Abstract
Senile osteoporosis is closely related to the loss of function of stem cells. In this study, we tried to investigate the potential of secretome from human umbilical cord-derived mesenchymal stem cells (hUCMSCs) in recovering stem cell ability from senescence and then delaying bone loss. We first harvested bone marrow-derived mesenchymal stem cells (BMSCs) from young and old rats and then compared their cellular characteristics such as cell growth, anti-senescence and differentiation. The results showed that these abilities were negatively affected by animal aging. Subsequently, aged BMSCs were exposed to secretome from hUCMSCs, and we found that this loss of cell potential can be modified by secretome treatment. Thereafter, the secretome was loaded into silk fibroin-based hydrogels and used for an in vivo animal study. The results showed that compared to the old untreated group, the bone formation capacity of aged rats was improved by local treatment of secretome-loaded silk fibroin hydrogels. In conclusion, these findings demonstrated that secretome from hUCMSCs has the capacity to recover stem cell potential and delay local bone loss in age-related osteoporosis, which could potentially be applied in osteoporosis therapy in the future.
Introduction
Osteoporosis is characterized by obvious defects in both quality and quantity of bone tissue, which leads to the loss of bone strength and the reduction of resistance to fracture [Citation1]. Clinically, this complex skeletal disorder can be divided into two types: primary and secondary osteoporosis. Primary osteoporosis is a systemic disease that is closely related to aging and menopause, and it includes postmenopausal and senile osteoporosis. Secondary osteoporosis represents a particular set of bone diseases that are caused by certain conditions or treatments [Citation2]. With the aging of the population, the prevalence of osteoporosis and related complications is increasing rapidly worldwide.
Although the detailed pathologic mechanism of osteoporosis remains unknown, much scientific evidence suggests that resident BMSCs are closely related to the occurrence of osteoporosis. There are deficiencies in BMSCs isolated from osteoporotic patients [Citation3], and disorders in BMSCs proliferation and osteoblastic differentiation abilities can ultimately give rise to osteoporosis [Citation4]. Obviously, BMSCs is a key part of the homeostatic machinery of bone turnover [Citation5]. Nevertheless, despite these observations, current therapies for osteoporosis are mostly concentrated on how to inhibit bone resorption; for instance, oestrogen, bisphosphonates, selective oestrogen receptor modulators and calcitonin are very popular in clinics, and they all are anti-resorptive agents. Although these therapies are effective to some degree, serious adverse effects of long-term usage should also be of concern: oestrogen can increase the risk of cardiovascular events and breast cancer, bisphosphonates can lead to osteonecrosis of the jaw and calcitonin may result in an increased risk of cancer [Citation6]. Moreover, these therapies neither encourage bone formation nor do they focus on the primary cause of osteoporosis. Therefore, more effective and safer therapies are needed.
Many reports have indicated that the replication and differentiation features of old stem cells can be altered by culturing them with extracellular matrix or conditioned medium from young stem cells [Citation7,Citation8], and through exposure to a young systemic environment, the aged progenitor cells can even be “rejuvenated” from an old state to a young state [Citation9]. Thus, the environment of stem cells is closely connected to the state of the cells. As an important part of stem cell niche, the stem cell secretome is defined as the complex set of molecules secreted by stem cells. It includes a variety of serum proteins, angiogenic factors, growth factors, hormones, cytokines and extracellular matrix proteins [Citation10]; and these bioactive factors exhibit diverse physiological functions such as immunomodulation, anti-inflammation, angiogenesis, anti-apoptosis and anti-oxidation [Citation10]. Currently, there is growing interest in the investigation of these bioactive factors.
In this study, we hypothesized that secretome from hUCMSCs was able to regulate the environment of old stem cells. We believe that through modulating the aging environment, the regenerative potential of BMSCs can be improved and the process of osteoporosis can be delayed. The treatment effect of secretome on old BMSCs was analyzed by examining the characteristics of BMSCs (), including cell proliferation, anti-senescence (SA-β-gal activity and oxidative stress) and cell differentiation (osteogenesis and adipogenesis). For in vivo analysis, silk fibroin hydrogels were chosen as vehicles, the bioactivity and release kinetics in vitro and biodegradation in vivo were tested. The effects of secretome on bone regeneration were measured by micro-CT and tissue staining ().
Figure 1. Stem cell secretome collection and cell potential analysis. (A) A schematic diagram of the experimental procedure in vitro. (B) The conditioned medium of hUCMSCs was gathered together and lyophilized. (C) Cell proliferation of stem cells from young and aged rats and effects of different concentrations of secretome on aged BMSCs were analyzed by MTT assay over a period of 5 days. (D) BMSCs were stained for SA-β-gal and the quantitative result was expressed in a bar graph. Scale bar = 100 μm. (E) The level of ROS in BMSCs was judged by fluorescence microscopy analyses after labelling with DCFH-DA. Scale bar = 200 μm. (F) Age-related gene changes in p16, p21 and p53 were detected by qRT-PCR. Representative results of three experiments are shown. *p < .05 vs O-C group.
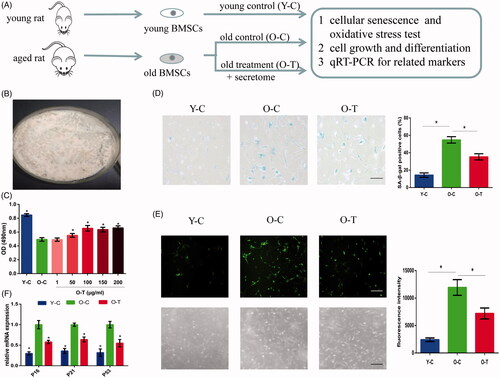
Figure 2. Osteogenic and adipogenic differentiation potential of BMSCs from young and aged rats and the effect of secretome on aged BMSC differentiation were investigated in vitro. (A, C) Osteogenic differentiation of BMSCs was examined using Alizarin Red S Staining and osteogenic specific markers including RUNX2 and OCN. (B) Adipogenic differentiation of BMSCs was investigated in vitro by taking Oil Red O staining. (D) Adipogenic markers including PPAR-γ2 and leptin were also tested. The bar graph represents quantitative data of staining intensities observed. Scale bar = 100 μm. Representative results of three experiments are shown. *p < .05 vs O-C group.
Materials and methods
Animals
Experiments were performed using young (3-month-old) and aged (15-month-old) male Sprague-Dawley rats, and the design of the animal experiments was approved by the ethical committee of Harbin Medical University.
Cell culture
BMSCs were isolated from young and aged male rats. All long bones were separated and the adhesion BMSCs were collected from bone fragments with growth medium (α-MEM supplemented with 10% foetal bovine serum (FBS), 100 U/mL of penicillin and 100 μg/mL of streptomycin). Cells had been characterized in our previous study [Citation11] and they were induced to differentiate into osteocytes and adipocytes in subsequent research, which verified the stem cell property of BMSCs. The hUCMSCs at passage one were provided by Hei Long Jiang Alliancells North Bioscience, and they were isolated and characterized by this company with ethical clearance.
Cells at passage three were used for the next stage of experiments. BMSCs were divided into three groups: the young control group (Y-C, which were isolated from young rats), the old control group (O-C, which were isolated from old rats) and the old treatment group (O-T, which were isolated from old rats and treated with secretome).
Preparation of stem cell secretome
To obtain the secretome, hUCMSCs were seeded in 75 cm2 culture flasks, when they reached 90% confluence the culture medium was replaced with serum-free DMEM. After 48 h, the medium was collected and centrifuged at 300×g for 5 min, then filtered with a 0.22 µm filter (Millipore, Billerica, MA), the serum-free conditioned medium from each flask was pooled, and the solution was lyophilized () and stored at −80 °C.
Cell viability assay
Cell viability was analyzed using a MTT assay. BMSCs were placed in a 96-well plate (5000 cells/well). In the treatment group, growth medium was added with different concentrations of secretome (ranging from 1 to 200 μg/ml), and then cells were cultured for 5 days. For the MTT assay, culture medium was replaced with 10 μl MTT (3–(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) reagent (5 g/l), after a four-hour incubation, the supernatant was replaced with 100 μl dimethyl sulfoxide, and the absorbance was measured at 490 nm.
Senescence-associated beta-galactosidase (SA-β-gal) staining
Since SA-β-gal is a biomarker of cellular senescence, the SA-β-gal assay has been widely used [Citation12] in research. The isolated BMSCs were cultured in a 6-well plate (1 × 105 cells/well) with or without secretome, after 5 days of incubation, SA-β-gal staining was performed, to quantify the percentage of SA-β-gal positive cells, BMSCs were observed under a phase contrast microscope and imaged.
Measurement of intracellular ROS
To examine BMSCs’ intracellular reactive oxygen species (ROS) levels, BMSCs were cultured with or without secretome in growth medium for 5 days, then growth medium was replaced with 10 µmol/L 2′,7-dichlorodihydrofluorescein diacetate (DCFH-DA, Solarbio, Beijing, China) and cells were incubated in a light-protected environment for 20 min. The accumulation of ROS in cells was viewed by fluorescence microscopy and imaged; the fluorescence intensity was quantified by a fluorescent microplate reader (Infinite M200 Pro, Tecan, Switzerland).
Osteogenic differentiation and alizarin red S staining
To examine osteogenic differentiation ability of different groups, BMSCs were exposed to osteogenic differentiation medium (DMEM supplemented with 10% FBS, 100 U/mL of penicillin, 100 μg/mL of streptomycin, 50 μM L-ascorbic acid, 100 nM dexamethasone and 10 mM β-glycerophosphate) with or without hUCMSC secretome. After 14 days of differentiation, BMSCs were stained with Alizarin Red S. In order to measure the degree of osteogenesis, the bound staining was eluted with 10% cetylpyridinium chloride and the absorbance of supernatants was measured at 540 nm.
Adipogenic differentiation and oil red O staining
To examine adipogenic differentiation ability of different groups, BMSCs were exposed to adipogenic differentiation medium (DMEM with 10% FBS, 100 U/mL of penicillin, 100 μg/mL of streptomycin, 1 μM dexamethasone, 0.5 mM isobutylmethylxanthine (IBMX), 10 μg/ml insulin and 200 μM indomethacin) for 14 days, and then the cells were analyzed by Oil red O staining. For quantification, the lipid droplets were extracted with 100% absolute isopropyl alcohol and the absorbance was measured at 540 nm.
Sonication-induced gelation and secretome capsulation
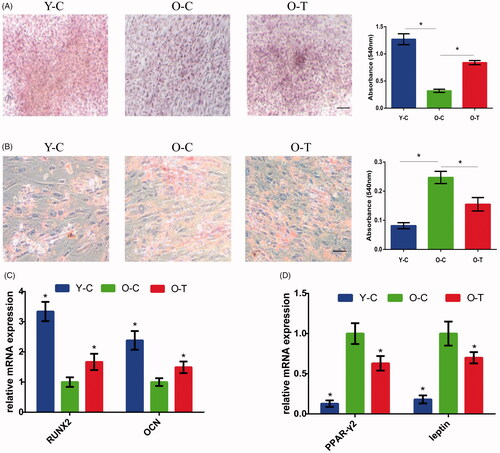
Silk fibroin is a natural polymer, after ultrasonication, silk fibroin structure changes from random coil-dominant to β-sheet-dominant, and a large number of interchain β-sheet structures form, such that silk fibroin transforms from solution-state to gel-state [Citation13]. During the process, cells or bioactive molecules can be added [Citation14] (). As shown in , the silk powder (Simatech Inc., Suzhou, China) was dissolved in deionized water and the final concentration of the silk fibroin was 5.0% (w/v). Then it was sonicated using a Branson 450 Sonifier (Sonics & Materials, Inc. Newtown, CT) at 30% amplitude for 30 s. The process was repeated after 30 min, and hUCMSC secretome was added when the silk solution had cooled to room temperature. This mixed solution was incubated at 37 °C for 8 h to allow gelation. The micro-structure of gel was imaged using Scanning electron microscopy (SEM).
Figure 3. Preparation of secretome-loaded silk fibroin hydrogels and morphology analysis. (A) Schematic illustration of silk sol-gel transition and secretome capsulation. (B) Silk powder was dissolved in deionized water and silk solution was transformed into silk hydrogel by ultrasonication. (C) Silk hydrogel morphology determined by SEM.
The bioactivity of released secretome in vitro
The bioactivity of released secretome was analyzed by MTT assay. Silk gel (100 μl) with different concentrations of secretome (g + s, ranging from 0 to 2000 μg/ml) was immersed in a microcentrifuge tube containing 1 ml growth medium and incubated at 37 °C. The supernatant was withdrawn at fifth day and the extraction solution containing released secretome was collected.
BMSCs from old rats were seed on a 96-well plate (5000 cells/well) and the medium was replaced with extraction solutions. After 5 days, cell viability was analyzed using MTT assay. Additionally, we use the growth medium with secretome (100 μg/ml) as a positive control group (PCG) but without secretome as a negative control group (NCG).
Release kinetics of secretome from silk gels in vitro
MSCs can secrete a variety of trophic factors such as transforming growth factor β (TGF-β), epithelial growth factor (EGF), fibroblast growth factor (FGF), insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF) and so on; these factors can promote cell proliferation and anti-apoptotic activity and stimulate bone regeneration [Citation15,Citation16]. In this study, TGF-β in secretome was used as a calibrator to test the release kinetics of secretome from silk gels. 500 μl silk gel encapsulated with secretome was prepared using 1-ml medical syringes. It was equally divided into five cylinder-shape segments (approximately 100 μl), and each segment was immersed in a microcentrifuge tube containing 1 ml PBS and incubated at 37 °C. At various time points (5, 10, 15, 20, 25, 30 days), the supernatant was collected, and tubes were replenished with fresh buffer continuously for up to 30 days. The total TGF-β in each segment and the release of TGF-β in the supernatant were quantified using a TGF-β ELISA Kit (Shanghai Jianglai Biotech, Shanghai, China) according to the manufacturer’s instructions and the cumulative release rate was calculated.
Intratibial injection
Rats were grouped as follows: young group (3-month-old, receiving PBS), old group (15-month-old, receiving PBS), secretome group (15-month-old, receiving secretome solution, 100 μg/ml), gel group (15-month-old, receiving silk gel treatment) and g + s group (15 month-old, receiving silk gel encapsulated with secretome). Each group contained six animals. PBS, secretome solution and silk gel were injected into the bone marrow (about 10 µl). Briefly, after anaesthesia with intraperitoneal injection of 4% chloral hydrate at a dose of l0 ml/kg, a 26-gauge needle was inserted into the bone marrow cavity of the right tibia, followed by injection of solution or gel into it (). After transplantation, the animals were returned to the same conditions of housing.
Bone density and bone structure analysis
Eight weeks later, all rats were sacrificed, and tibias from each rat were isolated and fixed in 10% formalin-buffered solution at room temperature, Bone microstructure was analyzed by micro-CT scanning (model 1172; Skyscan, Belgium). Images were acquired at 80 kV, 100 μA, 360° rotation scan, and the scan time for each specimen was approximately 20 min. For histology analysis, bone samples were decalcified in 9% formic acid and embedded in paraffin and sectioned (thickness = 5 μm), and sections were subjected to Masson Trichrome staining. The data collected were quantitatively represented as bone mineral density (BMD), trabecular bone volume expressed as percentage of total tissue volume (BV/TV), trabecular thickness (Tb.Th) and trabecular number (Tb.N). For biodegradation of silk gel, bone samples were collected at weeks 2 and 8 and then subjected to Masson Trichrome staining.
Gene expression analysis
All cells were harvested and total RNA was extracted using Trizol reagents (Invitrogen Life Technologies, Carlsbad, CA). 1 μg of total RNA was reverse transcribed with 0.5 μg of oligo(dT) primer. To quantify mRNA expression, PCR was performed with SYBR Green PCR MasterMix (ThermoScientific, Fremont, CA). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) served as an internal standard. All experiments were performed in triplicate to determine mRNA transcript levels of p16, p21, p53, Runt-related transcription factor 2 (RUNX2), Osteocalcin (OCN), peroxisome proliferator-activated receptor gamma2 (PPARγ2) and leptin. The primer pairs used are listed in . Relative gene expression was calculated by comparing the cycle threshold (CT) ratios to that of the reference gene.
Table 1. Primer sequences for qRT-PCR.
Statistical analysis
Every experiment was repeated at least three times and values were averaged. All results were represented as the mean ± standard deviation and all statistical analyses were carried out using SPSS19.0 (SPSS Inc., Chicago, IL). The one-way analysis of variance (ANOVA) with Tukey’s post hoc test for multiple group comparisons was used and p values <.05 was considered statistically significant.
Results
Effect of secretome on cell viability
To determine whether cell viability can be affected by senescence, the cell proliferation of BMSCs from young and old rats was examined. As shown in , compared to young BMSCs, aged stem cells showed an obvious reduction in cell proliferation ability. We then examined the effects of different concentrations of secretome on aged BMSCs. We cultured aged BMSCs supplemented with 1, 50, 100, 150 and 200 μg/ml hUCMSC secretome. After 5 days, the OD value showed an increasing dose-dependent tendency in response to secretome when the concentrations were less than 100 μg/ml; however, when the concentrations exceeded 100 μg/ml, there was no obvious difference. Based on this result, the dose of 100 μg/ml was chosen as the optimal concentration in the following experiments.
Evaluation of SA-β-gal staining and senescence biomarker changes in BMSCs
To determine the anti-aging ability of stem cells from different groups, SA-β-gal staining was used and senescence biomarkers were tested. As shown in , the SA-β-gal staining intensity of aged BMSCs was almost four fold higher than that of those from young rats, and in addition, a significant up-regulation of p16, p21 and p53 was detected. Then, we examined whether secretome treatment could delay stem cell senescence. The result showed that SA-β-gal staining positive rate was decreased dramatically after secretome treatment, and similarly, the expression of senescence biomarkers was also reduced.
Determination of intracellular reactive oxygen species level in BMSCs
ROS is a key modulator of stem cell state and function [Citation17] and excessive amounts of ROS will affect cell senescence and differentiation [Citation18,Citation19]. In this study, DCFH-DA fluorescence was used to determine intracellular ROS generation. The results showed that endogenous ROS levels were increased in BMSCs from old rats:the fluorescence increased by nearly fourfold compared to the young group (). Moreover, we discovered that secretome can efficiently regulate oxidative stress in aged cells, after secretome treatment, the levels of ROS in aged BMSCs were more close to that in the young group.
Influence of secretome on cell differentiation
Multipotent differentiation capacity is crucial for defining MSC, and many researchers believe there is a balance between osteoblastic and adipocyte differentiation in BMSCs [Citation20]. Therefore, we examined whether the osteogenic and adipogenic abilities of BMSCs were affected by senescence and whether the multipotent differentiation ability could be recovered through the supplementation with secretome. After inducing for 14 days, we could see the diminished formation of bone mineralization () while lipid deposit accumulation increased () with age, and compared to young BMSCs, an obvious down-regulation in osteogenesis markers including RUNX2 and OCN accompanied by a significant up-regulation in adipogenesis markers such as PPAR-γ2 and leptin was detected in aged BMSCs (). This result was in accordance with the hypothesis that aging environment is favourable for adipogenic differentiation rather than osteogenic differentiation [Citation21]. To determine whether secretome treatment could recover stem cell differentiation ability, aged BMSCs were induced with supplementation of secretome. The results showed that secretome treatment led to an elevated level of matrix mineralization () with a decreased level of lipid deposition (). In addition, the qRT-PCR results indicated that the expression levels of RUNX2 and OCN were up-regulated and the levels of PPAR-γ2 and leptin were down-regulated in the treatment group (). It is obvious that secretome treatment promoted osteogenesis rather than adipogenesis of aged BMSCs.
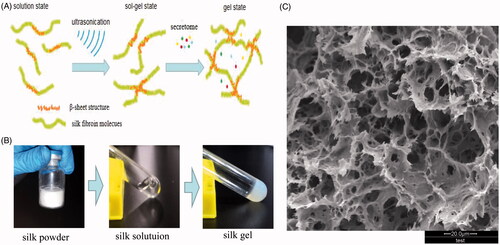
Figure 4. Silk fibroin hydrogels properties analysis and intratibial injection. (A) A schematic diagram of the experimental procedure in vivo. (B) The bioactivity of released secretome was analyzed by MTT assay, representative results of three experiments are shown. *p < .05 vs NCG. (C) Cumulative release rate of TGF-β from silk gels detected by ELISA. (D) Silk hydrogel was injected into the bone marrow cavity of a rat. (E) Histological study of degradation performance of silk gels at weeks 2 and 8. *Represents remaining gels. Scale bar = 100 μm.
The morphology of gel and bioactivity of released secretome in vitro
As shown in , the gel revealed high porosity and interconnectivity; this network structure can be characterized as a vehicle for encapsulation and delivery of cells and bioactive molecules. In order to test the bioactivity of released secretome in vitro, we cultured aged BMSCs with extraction solutions of different groups. After 5 days, the OD value showed an increasing dose-dependent tendency in response to released secretome when the concentrations were less than 1000 μg/ml; when the concentrations exceeded 1000 μg/ml, there was no obvious difference. Based on this result, the dose of 1000 μg/ml was chosen as the optimal concentration ().
The release profile in vitro and biodegradation in vivo
shows the cumulative release rate of delivered TGF-β when incubated in vitro. We can see that the release profile was sustained and orderly and there was no burst release during the in vitro culture, indicating that bioactive molecules encapsulated in silk hydrogels can be released slowly and continuously. This feature is important for choosing an ideal vehicle; accordingly, fabricating silk gel as a vehicle to deliver secretome was accepted in this research. shows the biodegradation process of silk gel in vivo. We can see that the gels degraded into small pieces and were surrounded by fibrous connective tissue or bone tissue, and there was a clear interface between silk gel and surrounding tissues.
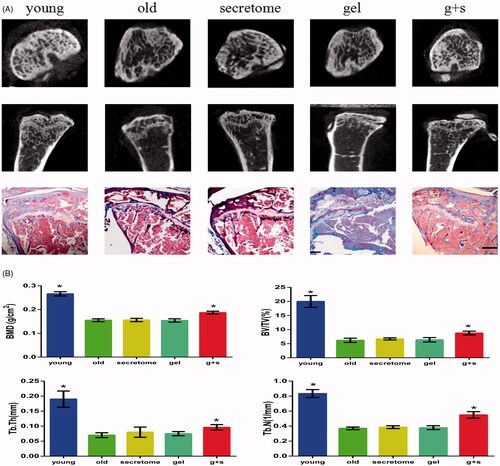
Reduction of bone mass attenuation in aged rats by secretome
In this study, micro-CT and Masson staining were used to analyze bone histomorphometry. As shown in , the young group possessed a higher BMD score, and the BV/TV, Tb.Th and Tb.N scores in the young group were also significantly higher than those from the aged group, which indicated that the bone mass of rats decreased with the increase of age. This condition did not change with the injection of secretome solution; we believe the reason is that the secretome solution had flushed out into the circulation before it exerted its effects, so we need a carrier which can degrade slowly and release secretome continuously at sites of osteoporosis. Based on our previous research, we chose silk fibroin hydrogels as the carrier. There was no difference in scores between the aged group and the silk gel group, which meant silk gels alone cannot work. We then injected silk gels encapsulated with secretome into the bone marrow, and after 8 weeks, we found a marked increase in the BMD score, the BV/TV, Tb.Th and Tb.N scores were also increased, indicating the effectiveness of secretome.
Figure 5. Micro-CT and Masson trichrome staining analysis of tibias from different groups. (A) Representative images from micro-CT and Masson staining of trabecular bone from the tibias. Scale bar =500 μm. (B) Bone characteristics were analyzed through bone mineral density (BMD), trabecular bone volume/total tissue volume (BV/TV), trabecular thickness (Tb.Th), and trabecular number (Tb.N). *p < .05 vs old group.
Discussion
Osteoporosis is a degenerative disease characterized by drastic bone loss which results in osteopenia and high fracture risk. Previous investigations have discovered that in osteoporosis patients, BMSC viability was reduced and the differentiation of BMSCs into adipocytes occurred more readily [Citation22], so there is a relationship between stem cell senescence and organ dysfunction [Citation23].
To recover BMSCs’ potential from senescence and promote bone regeneration, secretome from hUCMSCs was used in our research. hUCMSCs are a stem cell population obtained from Wharton’s jelly. Compared to other cell sources, hUCMSCs exhibit lower immune rejection and have higher multipotency capacity; moreover, hUCMSCs are isolated from umbilical cords, so during the collection process, there are no invasive procedures or ethical controversies [Citation24].
In previous studies, stem cell transplantation technology was used to promote bone regeneration; however, when cell therapy is employed, several limitations such as immune incompatibility and tumourigenicity should be taken into consideration. Furthermore, keeping transplant cells at a specific differentiation stage remains a major technical hindrance [Citation25]. When secretome from MSCs is employed, these limitations can be avoided. In addition, combined with proper vehicles, secretome can be applied directly to sites of osteoporosis with controlled dosage, space and time [Citation10,Citation24].
Methods of protein concentration include lyophilization, centrifugation, protein precipitation by trichloroacetic acid and ultrafiltration [Citation10]. Previous research has proven that lyophilization of the conditioned medium of stem cells can successfully conserve the original properties of secretome [Citation24,Citation26], so in order to better control dosage, the secretome of hUCMSCs was collected by lyophilization in our research.
Age-related osteoporosis is a complex disease, and in order to study it, physiologically similar models are needed. It has been reported that SD rats more than 9 months old can be used for establishing an age-related bone loss model [Citation27], and previous research has proven that male SD rats would develop osteoporosis naturally with age [Citation28,Citation29]. Therefore, aged SD rats were chosen as a model of senile bone loss in our research. However, it took a long time to develop characteristic features of bone aging.
In this study, we found that BMSCs obtained from old rats showed decreased growth viability compared with BMSCs from young rats, and this result indicated that age factors can affect cell viability, in accordance with other research [Citation22,Citation23]. To confirm the effect of hUCMSC secretome on aged BMSC viability, a broad range of concentrations was examined and the results showed that hUCMSC secretome can improve cell viability to some extent. As one of the most popular biomarkers for cell senescence [Citation30], SA-β-gal activity increased significantly in aged group, a result that was in accordance with the cell growth viability test. In addition, when hUCMSC secretome was added, the SA-β-gal activity decreased obviously, indicating the anti-senescence property of secretome.
In addition to testing cellular characteristics, the expression of senescence-related genes was also tested. p16, p21 and p53 have been proven to be very important for cell cycle progression [Citation31]. Previous reports have indicated that senescence could be triggered by p53 and its downstream target p21 in a telomere-dependent manner, and the up-regulation of p16 is related to permanence of the senescent state [Citation32]. Therefore, in this study, p16, p21 and p53 were chosen as senescence markers. The results showed that the expression of p16, p21 and p53 was significantly up-regulated in BMSCs from aged rats and this increase could be down-regulated by secretome treatment.
Although the mechanisms of senescence are not completely understood, the ROS theory is the most widely accepted theory of aging [Citation33]. Numerous studies have provided evidence for the positive correlation between oxidative and osteoporotic status [Citation34]. Increased levels of ROS will lead to induction of senescence-associated secretory phenotypes of MSCs [Citation35], it can also promote adipogenesis and blunt osteogenesis [Citation18]. In this study, BMSCs from aged rats displayed higher levels of ROS than those from young rats, and compared to the aged group, old BMSCs cultured with secretome showed a decrease of ROS, which proved that secretome could diminish endogenous oxidant production in cells.
It has been reported that senior citizens with osteoporosis have more adipogenesis in their bone marrow cavities [Citation20], researchers have also discovered that aging is accompanied by decreased bone regeneration and increased adipocyte differentiation [Citation36]. This imbalance between bone marrow adipogenesis and osteogenesis is due to BMSC aging [Citation37]. In this study, we demonstrated that secretome could modulate cell differentiation by promoting osteogenesis and inhibiting adipogenesis. Secretome treatment also elevated the expression of osteogenesis markers such as RUNX2 and OCN and suppressed adipogenesis regulators such as PPAR-γ2 and leptin. Collectively, secretome could promote osteogenesis rather than adipogenesis of aged BMSCs without genetic manipulation.
To inject secretome directly into sites of osteoporosis for local treatment, an appropriate carrier is required to prevent soluble secretion factors from being flushed out before they exert their effects. Our previous research has demonstrated the good biodegradation and biocompatibility of silk fibroin hydrogels [Citation13,Citation38], they can also be used as vehicles for encapsulation and delivery of cells and bioactive molecules [Citation14,Citation39]. The in vitro studies confirmed the bioactivity of secretome encapsulated in gels and the release process was sustained and orderly, and biodegradation in vivo has also been tested; therefore, silk hydrogels were chosen as vehicles to encapsulate and release secretome in vivo. To monitor the degree of osteoporosis, BMD scan and morphological validation by micro-CT and Masson staining were used. We found that transplantation of secretome-loaded silk fibroin hydrogels brought about a clear reversal of the negative impacts of osteoporosis. The BMD score, BV/TV, Tb.Th and Tb.N were all increased, which indicated the beneficial effects of secretome transplantation and the feasibility of silk fibroin hydrogels as a carrier.
Currently, it is known that umbilical cord mesenchymal stem cells can secrete a large number of bioactive molecules [Citation40]. Many of them, such as TGF-β, EGF, FGF, VEGF and IGF, favour bone regeneration [Citation41,Citation42], the antioxidant effects of secretome also contribute to bone remodelling [Citation34]. In this study, we have proved the feasibility of using secretome from hUCMSCs as a molecular cocktail to recover cell viability and enhance cell proliferation, osteoblast differentiation and bone regeneration. Future work will focus on better characterizing the secretome and defining which factors are in charge of its therapeutic effects.
Conclusions
In this research, the potential effects of secretome on bone regeneration were investigated. We found that the characteristics of cell growth, anti-senescence and cell differentiation of BMSCs and bone formation capacity of SD rats were all negatively affected by aging factors, and that this condition could be improved by secretome from hUMSCs by means of regulation of the aging environments of stem cells. Future work will focus on investigation of secretome and its components. This study supports the idea that secretome from hUMSCs has potential usage in the treatment of age-related osteoporosis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Hennemann A. Osteoporosis: prevention, diagnosis and therapy. Med Monatsschr Pharm. 2002;25:164–167.
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325.
- Rodríguez JP, Montecinos L, Ríos S, et al. Mesenchymal stem cells from osteoporotic patients produce a type I collagen deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2015;79:557–565.
- Jones G, Nguyen T, Sambrook PN, et al. Symptomatic fracture incidence in elderly men and women: the Dubbo Osteoporosis Epidemiology Study (DOES). Osteoporosis Int. 1994;4:277–282.
- Seeman E. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261.
- Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabet Endocrinol. 2017;5:898–9075.
- Sun Y, Li W, Lu Z, et al. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. Faseb J. 2011;25:1474–1485.
- Mirsaidi A, Genelin K, Vetsch JR, et al. Therapeutic potential of adipose-derived stromal cells in age-related osteoporosis. Biomaterials. 2014;35:7326–7335.
- Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764.
- Tran C, Damaser MS. Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv Drug Deliv Rev. 2015;82:1–11.
- An G, Zhang WB, Ma DK, et al. Influence of VEGF/BMP-2 on the proliferation and osteogenetic differentiation of rat bone mesenchymal stem cells on PLGA/gelatin composite scaffold. Europ Rev Med Pharmacol Sci. 2017;21:2316.
- Burova E, Borodkina A, Shatrova A, et al. Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium. Oxid Med Cell Longev. 2013;2013:1–12.
- Wang Y, Liang M, Zheng Z, et al. Adhesion prevention after laminectomy using silk‐polyethylene glycol hydrogels. Adv Healthcare Mater. 2015;4:2120–2127.
- Wang X, Kluge J, Leisk GG, et al. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29:1054–1064.
- Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260.
- Ramaswamy Y, Lim KS, Zreiqat H, et al. Stem cells for bone regeneration: role of trophic factors. In: Alessandro Rozim Zorzi, Joao Batista de Miranda, editors. Advanced Techniques in Bone Regeneration. London: IntechOpen; 2016. p. 357–374.
- Ludin A, Gur-Cohen S, Golan K, et al. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antiox Redox Signal. 2014;21:1605–1619.
- Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev. 2015;24:1150–1163.
- Chiba Y, Shimada A, Kumagai N, et al. The senescence-accelerated mouse (SAM): a higher oxidative stress and age-dependent degenerative diseases model. Neurochem Res. 2009;34:679–687.
- Moerman EJ, Teng K, Lipschitz DA, et al. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389.
- Zhang W, Ou G, Hamrick M, et al. Age-related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. J Bone Miner Res. 2008;23:1118–1128.
- Rodríguez JP, Garat S, Gajardo H, et al. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 2015;75:414–423.
- Liu HY, Huang CF, Lin TC, et al. Delayed animal aging through the recovery of stem cell senescence by platelet rich plasma. Biomaterials. 2014;35:9767–9776.
- Wang KX, Xu LL, Rui YF, et al. The effects of secretion factors from umbilical cord derived mesenchymal stem cells on osteogenic differentiation of mesenchymal stem cells. PLoS One. 2015;10:e0120593
- Liu HY, Wu AT, Tsai CY, et al. The balance between adipogenesis and osteogenesis in bone regeneration by platelet-rich plasma for age-related osteoporosis. Biomaterials. 2011;32:6773–6780.
- Eiró N, Sendon-Lago J, Seoane S, et al. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget. 2014;5:10692–10708.
- Zhu CT, Li T, Zhang P, et al. Beneficial effects of low-level laser irradiation on senile osteoporosis in rats. Eur Rev Med Pharmacol Sci. 2007;21:5230–5238.
- Pietschmann P, Skalicky M, Kneissel M, et al. Bone structure and metabolism in a rodent model of male senile osteoporosis. Exp Gerontol. 2007;42:1099–1108.
- Xu R, Shen X, Si Y, et al. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell. 2018;17:e12794.
- Zhou Y, Dong Y, Xu QG, et al. Mussel oligopeptides protect human fibroblasts from hydrogen peroxide (H2O2)-induced premature senescence. Arch Gerontol Geriatr. 2014;58:293–299.
- Pellizzaro C, Coradini D, Daniotti A, et al. Modulation of cell cycle-related protein expression by sodium butyrate in human non-small cell lung cancer cell lines. Int J Cancer. 2001;91:654–657.
- Cheng H, Qiu L, Ma J, et al. Replicative senescence of human bone marrow and umbilical cord derived mesenchymal stem cells and their differentiation to adipocytes and osteoblasts. Mol Biol Rep. 2011;38:5161–5168.
- Krause KH. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp Gerontol. 2007;42:256–262.
- Guicheux J, Wittrant Y, et al. Oxidative stress in bone remodelling and disease. Trends Mol Med. 2009;15:468–477.
- Zhou L, Chen X, Liu T, et al. SIRT1-dependent anti-senescence effects of cell-deposited matrix on human umbilical cord mesenchymal stem cells. J Tissue Eng Regen Med. 2018;12:e1008–e1021.
- Lecka-Czernik B, Rosen CJ, Kawai M, et al. Skeletal aging and the adipocyte program. Cell Cycle. 2010;9:3672–3678.
- Justesen J, Stenderup K, Eriksen EF, et al. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int. 2002;71:36–44.
- Leng X, Liu B, Su B, et al. In situ ultrasound imaging of silk hydrogel degradation and neovascularization. J Tissue Eng Regen Med. 2017;11:822–830.
- Ma D, An G, Liang M, et al. A composited PEG-silk hydrogel combining with polymeric particles delivering rhBMP-2 for bone regeneration. Mater Sci Eng C Mater Biol Appl. 2016;65:221–231.
- Bai L, Li D, Li J, et al. Bioactive molecules derived from umbilical cord mesenchymal stem cells. Acta Histochemica. 2016;118:761–769.
- Yun YR, Jang JH, Jeon E, et al. Administration of growth factors for bone regeneration. Regen Med. 2012;7:369–385.
- Erlebacher A, Filvaroff EH, Ye JQ, et al. Osteoblastic responses to TGF-beta during bone remodeling. Mol Biol Cell. 1998;9:1903.
