values are mean of triplicates ± SD.
Elicitation of cell suspension cultures of Linum usitatissimum with ZnO-NPs also enhanced accumulation of the secondary metabolic compounds lignans and neolignans. This accumulation correlated with the TPC, as both lignans and neolignans are also polyphenols by nature. We obtained the highest lignans content/production (SDG 25.01 mg/g and 284.12 mg/l; LDG 7.65 mg/g and 86.97 mg/l) after 25 days of initial culturing when cell suspension cultures were repeatedly treated with ZnO-NPs on day 0 and 15; whereas highest neolignans content/production (DCG 36.44 mg/g and 493.28 mg/l; GCGG 22.73 mg/g and 307.69 mg/l) occurred after 30 days of initial culturing when cell suspension cultures were repeatedly treated with ZnO-NPs on day 0 and 25 ( and ). The mechanism underlying enhanced production of secondary metabolites in response to ZnO-NPs is still unclear, but a number of reports are available on their role as a potential abiotic elicitor during in vitro cultures such as Sharafi et al. [Citation33], Karimi et al. [Citation21] and Javed et al. [Citation34]. Bhardwaj et al. [Citation35] made an attempt to decipher the transcriptional regulation of ZnO-NPs induced enhanced accumulation of bacoside A content in cell suspension cultures of Bacopa monnieri. They suggested that reduced transcriptional levels of HMG-CoA reductase gene in response to ZnO-NPs might be a result of diverging biosynthetic pathway from mevalonate to isoprenoid pathway. Furthermore, Mosavat et al. [Citation36] have recently reported elicitation of thymol in callus cultures of Thymus kotschyanus and carvacrol in callus cultures of Thymus daenesis under ZnO-NPs stress.
Table 1. Temporal effects of repeated elicitation with biogenic ZnO-NPs on content of DCG: dehydrodiconiferyl alcohol glucoside; GGCG: guaiacylglycerol-b-coniferyl alcohol ether glucoside; SDG: secoisolariciresinol diglucoside; and LDG: lariciresinol diglucoside.
Table 2. Temporal effects of repeated elicitation with biogenic ZnO-NPs on production of DCG: dehydrodiconiferyl alcohol glucoside; GGCG: guaiacylglycerol-b-coniferyl alcohol ether glucoside; SDG: secoisolariciresinol diglucoside; and LDG: lariciresinol diglucoside.
Conclusion
Biotechnological approach towards enhanced production of commercially important secondary metabolites from a wide range of medicinal plants is so far the best methodology as it offers flexibility in terms of manipulating growing/operational conditions and reproducibility [Citation37]. Application of ZnO-NPs in Linum usitatissimum derived cell suspension cultures proved a simple but effective procedure to enhance biomass and most important, accumulation of polyphenols—lignans and neolignans—over a short period of time, possibly as a result of ZnO-NPs induced metabolic shuffling. Results obtained during this study support the positive role of ZnO-NPs as an elicitor during in vitro established cultures of a variety of medicinal plants.
Authors contribution
AZ did the research work, data analyses and manuscript write-up. WA and MN assisted AZ with experiments. BHA conceived the idea, supervised the research work and reviewed the manuscript. CH performed chemical synthesis of standards and HPLC analysis.
Acknowledgements
Dr. Bilal Haider Abbasi acknowledges research fellowship of Le Studium-Institute for Advanced Studies, Loire Valley, Orléans, France. This research was in part supported by Cosmetosciences, a global training and research program dedicated to the cosmetic industry. Located in the heart of the cosmetic valley, this program led by University of Orléans, France is funded by the Region Centre-Val de Loire. Adnan Zahir acknowledges the financial assistance of Higher Education Commission of Pakistan for his PhD degree.
Disclosure statement
The authors declare no conflict of interest.
References
- Muir AD, Westcott ND. Flax: The genus linum. London (UK): CRC Press; 2003.
- Zahir A, Ahmad W, Nadeem M, et al. In vitro cultures of Linum usitatissimum: Synergistic effects of mineral nutrients and photoperiod regimes on growth and biosynthesis of lignans and neolignans. J Photochem Photobiol B. 2018;187:141–150.
- Wallis A. F. A. Structural diversity in lignans and neolignans. In: Lewis NG, Sarkanen S, editors. Lignin and lignan biosynthesis. Washington (DC): American Chemical Society; 1998.
- Murthy HN, Lee E-J, Paek K-Y. Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss Organ Cult. 2014;118:1–16.
- Namdeo A. Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn Rev. 2007;1:69–79.
- Zahir A, Nadeem M, Ahmad W, et al. Chemogenic silver nanoparticles enhance lignans and neolignans in cell suspension cultures of Linum usitatissimum L. Plant Cell Tiss Organ Cult. 2018;1–8.
- Fazal H, Abbasi BH, Ahmad N, et al. Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L. Appl Biochem Biotechnol. 2016;180:1076–1092.
- Zaka M, Abbasi BH, Rahman L-u, et al. Synthesis and characterisation of metal nanoparticles and their effects on seed germination and seedling growth in commercially important Eruca sativa. IET Nanobiotechnol. 2016;10:134–140.
- Marschner H. Mineral nutrition of higher plants. 2nd ed. London (UK): Academic Press; 1995.
- Peck AW, McDonald GK. Adequate zinc nutrition alleviates the adverse effects of heat stress in bread wheat. Plant Soil. 2010;337:355–374.
- Tavallali V, Rahemi M, Eshghi S, et al. Zinc alleviates salt stress and increases antioxidant enzyme activity in the leaves of pistachio (Pistacia vera L.‘Badami’) seedlings. Turk J Agric For. 2010;34:349–359.
- Abbasi BH, Anjum S, Hano C. Differential effects of in vitro cultures of Linum usitatissimum L.(Flax) on biosynthesis, stability, antibacterial and antileishmanial activities of zinc oxide nanoparticles: a mechanistic approach. RSC Adv. 2017;7:15931–15943.
- Ul-Haq I, Ullah N, Bibi G, et al. Antioxidant and cytotoxic activities and phytochemical analysis of Euphorbia wallichii root extract and its fractions. Iran J Pharm Res. 2012;11:241–249.
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent methods in enzymology. Methods Enzymol. 1999;299:152–178.
- Lee SK, Mbwambo Z, Chung H, et al. Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen. 1998;1:35–46.
- Corbin C, Fidel T, Leclerc EA, et al. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason Sonochem. 2015;26:176–185.
- Gupta SD, Agarwal A, Pradhan S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: an insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol Environ Saf. 2018;161:624–633.
- Tripathi DK, Singh S, Singh S, et al. Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol Biochem. 2017;110:167–177.
- Navarro E, Baun A, Behra R, et al. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology. 2008;17:372–386.
- Thuesombat P, Hannongbua S, Akasit S, et al. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol Environ Saf. 2014;104:302–309.
- Karimi N, Behbahani M, Dini G, et al. Enhancing the secondary metabolite and anticancer activity of Echinacea purpurea callus extracts by treatment with biosynthesized ZnO nanoparticles. Adv Nat Sci: Nanosci Nanotechnol. 2018;9:045009.
- Khanm H, Vaishnavi B, Shankar A. Raise of nano-fertilizer era: effect of nano scale zinc oxide particles on the germination, growth and yield of tomato (Solanum lycopersicum). Int J Curr Microbiol Appl Sci. 2018;7:1861–1871.
- Panwar J. Positive effect of zinc oxide nanoparticles on tomato plants: A step towards developing nano-fertilizers. In: International Conference on Environmental Research and Technology (ICERT); 2012.
- Latef A, Alhmad MFA, Abdelfattah KE. The possible roles of priming with zno nanoparticles in mitigation of salinity stress in Lupine (Lupinus termis) plants. J Plant Growth Regul. 2017;36:60–70.
- Tarafdar J, Raliya R, Mahawar H, et al. Development of zinc nanofertilizer to enhance crop production in pearl millet (Pennisetum americanum). Agric Res. 2014;3:257–262.
- Chanu TT, Upadhyaya H. Zinc oxide nanoparticle-induced responses on plants: a physiological perspective. Nanomater Plants Algae Microorg. 2019;43–64.
- Venkatachalam P, Jayaraj M, Manikandan R, et al. Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Biochem. 2017;110:59–69.
- Bonnet M, Camares O, Veisseire P. Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L. cv Apollo). J Exp Bot. 2000;51:945–953.
- Zahir A, Abbasi BH, Adil M, et al. Synergistic effects of drought stress and photoperiods on phenology and secondary metabolism of Silybum marianum. Appl Biochem Biotechnol. 2014;174:693–707.
- Ahmad N, Fazal H, Abbasi BH, et al. DPPH free radical scavenging activity and phenotypic difference in hepatoprotective plant (Silybum marianum L.). Toxicol Ind Health. 2013;29:460–467.
- García-López J, Zavala-García F, Olivares-Sáenz E, et al. Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 2018;8:215.
- Zafar H, Ali A, Ali JS, et al. Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response. Front Plant Sci. 2016;7:535.
- Sharafi E, Khayam Nekoei S, Fotokian MH, et al. Improvement of hypericin and hyperforin production using zinc and iron nano-oxides as elicitors in cell suspension culture of St John’s wort (Hypericum perforatum L.). J Med Plants Byprod. 2013;2:177–184.
- Javed R, Usman M, Yücesan B, et al. Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant Physiol Biochem. 2017;110:94–99.
- Bhardwaj P, Goswami N, Narula P, et al. Zinc oxide nanoparticles (ZnO NP) mediated regulation of bacosides biosynthesis and transcriptional correlation of HMG-CoA reductase gene in suspension culture of Bacopa monnieri. Plant Physiol Biochem. 2018;130:148–156.
- Mosavat N, Golkar P, Yousefifard M, et al. Modulation of callus growth and secondary metabolites in different Thymus species and Zataria multiflora micropropagated under ZnO nanoparticles stress. Biotechnol Appl Biochem. 2019. doi:10.1002/bab.1727.
- Anjum S, Abbasi BH, Doussot J, et al. Effects of photoperiod regimes and ultraviolet-C radiations on biosynthesis of industrially important lignans and neolignans in cell cultures of Linum usitatissimum L.(Flax). J Photochem Photobiol B. 2017;167:216–227.

