Abstract
Non-viral nanocarrier affords a platform for drug and siRNA combination, the focus of which is to load drug and siRNA into a single carrier, allowing for co-delivery and a synergistic effect at tumour site. In our previous study, pH-sensitive carboxymethyl chitosan-modified liposomes (CMCS-SiSf-CL) were assembled for sorafenib (Sf) and Cy3-siRNA co-loaded. The present study evaluated in vitro and in vivo co-delivery of the co-loaded liposomes. Further, in vitro inhibiting hepatocellular carcinoma of the pH-sensitive sorafenib (Sf) and VEGF-siRNA co-loaded liposomes was discussed. The experimental results demonstrated co-delivery and penetration into 2-dimensional (2D) cultured HepG2 cells, 3-dimensional (3D) cultured HepG2 tumour spheroids and tumour regions of H22 tumour-bearing mice. Compared with free siRNA and single loaded carrier, co-delivery liposomes exhibited enhanced VEGF downregulating effect, inducing cell early apoptosis. Therefore, the CMCS-SiSf-CL delivery system can lay the foundation for the co-delivery systems development and provide new area for HCC therapy.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common human malignancy and the third leading cause of cancer mortality worldwide owing to its high morbidity and mortality rates [Citation1,Citation2]. The mainly clinical treatment for HCC is still surgical resection in combination with chemotherapy. Due to the imperceptible initiation, patients with moderate or advanced HCC have a poor prognosis and miss the best time for surgical treatment. Therefore, new chemotherapy drugs and chemotherapy regimens have been emerging with the development of tumour molecular biology, cytology, and physiology mechanism. Tumourigenesis and growth depends on blood and oxygen supplied with tumour vessels. Blocking the blood and oxygen supply is a widely used antitumoural strategy. It is considered that epidermal growth factor (VEGF) and the receptor (VEGFR) play an important role in angiogenesis and proliferation of HCC [Citation3]. Down-regulating VEGF expression or inhibiting VEGFR activity through different methods may effectively suppress tumour angiogenesis and growth. Sorafenib (Sf), a multikinase inhibitor, blocks VEGFRs and PDGFRs that are involved in angiogenesis and cell proliferation [Citation4], which is widely used as the first-line therapy for advanced HCC. Unfortunately, the low solubility and bioavailability of Sf cause various side effects, such as gastrointestinal symptoms, hypertension, and hand–foot skin reaction [Citation5]. These adverse reactions occur in almost 30% of patients, resulting in dose reductions or discontinuation [Citation6]. Thus, an efficient strategy to enhance therapeutic effect and reduce side effects of Sf is required.
Recently, a combination of chemotherapeutic drugs and siRNA has attracted broad attention owing to enhanced antitumor efficacy over single administration. The combination therapy could simultaneously inhibit tumour growth via the drugs and specifically silence oncogene via the addition of siRNA, reducing drug doses and overcoming the major limitation of traditional chemotherapy drugs. Up until now, considerable efforts have been devoted to co-load drug and siRNA by a single carrier, which offers several advantages for combination therapy, such as ensuring synchronized pharmacokinetics, allowing for targeted cells to receive both agents at a ratiometric dose and ensuring the two therapeutic agents work synergistically within the same cell to produce an enhanced anti-cancer effect [Citation7]. Moreover, simultaneous delivery via a single carrier would guarantee the preset administration dose of two distinct payloads in vivo through tuning the optimal ratio in vitro [Citation7].
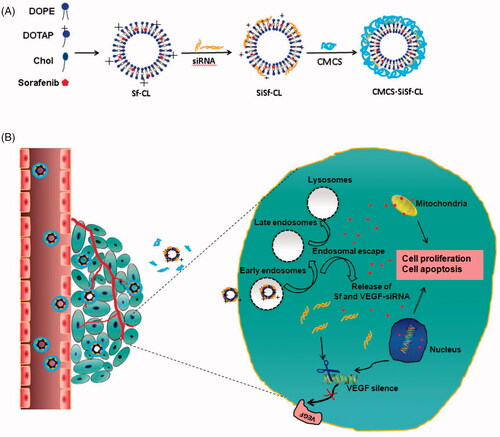
Since siRNA is water soluble biomacromolecules and negatively charged surface, while many chemotherapy drugs are hydrophobic small molecules, it is a great challenge to establish applicable co-loaded nanocarriers. During the recent past, there has been a remarkable progress in constructing drug/siRNA co-loaded delivery systems. The representative candidates, including liposomes/lipid nanoparticles [Citation8], cationic polymer/micelle [Citation9], and inorganic nanoparticles [Citation10], have been extensively studied and discussed. Among these nanocarriers, cationic liposomes are one of the most successful co-loaded systems: (1) low toxicity and ease of preparation; (2) the ability of loading hydrophobic, amphipathic, and hydrophilic drugs; (3) forming positively lipoplex with siRNA and facilitating cellular uptake; (4) aiding in endosomal escape of siRNA [Citation11,Citation12]. Though these advantages provide promising prospects for drug/siRNA co-loading and co-delivery, the barriers in vivo should be taken into account to realize controlled release of the two agents. Currently, pH-sensitive strategies have been applied to drug or gene delivery according to low extracellular pHex or endosomal pHen [Citation13]. pH-sensitive materials with weakly acidic or basic titratable functional groups are often introduced into the structures of pH-sensitive nanocarriers, accepting protons, changing their structures and triggering drug/gene release in the microenvironmental pH [Citation13]. Therefore, it is desirable to choose corresponding materials based on pH value of the targeting region, which would promote triggering of release at the target. In our previous work, Sf and Cy3-siRNA co-loaded cationic liposomes (SiSf-CL) were constructed. Then pH-sensitive material, carboxymethyl chitosan (CMCS), was used to modify the positively charged complex at physiological pH via electrostatic interaction to develop a CMCS-modified pH-sensitive Sf/Cy3-siRNA co-loaded cationic liposome (CMCS-SiSf-CL). CMCS-SiSf-CL was demonstrated to pH-sensitive disassemble in tumour slight acid environment (pH 6.5), inducing higher Sf release rate and Cy3-siRNA cellular uptake efficiency. In vivo study showed that the carrier could deliver Sf to tumour to generate antitumor effect and did not injure normal organs. Therefore, in this study, siRNA with VEGF target was chose to co-load with Sf via CMCS-SiSf-CL, resulting in improving anti-tumour efficiency through the co-inhibition of VEGF/VEGFR signal pathway (). In vitro and in vivo co-delivery of the co-loaded liposomes was evaluated on 2D cultured cells, 3D cultured tumour spheroids, and tumour tissue. Then, in vitro inhibiting hepatocellular carcinoma of CMCS-SiSf-CL was assessed.
Methods
Materials
Sf was purchased from Biochempartner Co., Ltd. (Shanghai, People’s Republic of China). N-[1-(2,3-dioleyloxy) propyl]-N,N,N-trimethylammonium chloride (DOTAP), 1, 2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) was purchased from Avanti Polar Lipids (Alabaster, AL). Cholesterol (CHOL) was purchased from Sigma-Aldrich Co. (St Louis, MO). CMCS (molecular weight, 50,000; degree of carboxymethyl substitution, 60%; degree of deacetylation, 85%) was obtained from Jinan Haidebei Biological Engineering Co., Ltd. (Jinan, Shandong, People’s Republic of China). Human VEGF-siRNA, labelled nonspecific siRNA (Cy3-siRNA or Cy5-siRNA) and unlabelled nonspecific control sequences (siN.C.) were obtained from Ribobio Co., Ltd. (Guangzhou, Guangdong, People’s Republic of China). The sequences of Cy3-siRNA and Cy5-siRNA were: sense 5′-Cy3-UUCUCCGAACGUGUCACGUdTdT-3′; antisense: 5′-AAGAGGCUUGCACAGUGCAdTdT-3′. The target sequences of VEGF-siRNA were: sense 5′-GGCGUCGCACUGAAACUUUdTdT-3′; antisense 5′-AAAGUUUCAGUGCGACGCCdTdT-3′. siRNA were dissolved in RNase-free water. LysoTracker Red and Green were purchased from Beyotime Co., Ltd. (Haimen, Zhejiang, People’s Republic of China). Coumarin-6 was purchased from Sigma-Aldrich Co (St Louis, MO). All other chemicals were of analytical grade and used without further purification.
Human hepatocellular liver carcinoma cell lines HepG2 and murine hepatic cancer cell line H22 were kindly provided by Institute of Immunopharmacology and Immunotherapy of Shandong University (Jinan, Shandong, People’s Republic of China) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% FBS and incubated at 37 °C and 5% CO2.
Kunming mice (weight, 18–22 g) were provided by Medical Animal Test Center of the New Drugs Evaluation Center, Shandong University. All animal experiments complied with the requirements of the Animal Management Rules of the Ministry of Health (China) and approved by the Laboratory Animal Ethics Review Committee of Qilu Medical College of Shandong University.
Preparation of liposomes
Sf-CL was prepared by the thin-film hydration method as our previous report [Citation7]. Briefly, Sf or hydrophobic fluorescent probe (Coumarin-6), DOPE, DOTAP and cholesterol (1:2.6:6.7:3.2, mol/mol) were dissolved with absolute ethanol in a pear-shaped flask and then evaporated to form a dry film at 40 °C. The film was hydrated with RNase-free deionized water at 40 °C. The resulting suspension was extruded five times through polycarbonate membranes (400, 200, and 100 nm) using a LiposoFast™ basic extruder (Avestin, Ottawa, ON, Canada). SiSf-CL was prepared by mixing Sf-CL with siRNA (Cy3-siRNA, Cy5-siRNA, siN.C., and VEGF-siRNA) solution for 30 min at room temperature. CMCS-SiSf-CL was prepared by adding CMCS solution in SiSf-CL. Blank liposomes were prepared by the same method without involving Sf and siRNA ().
Intracellular uptake and endosomal escape
The intracellular uptake and distribution of co-loaded liposomes were examined by confocal laser scanning microscopy (CLSM, LSM780, Carl Zeiss, Jena, Germany). For the microscopic analysis, Cy3-siRNA and coumarin-6 were used to mimic co-delivery of siRNA and Sf. HepG2 cells were seeded on glass bottom cell culture dishes containing complete 1640 medium and incubated for 24 h. Following two washes with cold PBS, the co-loaded liposomes were diluted with serum-free medium at pHs 6.5 or 7.4 and incubated with cells for 1 h at 37 °C under a 5% CO2-containing atmosphere. The final concentration of Cy3-siRNA and coumarin-6 were 50 nM and 0.57 μM, respectively. After incubation, the cells were washed three times with ice cold PBS and fixed with 4% formaldehyde for 15 min, and the nuclei were stained with Hoechst 33342 for 10 min. Finally, samples were examined under CLSM with an excitation wavelength of 543 nm for Cy3-siRNA, 488 nm for coumarin-6, and 346 nm for Hoechst 33342.
To visualize the endosomal release of liposomal Cy3-siRNA and the intracelluar distribution of coumarin-6, HepG2 cells were incubated for 3 h with the co-loaded liposomes (pH 6.5 or 7.4). Then, endosome/lysosome labelling was performed for 1 h using LysoTrackerTM Red or Green at a concentration of 100 nM. After nuclear staining, the cells were observed by a CLSM. The LysoTracker Red and Green were excited by 561 nm and 488 nm lasers, respectively.
3D tumour spheroids formation
An agarose solution (2%, w/v) was prepared in serum-free RPMI-1640 by heating the solution to 90 °C and incubated for 30 min [Citation14,Citation15]. Each well of 96-well culture plates was coated with 100 µL of the sterilized solution. HepG2 cells were seeded into each well at the density of 2 × 103 cells/100 μL per well in the culture medium. The 96-well plates were centrifuged at 800 rcf for 5 min at room temperature after seeding, and then the resulting cellular aggregates were cultured to grow up at 37 °C in the presence of 5% CO2 for several days. About 50 µL of fresh complete medium were added every 3 d into the wells until the diameter of spheroids was about 400 µm. The uniform and compact tumour spheroids were selected for further tests.
Penetration in vitro 3D tumour spheroids
The tumour spheroids were incubated with CMCS-SiSf-CL in serum-free medium (pH 6.5 or 7.4). The final concentration of coumarin-6 and Cy3-siRNA was 0.57 μM and 50 nM, respectively. After 5 h of incubation, the treated spheroids were rinsed with ice-cold PBS three times and fixed in 4% paraformaldehyde for 30 min. Subsequently, spheroids were transferred to 15 mm glass bottom cell culture dishes and covered by mounting medium. Fluorescent intensity was observed by CLSM. Z-stack images were obtained by tomoscan in depth from the top of the spheroid to the middle with 3.4 μm intervals.
In vivo co-delivery into tumour tissue
To further estimate the in vivo co-delivery ability of CMCS-SiSf-CL into the tumour region, cryo-section observations were carried out in H22-bearing female KM mice. The mice were subcutaneously injected into the right armpits with 0.1 ml of cell suspension containing 1 × 106 H22 cells. When the tumour grew to about 70 mm3, mice were intravenously administered with coumarin-6/Cy5-siRNA co-loaded SiSf-CL and coumarin-6/Cy5-siRNA co-loaded CMCS-SiSf-CL. The administration dose for coumarin-6 and Cy5-siRNA was 342.3 μg/kg and 1.0 mg/kg body weight, respectively. Six hours later, the mice were sacrificed, and the tumours were collected, embedded, and cryo-sectioned with the thickness of 8 μm. The nuclei were stained with Hochest 33324 and were imaged under confocal microscope with the excitation wavelength of 649 nm for Cy5-siRNA, 488 nm for coumarin-6, and 346 nm for Hoechst 33342.
Determination of VEGF expression in cells
For in vitro VEGF silencing study, HepG2 cells were seeded at a density of 1.5 × 105 per well in six-well plated until 70% confluence was reached. Then free VEGF-siRNA, free Sf, CMCS-Sf-CL, CMCS-Si-CL, SiSf-CL, CMCS-SiSf-CL, CMCS-SiSf-CL (pH 7.4), CMCS-SiSf-CL (pH 6.5), or CMCS-NCsi-CL was added to each well and incubated for 6 h. The final concentrations of Sf and VEGF-siRNA were 100 nM and 2.8 μM, respectively. Then, the medium was replaced with fresh complete 1640 medium and incubated for another 42 h. Finally, the cellular total RNA and proteins in each well were, respectively, extracted using different reagents as described below.
Total mRNA was extracted with Trizol (Invitrogen, Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s protocol and then quantified using a Nanodrop (Thermo Fisher Scientific, Waltham, MA). The complementary DNA (cDNA) was synthesized based on the isolated mRNA using first-strand cDNA Synthesis Kit (Beyotime, Haimen, Zhejiang, People’s Republic of China) and then used for RT-PCR kit with Taq (Beyotime, Haimen, Zhejiang, People’s Republic of China). The PCR was performed at 95 °C for 1 min. 50 cycles at 94 °C for 15 s. 60 °C for 45 s. 72 °C for 15 s. The relative VEGF gene expression level was calculated using the formula of 2− ΔΔCT) method. All experiments were performed in triplicates.
For western-blot analysis, the cells were lysized with lysis buffer (Beyotime, Haimen, Zhejiang, People’s Republic of China) on ice for 20 min. The total protein was determined by a BCA protein assay kit (Beyotime, Haimen, Zhejiang, People’s Republic of China) and separated by a 10% sodium dodecyl sulphate polyacrylamide gel (SDS-PAGE), then transferred to a transferred to polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA), incubated with anti-VEGF antibody or anti-GAPDH antibody (1:1000, Ebioscience, Thermo Fisher Scientific, Waltham, MA) as a control overnight at 4 °C, and cultured with the goat anti-rabbit secondary antibody (1:1000, Wanleibio, Shenyang, Liaoning, China) for 1 h. The blotting images were obtained from a Bio-Rad imaging system (Chemidoc TM MP imaging system, Hercules, CA).
Cytotoxicity test
HepG2 cells were cultured in 96-wells plates at a density of 5000 cells/well overnight. Then the cells were treated with the formulations mentioned above for 48 h. Subsequently, 20 μL of MTT solution was added to each well. After incubation for additional 4 h, the culture medium was replaced with 150 μL dimethyl sulphoxide per well. The absorbance was measured at 570 nm using a microplate reader (ELx808, BioTek; Winooski, VT). Cell viability was normalized to that of the cultured PBS-treated cells which served as the indicator of 100% cell viability. Results were the mean of three test runs.
Cell apoptosis test
HepG2 cells were cultured in 12-wells plates at a density of 1.5 × 105 cells/well overnight. The cells were exposed to aforementioned formulations 48 h post-seeding. Then the cells were trypsinized, collected and stained with Annexin V-FITC/PI apoptosis detection kit (Wanleibio, Shenyang, Liaoning, China) according to the manufacturer’s protocol. The proportions of apoptotic cells were analyzed by flow cytometry. The untreated cells were used as control.
Results and discussion
In vitro pH-sensitive co-delivery in 2D cultured cells
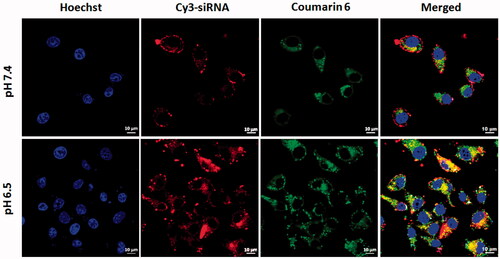
The sufficient synergistic effect of chemotherapy drug and siRNA primarily requires co-location in tumour cells. Therefore, the carrier must co-load drug/siRNA as well as co-deliver both into the same cell population, which is considered as an effective delivery platform to generate combination therapy. Our previous research had demonstrated that CMCS could pH-sensitive disassemble from cationic SiSf-CL in pH 6.5 culture condition, resulting in efficient cellular uptake of Cy3-siRNA [Citation7]. To further demonstrate the co-delivery of siRNA and Sf in 2D cultured cells, confocal laser scanning microscopy was employed to visualize the intracellular distribution of the co-loaded liposomes (Cy3-siRNA and coumarin-6 to replace siRNA and Sf, respectively) in HepG2 cells. The nucleus was stained with Hoechst 33428 (blue fluorescence). As is shown in , after 1 h incubation, both red and green fluorescence was observed in cytoplasm of HepG2 cells at pH 6.5 and pH 7.4. The yellow fluorescence in the merged image showed that Cy3-siRNA and coumarin-6 could be co-delivered into cells by CMCS-SiSf-CL. The negative charged CMCS-SiSf-CL was lacked of endocytosis, inducing slightly intracellular distribution of coumarin-6 and Cy3-siRNA in normal culture condition. On the contrast, the fluorescence was stronger in pH 6.5 culture medium than that in pH 7.4, which indicated that co-loaded liposomes were more efficient at acid environment in comparison with than at neutral environment.
Intracellular distribution and endosomal escape
In the process of siRNA transfection, siRNA nanocarriers will be entrapped in early endosomes after endocytosis, and then rapidly maturate into late endosomes and ultimately fuse with other late endosomes [Citation16,Citation17]. Therefore, endosomal escape is a critical step for siRNA to enter in cytoplasm and achieve gene silencing before digestion in late endosomes. In order to investigate the endosomal escape of endocytosed siRNA, the endosomes in HepG2 cells was labelled with Lysotracker Red or Lysotracker Green after transfection with the co-loaded liposomes. As is shown in , the red fluorescence of Cy3-siRNA was partially dispersed in cytoplasm and slightly overlapped green fluorescence of Lysotracker Green after incubation for 3 h with Cy3-siRNA single-loaded CMCS-Si-CL, indicating that siRNA had successfully escaped from endosome into cytoplasm following 3 h incubation. Additionally, the red fluorescence around cytoplasm was stronger in pH 6.5 than that in 7.4 owing to higher cellular uptake of Cy3-siRNA in acid condition.
Figure 3. Confocal laser scanning microscopy images of the lysosome escape of CMCS-modified liposomes loaded with Cy3-siRNA (A) and CMCS modified liposomes loaded with coumarin-6 (B). HepG2 cells were incubated with CMCS-modified liposomes for 3 h at pHs 7.4 and 6.5.
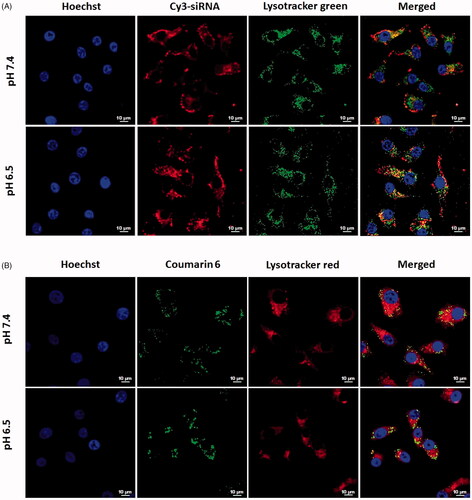
DOPE is a membrane-fusogenic lipid that fuses with endosome membrane and destabilizes the membrane by converting to a hexagonal phase in the acid endosomes [Citation18]. On the other hand, the cationic lipid DOTAP may form ion-pairs with the anionic endosomal lipids, further destabilizing the endosomes and releasing siRNA into the cytoplasm to silence target-gene expression [Citation19]. Consequently, it is speculated that DOPE and DOTAP in the CMCS modified liposomes ensures the endosomal escape of condensed siRNA.
For the coumarin-6 loaded liposomes, the cells showed some yellow fluorescence, which was colocalization of the red fluorescence and the green (). This may due to the physicochemical properties and the release of small hydrophobic molecular coumarin-6 from liposomes [Citation20,Citation21].
Penetration in vitro 3D tumour spheroids
A main barrier to drug/gene delivery in vivo is the interstitial transport which impedes the carriers’ diffusion and penetration into the solid tumour [Citation22]. Comparing with traditional 2D cell culture, the 3D cell model may provide a more physiologically relevant microenvironment that can simulate tumour microenvironment in vivo [Citation23].
To further estimate the tumour penetration of siRNA and drug by the co-loaded liposomes, HepG2 cell 3D spheroids of 400 μm were formed and used to confirm the penetration of the co-loaded liposomes in vivo-like tumour. As indicated in , Cy3-siRNA and coumarin-6 could penetrate into tumour spheroids via co-loaded liposomes both in 7.4 and 6.5 environments after 5 h incubation. The fluorescence was observed at 84 nm depth in pH 7.4 condition and 107.8 nm in pH 6.5 (). At the same depth range of Z-stack scanning, the merged fluorescence intensity was much stronger at pH 6.5 in compare with that at pH 7.4, indicating the more efficient tumour penetration of co-loaded liposomes in acid environment.
Figure 4. Penetration into 3D HepG2 tumour spheroids after incubation with CMCS-modified Cy3-siRNA/coumarin-6 co-loaded liposomes for 5 h at pH 7.4 (A, B, C) and pH 6.5 (D, E, F). (A, D) Laser scanning confocal images of 3D HepG2 tumour spheroids. (B, E) Z-stack images were obtained from the top to the equatorial plane of tumour spheroids in 3.4 mm thickness; (C, F) 3D images of penetration into tumour spheroids.
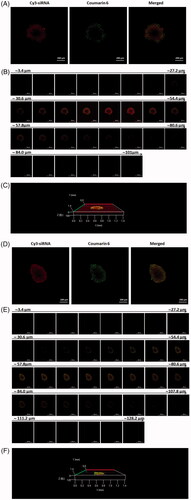
The delivery efficiency of nanocarriers in tumour spheroids involves diffusive transport in tumour interstitium and cellular uptake in tumour cells [Citation24]. It is reported that anionic liposomes at - 10 mV and cationic liposomes with DOPE molar ratio >15% could penetrate deeply into tumour spheroids, while the internalization efficiency of cationic liposomes in 2D cultured monolayer cells was significantly higher than that of anionic liposomes [Citation24,Citation25]. Herein zeta potential is presumably not the crucial parameter to tumour penetration. With respect to this study, CMCS modified co-loaded liposomes delivered drug and gene into tumour spheriod both at pH 7.4 (negatively charged) and pH 6.5 (positively charged). Meanwhile the merged fluorescence intensity was obviously stronger in pH 6.5 condition compared with that in pH 7.4 condition at the same interval of Z-stack scan. These results indicate that charge reversal of CMCS-SiSf-CL in tumour acid microenvironment could co-deliver drug/siRNA into tumour interstitium, promoting the intracellular uptake and further realizing intracellular co-delivery.
In vivo co-delivery into tumour tissue
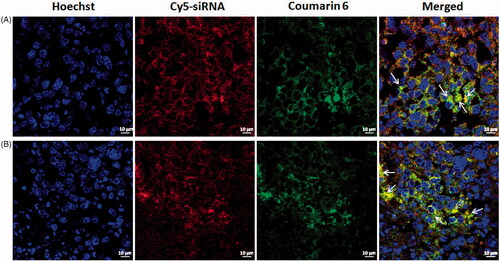
To test in vivo co-delivery of co-loaded liposomes, tumour frozen sections were assessed using CLSM after Cy5-siRNA/coumarin-6 co-loaded cationic liposomes (SiSf-CL) and CMCS modified Cy5-siRNA/coumarin-6 co-loaded liposomes (CMCS-SiSf-CL) were administered systemically into H22 tumour-bearing mice. The yellow region (arrow) in merged images of suggested that Cy5-siRNA and coumarin-6 could be co-delivered into intracellular space of tumour tissue and cytoplasm following CMCS-SiSf-CL injection, which approaching to that of SiSf-CL treatment. The results demonstrated that CMCS-SiSf-CL accumulated in tumour site via EPR effect, then pH-sensitive CMCS fell off, realizing the effective endocytosis of Sf and siRNA loaded in exposed cationic liposomes.
In vitro gene silencing and Western blotting
In order to confirm the transfection dose, VEGF-siRNA was transfected by blank cationic liposomes with final concentration of 20 nM, 50 nM, and 100 nM. As shown in , the transcriptional VEGF mRNA level was dose dependent and the lowest at the concentration of 100 nM; therefore, siRNA transfection dose was set as 100 nM.
Figure 6. VEGF mRNA determined at 48 h by real-time PCR after treatment with various formulations in HepG2 cells. (A) VEGF mRNA determined after treatment with different dose of CL/siRNA. (B) VEGF mRNA determined after treatment with free siRNA, free Sf, CMCS-Sf-CL, CMCS-Si-CL, SiSf-CL, CMCS-SiSf-CL (pH 7.4), CMCS-SiSf-CL (pH 6.5),and CMCS-NCsi-CL in HepG2 cells. Non-treated cells serve as control. The data are presented as the mean ± SD (n = 3), *p < .05, ***p < .005.
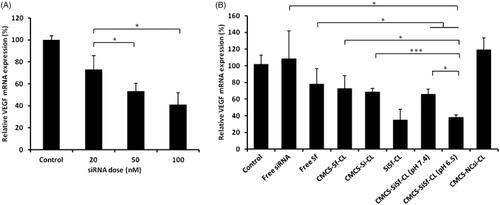
As shown in , there was almost no gene silence on free VEGF-siRNA and siN.C. groups. The cells treated with free VEGF-siRNA showed almost the same VEGF mRNA level as the normally cultured cells (control group), indicating that free siRNA was not cellular internalized. VEGF mRNA expression of free Sf and CMCS-Sf-CL was 78.31% ± 18.18% and 73.12% ± 14.99%, respectively, demonstrating some VEGF gene silencing efficacy of Sf. VEGF mRNA expression of CMCS-SiSf-CL in pH 7.4 and pH 6.5 culture condition was lower than that of free siRNA and Sf groups (p < .05). The results indicated that the co-loaded liposomes could deliver siRNA and Sf into the cytoplasm efficiently, resulting in high level of gene silencing. In addition, cells treated with CMCS-SiSf-CL at pH 6.5 showed a statistically decreased mRNA level (38.41 ± 2.27%) compared with CMCS-Si-CL (p < .001), CMCS-Sf-CL (p < .05), and CMCS-SiSf-CL at pH 7.4 (p < .05). The result evidenced the pH-sensitive CMCS peeling in acid environment and co-delivery VEGF-siRNA and Sf. In contrast, non-specific control sequences delivered by CMCS-NCSi-CL had no significant effect on VEGF expression.
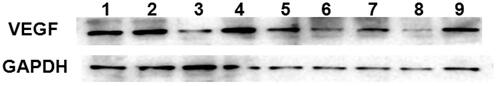
VEGF gene expression at the protein level determined by Western blot assay showed similar results. As shown in , compared with the control cells or cells incubated with CMCS-NCsi-CL, cells incubated with CMCS-Si-CL, SiSf-CL, and CMCS-SiSf-CL (pH 6.5 and 7.4) showed down**-regulated VEGF expression. VEGF protein expression of SiSf-CL and CMCS-SiSf-CL (pH 6.5) groups was significantly lower than that of the CMCS-SiSf-CL (pH 7.4) group. In addition, the VEGF expression in the SiSf-CL and the CMCS-SiSf-CL (pH 6.5) group was the lowest, suggesting the synergistic inhibition of the co-loaded liposomes. These data above underlined the significance of silencing VEGF gene in the chemotherapy of HCC and confirmed that CMCS modified liposomes could efficiently deliver VEGF-siRNA into cells to produce mRNA and protein levels of specific inhibition.
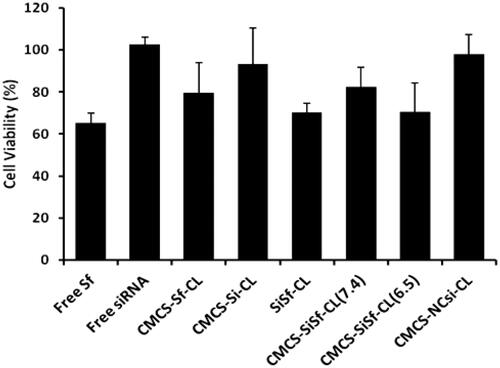
In vitro cytotoxicity
The cytotoxicity of different formulations in HepG2 cells following transfection was assessed using MTT assay at VEGF-siRNA concentration of 100 nM and Sf concentration of 2.8 μM after 48 h incubation. As shown in , free VEGF-siRNA, CMCS-Si-CL, and CMCS-NCsi-CL did not exhibit significant cytotoxicity against HepG2 cells at 48 h. The free Sf and the CMCS-Sf-CL group achieved 65.35% ± 4.64% and 79.56% ± 14.39% cell viability, respectively. Cell viability of the co-loaded formulations, including SiSf-CL, CMCS-SiSf-CL at pH 7.4 and pH 6.5, were above 70%, had no significant difference compared with that of free Sf and CMCS-Sf-CL groups. The results indicated that VEGF-siRNA had no significant inhibition on cells, which resulted in higher cell viability during transfection.
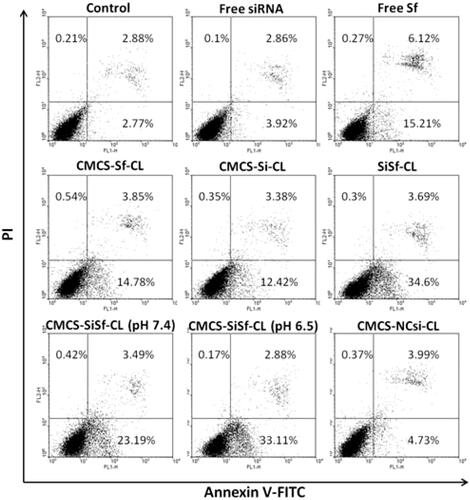
Cell apoptosis test
The effect of VEGF siRNA complexes on apoptosis was investigated by Annexin V-FITC and PI double staining and analyzed via flow cytometry. As shown in , the cells exhibited very few cell apoptosis incubated with CMCS-NCsi-CLcomplexes after 72 h. Cells treated with free Sf and CMCS-Sf-CL resulted in apoptosis (21.6% and 19.7%, respectively), and the free Sf group displayed late stage apoptotic (6.12%). SiSf-CL and CMCS-SiSf-CL at pH 7.4 and pH 6.5 induced early apoptosis. Moreover, CMCS-SiSf-CL induced 36.16% apoptosis cells at pH 6.5, which was higher than that of CMCS-SiSf-CL at pH 7.4 (27.1%) and approximately to that of SiSf-CL (38.59%). CMCS-Si-CL group showed 16.15% of apoptosis cells, which was not consistent with cytotoxicity assay described above. The results suggested that VEGF-siRNA uptake via the liposomes specific silenced VEGF gene expression and then induced early apoptosis of cells. It has been reported that VEGF-siRNA downregulated VEGF expression, triggering decrease of BCL-2/Bax ratio and release of cytochrome c from the mitochondrial and thereby inducing apoptosis, which finally resulted in highly cell apoptosis and cell death [Citation26,Citation27].
Conclusion
Above all, the study constructed CMCS modified Sf and VEGF-siRNA co-loaded liposomes. The obtained system CMCS-SiSf-CL was demonstrated to efficiently co-deliver Sf/siRNA in 2D cultured monolayer cells and penetrate into 3D cultured tumour spheroids at slightly acid environment (pH 6.5). The frozen sections of mice tumour tissues confirmed showed that either SiSf-CL or CMCS-SiSf-CL could co-deliver Sf and siRNA into tumour regions. What is more, CMCS-SiSf-CL at pH 6.5 downregulated VEGF expression significantly compared with free VEGF-siRNA, free Sf, Sf single-loaded CMCS-Sf-CL and VEGF-siRNA single-loaded CMCS-Si-CL in vitro. These results suggested that the co-delivery of Sf and VEGF-siRNA via CMCS-SiSf-CL would be a promising approach for HCC therapy.
Disclosure statement
The authors declare no competing financial interest.
Additional information
Funding
References
- Lipinski KA, Barber LJ, Davies MN, et al. Cancer evolution and the limits of predictability in precision cancer medicine. Trends Cancer. 2016;2:49–63.
- Yuppa DP. The emperor of all Maladies: a biography of cancer. Psychooncology. 2012;21:e7–e8.
- Park YN, Kim YB, Yang KM, et al. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061–1065.
- Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858.
- Jang HJ, Lee SA, Seong S, et al. Combined treatment for lung metastasis from hepatocellular carcinoma: a case report. Explore. 2018;14:385-388.
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34.
- Yao Y, Su Z, Liang Y. et al. pH-sensitive carboxymethyl chitosan-modified cationic liposomes for sorafenib and siRNA co-delivery. Int J Nanomed. 2015;10:6185–6197.
- Guan JB, Sun J, Sun FL, et al. Hypoxia-induced tumor cell resistance is overcome by synergistic GAPDH-siRNA and chemotherapy co-delivered by long-circulating and cationic-interior liposomes. Nanoscale. 2017;9:9190–9201.
- Wang TQ, Yu XY, Han LQ, et al. Tumor microenvironment dual-responsive core-shell nanoparticles with hyaluronic acid-shield for efficient co-delivery of doxorubicin and plasmid DNA. Int J Nanomed. 2017;12:4773–4788.
- Moller K, Muller K, Engelke H, et al. Highly efficient siRNA delivery from core-shell mesoporous silica nanoparticles with multifunctional polymer caps. Nanoscale. 2016;8:4007–4019.
- Duan XM, Mu MJ, Yan JF, et al. Co-delivery of Aurora-A inhibitor XY-4 and Bcl-xl siRNA enhances antitumor efficacy for melanoma therapy. IJN. 2018;13:1443–1456.
- Tsouris V, Joo MK, Kim SH, et al. Nano carriers that enable co-delivery of chemotherapy and RNAi agents for treatment of drug-resistant cancers. Biotechnol Adv. 2014;32:1037–1050.
- Kanamala M, Wilson WR, Yang M, et al. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review. Biomaterials. 2016;85:152–167.
- Dong D, Gao W, Liu Y, et al. Therapeutic potential of targeted multifunctional nanocomplex co-delivery of siRNA and low-dose doxorubicin in breast cancer. Cancer Lett. 2015;359:178–186.
- Ju C, Mo R, Xue J, et al. Sequential intra-intercellular nanoparticle delivery system for deep tumor penetration. Angew Chem Int Ed. 2014;53:6253–6258.
- Lin Q, Chen J, Zhang Z, et al. Lipid-based nanoparticles in the systemic delivery of siRNA. Nanomedicine. 2014;9:105–120.
- Ma D. Enhancing endosomal escape for nanoparticle mediated siRNA delivery. Nanoscale. 2014;6:6415–6425.
- Du Z, Munye MM, Tagalakis AD, et al. The role of the helper lipid on the DNA transfection efficiency of lipopolyplex formulations. Sci Rep. 2014;4:7107.
- Zhao Y, Gao H, He J, et al. Co-delivery of LOX-1 siRNA and statin to endothelial cells and macrophages in the atherosclerotic lesions by a dual-targeting core-shell nanoplatform: a dual cell therapy to regress plaques. J Control Release. 2018;283:241–260.
- Shen J, Sun H, Meng Q, et al. Simultaneous inhibition of tumor growth and angiogenesis for resistant hepatocellular carcinoma by co-delivery of sorafenib and survivin small hairpin RNA. Mol Pharm. 2014;11:3342–3351.
- Yang ZZ, Li JQ, Wang ZZ, et al. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials. 2014;35:5226–5239.
- Stapleton S, Jaffray D, Milosevic M. Radiation effects on the tumor microenvironment: implications for nanomedicine delivery. Adv Drug Deliv Rev. 2017;109:119–130.
- Fitzgerald KA, Malhotra M, Curtin CM, et al. Life in 3D is never flat: 3D models to optimise drug delivery. J Control Release. 2015;215:39–54.
- Wientjes MG, Yeung BZ, Lu Z, et al. Predicting diffusive transport of cationic liposomes in 3-dimensional tumor spheroids. J Control Release. 2014;192:10–18.
- Gao Y, Li M, Chen B, et al. Predictive models of diffusive nanoparticle transport in 3-dimensional tumor cell spheroids. AAPS J. 2013;15:816–831.
- Ge YL, Zhang X, Zhang JY, et al. The mechanisms on apoptosis by inhibiting VEGF expression in human breast cancer cells. Int Immunopharmacol. 2009;9:389–395.
- Miao RC, Xu XS, Wang ZX, et al. Synergistic effect of nutlin-3 combined with aspirin in hepatocellular carcinoma HepG2 cells through activation of Bcl-2/Bax signaling pathway. Mol Med Rep. 2018;17:3735–3743.