Abstract
Osteosarcoma is a common malignant bone tumour in adolescents and old people, with highly invasive and metastatic features and poor prognosis. This study aimed to explore the role of miR-384 in osteosarcoma MG63 cells by targeting SLBP. Cell viability, migration and invasion, apoptosis, as well as apoptosis-related factors were evaluated by CCK-8 assay, Transwell assay, flow cytometer and Western blotting, respectively. Dual-luciferase reporter assay was used to determine the target of miR-384. SLBP level was analyzed using qRT-PCR and Western blotting. Important factors of MEK/ERK and PI3K/AKT signalling pathways were analyzed using Western blotting. We found that miR-384 was down-regulated in osteosarcoma tissue samples and cell lines (MG63, U2OS and OS732). miR-384 overexpression inhibited viability, migration and invasion, but promoted apoptosis of MG63 cells; whereas, miR-384 silence exhibited the contrary effects on MG63 cells. SLBP was a target of miR-384. Knockdown of SLBP reversed the promoting effect of miR-384 silence on cells, indicating that miR-384 silence promoted growth and metastasis of MG63 cells by up-regulating SLBP. In conclusion, knocking down miR-384 promoted the growth and metastasis of osteosarcoma MG63 cells by up-regulating SLBP. To conclude, miR-384-SLBP may be a potential therapeutic target for osteosarcoma therapy.
Introduction
Osteosarcoma, derived from primitive bone-forming mesenchymal cells, is the most common primary bone malignancy and occurs most frequently in adolescents, but there is a second incidence peak in older adulthood [Citation1,Citation2]. The survival is correlated with the age of patients, with the poorest survival among the elderly [Citation3]. To date, complete surgical excision is important to ensure an optimum outcome [Citation4]. However, many factors have effects on the outcome, including size of the tumour, tumour staging, presence of metastases, local recurrence, chemotherapy regimen, anatomic location, and percentage of tumour cells destroyed by neoadjuvant chemotherapy [Citation4]. Although the therapy for this disease continues to be updated and changed, the overall survival still remains poor [Citation5]. Some new studies have demonstrated that certain oncogenes or tumour suppressors were deregulated in osteosarcoma [Citation6,Citation7]. Thus, investigating and discovering potential therapeutic targets may improve the survival of osteosarcoma patients.
microRNAs (miRNAs/miRs) are short non-coding single-stranded RNA molecules with ∼22 nucleotides. miRNAs can inhibit protein expression at a post-transcriptional level, mostly via base pairing with the 3'-untranslated regions (3’UTR) of the target mRNA, thereby leading to its reduced translation and/or degradation [Citation8,Citation9]. In spite of controlling basic biological functions, some miRNAs may function as “oncomiRs” (miRNAs that are related to cancer) [Citation10]. In osteosarcoma, some miRNAs have been found abnormal expressed, like miR-143, miR-199a, miR-21, and miR-221 [Citation11–14]. They acted as oncogenes or tumour suppressors in osteosarcoma.
Aberrant expression of miR-384 has been found in some kinds of cancers, including renal cell carcinoma [Citation15], non-small-cell lung cancer [Citation16], colorectal cancer [Citation17] and hepatocellular carcinoma [Citation18], indicating that miR-384 might play a critical role in tumorigenesis. Moreover, it exerted anti-proliferation, anti-invasion, anti-metastasis, and pro-apoptotic activities in these cancers via regulating its target genes like AEG1, KRAS, CDC42, and IRS1 [Citation15–18], revealing the cancer suppressive role of miR-384.
To our knowledge, the role of miR-384 in osteosarcoma has not been reported. Therefore, the present study focused on the effects of miR-384 on osteosarcoma cells as well as the underlying molecular mechanisms via which down-regulating miR-384 promoted growth and metastasis of osteosarcoma cells. Human osteosarcoma cell line MG63 was used in this study. The expression of miR-384 in MG63 cells was overexpressed and suppressed by transfection with miR-384 mimic and miR-384 inhibitor, respectively. Thereafter, cell viability, cell migration and invasion, apoptotic cells rate, and the expression of apoptosis-associated factors were detected. Moreover, the regulation of miR-384 and SLBP was evaluated to reveal whether SLBP was involved in roles of miR-384 in MG63 cells.
Methods
Clinical specimens
To examine miR-384 level, clinical human osteosarcoma tissues and the corresponding normal tissues (12 males and 8 females) were obtained from 20 patients hospitalized at China-Japan Union Hospital of Jilin University (Jilin, China). The ages of the patients were 8–22 years old. The tumour sizes were among 5–10 cm. The anatomic sites included femur (n = 10), tibia (n = 7) and humeral bone (n = 13). The clinical specimens were divided into two groups: grade I-II A (n = 8) and grade II B-III (n = 12) based on the WHO 2007 classification of tumours. No patients received any therapies before surgery. Informed consents from all patients or their guardians were obtained, and the present research was approved by the Medical Ethics Committee of the China-Japan Union Hospital of Jilin University.
Cell culture
The human osteoblast cell line hFOB1.19 and three osteosarcoma cell lines MG63, U2OS, OS732 attained from Shanghai Institutes for Biological Sciences Cell Resource Center (Shanghai, China) were incubated in high glucose Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Carlsbad, CA, USA) with the supplement of 10% fetal bovine serum (FBS; Gibco). All cell lines were incubated at 37 °C in a humidified incubator with 5% CO2.
Cell transfection assay
miR-384 mimic, miR-384 inhibitor, and their corresponding negative control (NC) were synthesized by GenePharma Co. (Shanghai, China). The siRNA of SLBP (si-SLBP) was designed and synthesized by GenePharma. Cell transfections were conducted using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol.
Cell counting kit-8 (CCK-8) assay
To evaluate cell viability, 96-well plate was added with 100 μL cells, approximately 5,000 cells/well. Ten microlitres CCK-8 solution (Dojindo Molecular Technologies, Gaithersburg, MD) was added to each well. The cultures were incubated for 2 h at 37 °C in humidified 95% air and 5% CO2. After incubation, Microplate Reader (Bio-Rad, Hercules, CA) was used to measure the absorbance of cultures (at 450 nm).
Migration and invasion assay
The behaviours of migration and invasion were determined using Transwell assay. Transwell chambers with a pore size of 8 μm were used. In the migration assay, transfected cells were suspended in 100 μL serum-free medium (5 × 105 cells/mL) in the upper chamber, and 600 μL complete medium was in the lower chamber. After 48 h (37 °C, 5% CO2), cells on the upper membrane surface were removed. Migrated cells on the lower side of the membrane were stained with crystal violet and counted. In the invasion assay, the Transwell membrane was coated with 80 μL of Matrigel solution (500 ng/μL; BD, Franklin Lakes, NJ, USA). Invasion assay was also conducted referring to the step above.
Apoptosis assay
Cells were resuspended in phosphate buffered saline (PBS) (Gibco) and washed twice with PBS. Cells were added with 100 μL binding buffer and 10 μL fluorescein isothiocynate (FITC)-conjugated Annexin V (20 μg/mL). Then, they were incubated at room temperature in the dark for 30 min. Cultures were added with 5 μL PI (50 μg/mL) and further incubated for 5 min in the dark. Afterwards, 400 μL binding buffer was added in the cultures. The quantitative detection was immediately performed using a FACScan (Beckman Coulter, Fullerton, CA, USA) and the data were analyzed using FlowJo software.
qRT-PCR
RNAs were isolated from tested cells and tissues using TRIzol reagent (Invitrogen). For detecting miR-384 expression, 10 ng RNA was reversely transcribed using TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA). The qRT-PCR was performed on Bio-Rad CFX Connect Real-Time PCR Detection System using SsoFast Probes Supermix (Bio-Rad, Hercules, CA). miR-384 expression was normalized to U6. For detecting SLBP expression, 3 μg of total RNA was used to synthesize first strand cDNAs by a First Strand cDNA Synthesis Kit (Roche Diagnostics GmbH, Mannheim, Germany). Quantitative PCR was performed using an SYBR Green RT-PCR Kit and a Thermal Cycler Dice Real Time System (Takara Bio, Shiga, Japan). SLBP expression was normalized to GAPDH. Expression levels of genes were calculated using 2−ΔΔCt method.
Dual luciferase activity assay
The 3’UTR of human SLBP cDNA containing the putative target site for miR-384 was chemically synthesized and inserted at the XbaI site as a wild type (wt) with a corresponding mutant type (mt). Twenty-four hours before transfection, cells were plated in 24-well plates with 1 × 105 cells/well. Two hundred ng of pGL3-SLBP-3’-UTR and 80 ng pRL-TK (Promega, Madison, WI. USA) were transfected in combination with 60 pmol of miR-384 mimic or mimic NC using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol, respectively. Luciferase activity was measured 24 h after transfection using the Dual Luciferase Reporter Assay System (Promega). Firefly luciferase activity was normalized to renilla luciferase activity for each transfected well.
Western blotting
MG63 cells were plated in 6-well plates (6 × 105 cells/well), and 72 h after the transfection assay, cells were harvested and homogenized with RIPA lysis buffer (Thermo, IL, USA). Total protein was separated by denaturing 10% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membrane. The primary antibodies for Bcl-2, Bax, pro caspase-3, cleaved caspase-3, SLBP, MEK, p-MEK, ERK, p-ERK, PI3K, p-PI3K, AKT, p-AKT and GAPDH were purchased from Abcam (Cambridge, UK). The membranes were incubated with primary antibodies overnight at 4 °C and probed with the sheep anti-rabbit secondary antibody (conjugated to horseradish peroxidase; Abcam) for 1 h at room temperature. Protein levels were normalized to GAPDH. The bands were visualized with Enhanced Chemiluminescence system (ECL, Amersham Pharmacia).
Statistical analysis
Data are expressed as means ± SD, and p < 0.05 is considered as statistically significant by Student’s t-test or one-way analysis of variance (ANOVA) according to analysis of SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA).
Results
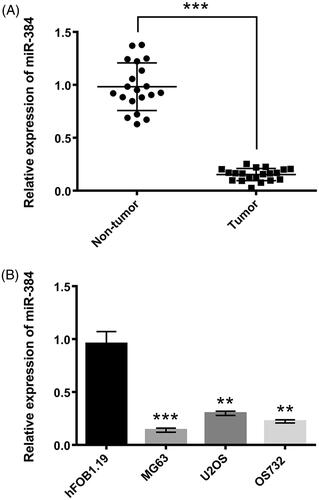
miR-384 were down-regulated in osteosarcoma tissues and cell lines
miR-384 levels were detected in 20 pairs of osteosarcoma and adjacent normal tissues via qRT-PCR. miR-384 expression was dramatically down-regulated in osteosarcoma tissues in comparison to normal tissues (p < .001, ). Similarly, miR-384 expression was significantly decreased in osteosarcoma cell lines, MG63 (p < .001), U2OS (p < .01), and OS732 (p < .01, ), as in comparison to osteoblast cell line hFOB1.19. As MG63 cell line expressed miR-384 at the lowest level among the tested cell lines, it was used for subsequent study.
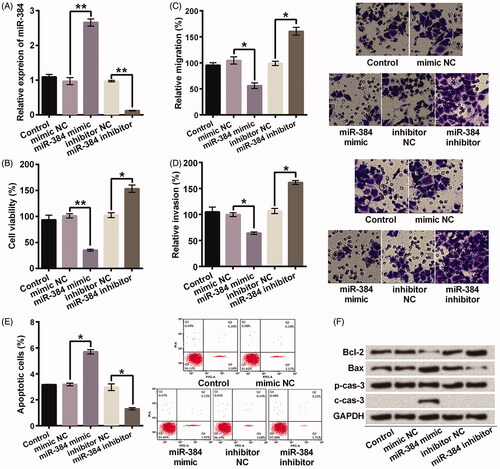
miR-384 overexpression suppressed growth and metastasis of MG63 cells and miR-384 silence exhibited the contrary effect
miR-384 was overexpressed or silenced after transfection with miR-384 mimic or inhibitor (). Afterwards, the effects of miR-384 overexpression or silence on viability, migration, invasion and apoptosis of MG63 cells were analyzed. Elevating expression of miR-384 significantly suppressed viability (p < .01, ), migration (p < .05, ), invasion (p < .05, ), but significantly enhanced apoptotic cell rate (p < .05, ). Besides, elevating expression of miR-384 obviously decreased expression of Bcl-2 (anti-apoptotic protein) and increased expressions of Bax and cleaved caspase-3 (pro-apoptotic proteins, ). Inversely, miR-384 silence promoted viability, migration, invasion, but reduced apoptosis (All p < .05, ). Bcl-2 expression was increased, Bax expression was decreased, and no cleaved caspase-3 was detected after knocking down miR-384 (). Thus, miR-384 overexpression suppressed growth and metastasis of MG63 cells and miR-384 silence exerted the contrary effect.
Figure 2. miR-384 inhibited growth and metastasis of MG63 cells. (A) Relative miR-384 expression was determined after transfection assay. miR-384 was examined using qRT-PCR. miR-384 (B) inhibited cell viability, (C) migration and (D) invasion, as well as (E) promoted apoptosis and (F) influenced apoptosis-related proteins. However, knocking down miR-384 showed the contrary effects. Cell viability was assessed by CCK-8; Migration and invasion were evaluated by transwell assay; Apoptotic cells were observed with flow cytometer; Proteins involved in apoptosis progression was detected using Western blotting. Means ± SD were shown. *p < .05, **p < .01.
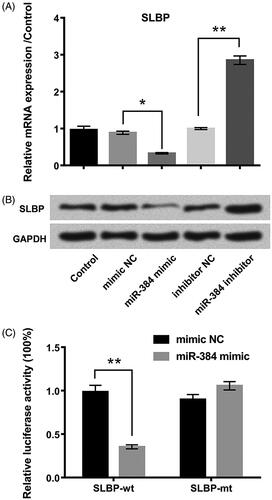
SLBP was a target of miR-384
As shown in , SLBP expression was negatively regulated by miR-384. SLBP mRNA (p < .05) () and protein () levels were lower in miR-384-overexpressed cells but higher (p < .01) in miR-384-silenced cells compared with the corresponding controls. SLBP might be the potential miR-384 target. We constructed pGL3-report luciferase vectors containing the wt or mt miR-384 binding site of SLBP 3′-UTR. Luciferase assay showed that miR-384 overexpression largely decreased the activity of the wild-type reporter but not that of the mutant reporter (), thereby suggesting that miR-384 inhibits the 3′-UTR function of SLBP. Data suggested that SLBP was a target of miR-384 and negatively regulated by miR-384.
Figure 3. SLBP was a direct target of miR-384. SLBP expression was negatively regulated by miR-384 at (A) mRNA and (B) protein levels. mRNA and protein were examined using qRT-PCR and Western blotting, respectively. (C) Luciferase activities of reporter constructs containing wild-type or mutant 3′-UTR of SLBP were assayed and normalized to those of Renilla. Means ± SD were shown. *p < .05, **p < .01.
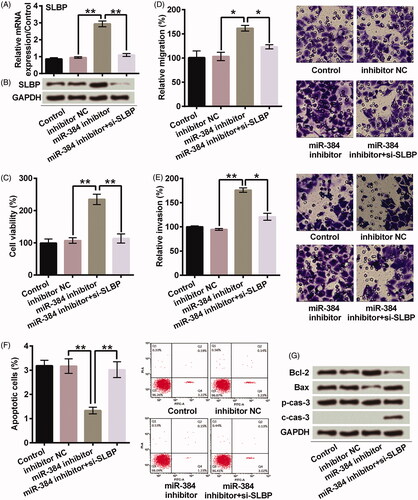
Suppressing miR-384 promoted growth and metastasis of MG63 cells by targeting SLBP
SLBP was up-regulated after miR-384 silence; however, it was recovered after miR-384 and SLBP were both knocked down at mRNA (p < .01) () and protein () levels. Knocking down SLBP significantly impaired the promoting effects of miR-384 silence on viability (p < .01, ), migration (p < .05, ) and invasion (p < .05, ) of MG63 cells. Meanwhile, knocking down SLBP significantly impaired the inhibiting effect of miR-384 silence on apoptosis of MG63 cells (p < .01, ). Bcl-2 was decreased, Bax was increased, and cleavage of caspase-3 was promoted in miR-384 inhibitor + si-SLBP group in comparison to miR-384 inhibitor group (). These results suggested that miR-384 silence might enhance the growth and metastasis of MG63 cells via up-regulating SLBP.
Figure 4. miR-384 silence promoted the growth and metastasis of MG63 cells by elevating expression of SLBP. Transfection of si-SLBP inhibited SLBP expression in miR-384 silenced cells at (A) mRNA (B) and protein levels. SLBP was determined at both mRNA and protein levels using qRT-PCR and Western blotting. SLBP silence abolished the promoting effects of miR-384 silence on (C) cell viability, (D) cell migration and (E) invasion. SLBP silence reduced (F) the inhibitory effect of miR-384 silence on apoptosis and (G) the effect on apoptosis-related proteins. CCK-8 assay was applied for cell viability; Transwell assay was conducted to examine migration and invasion behaviours; Apoptotic cells were observed under a flow cytometer; Western blotting assay was used to quantify protein associated with apoptosis. Means ± SD were shown. *p < .05, **p < .01.
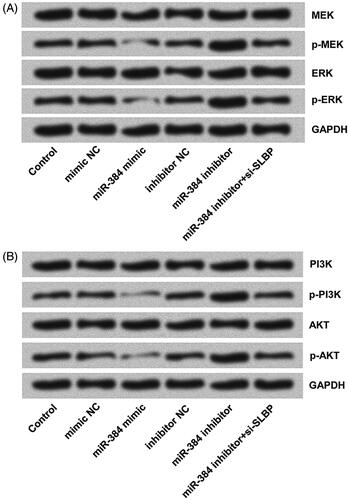
miR-384 silence activated MEK/ERK and PI3K/AKT signalling pathways in MG63 cells by targeting SLBP
MEK/ERK and PI3K/AKT signalling pathways were closely related to tumorigenesis, progression, invasion and metastasis. Activations of MEK/ERK and PI3K/AKT were detected using Western blotting. The phosphorylated expression of MEK and ERK (), as well as phosphorylation of PI3K and AKT (), decreased obviously after miR-384 overexpression as compared with mimic NC but increased remarkably following miR-384 knockdown as compared with inhibitor NC. Furthermore, we found that down-regulating SLBP inhibited the phosphorylation-promoting effects of miR-384 silence on MEK and ERK (), as well as PI3K and AKT (). According to these results, we can infer that miR-384 silence might promote osteosarcoma cells by activating MEK/ERK and PI3K/AKT signalling pathways via up-regulating SLBP.
Figure 5. miR-384 silence activated (A) MEK/ERK and (B) PI3K/AKT signalling pathways in MG63 cells by up-regulating SLBP. However, miR-384 overexpression inhibited the activations of both pathways. MEK, ERK, PI3K and AKT, as well as their phosphorylated forms, were analyzed using Western blotting assay.
Discussion
Osteosarcoma is a common malignant bone tumour in adolescents and the elderly [Citation1]. Up to now, combined neoadjuvant chemotherapy and surgery remain the principal treatment for osteosarcoma patients; however, patient survival rates are still low [Citation19]. Finding new prognostic molecular markers and developing novel therapeutic methods are important. Acquired evidence indicate that miRNAs play essential roles in cancer development, including osteosarcoma [Citation20]. Although the roles of miR-384 in many types of cancers are investigated previously [Citation15,Citation16], the effect of miR-384 on osteosarcoma is rarely reported. The present study focused on the function role and the underlying mechanism of miR-384 in osteosarcoma.
It is widely reported that the decrease of miR-384 was found in cancer patients. Our data showed that miR-384 was down-regulated in osteosarcoma tissues and cell lines MG63, U2OS and OS732, indicating it might be related to the progress of osteosarcoma. Increasing evidence show that miR-384 acts as a tumour suppressor gene in many types of cancers. For example, it exerts suppressive effect on human hepatocellular carcinoma cell proliferation [Citation18]. It inhibits the invasive and migrating abilities of colorectal cancer cells in vitro and the metastatic potential in vivo [Citation17]. Conversely, low-levelled expression of miR-384 can promote hepatic carcinoma cell proliferation, migration and invasion [Citation21]. miR-384 suppression is closely correlated with the invasive depth, lymph node and distant metastasis of colorectal cancer [Citation17]. Our results coincided with previous studies, which showed that ectopic overexpression of miR-384 promoted apoptosis, and inhibited cell viability, migration and invasion; whereas, the suppression of miR-384 inhibited apoptosis, enhanced cell viability, migration and invasion. Thus, our data demonstrated that miR-384 might suppress the progression of osteosarcoma.
miR-384 played an important role in mediating growth and metastasis of MG63 cells. Furthermore, we explored the molecular mechanism of miR-384 silence promoting growth, invasion and metastasis of MG63 cells. miRNAs can regulate the expression of massive target genes by targeting the homologous sequences of mRNAs with the 3’UTR to promote RNA degradation [Citation9]. miRNAs function in cancers by targeting their specific target gene [Citation22,Citation23]. miR-384 was showed to inhibit the growth of hepatocellular carcinoma cells through directly inhibiting IRS1 expression [Citation18]. Wang et al. also showed that miR-384 represses the expression of KRAS and CDC42 by directly targeting their 3’-UTR and then mediated colorectal cancer metastasis [Citation17]. In this paper, SLBP was identified as a direct functional target of miR-384. miR-384 negatively regulated SLBP expression. Moreover, promotion of cell growth and metastasis caused by miR-384 inhibition was counteracted by silencing SLBP expression with siRNAs; thereby, we inferred that suppression of miR-384 promoted the development of osteosarcoma likely by up-regulating SLBP.
SLBP is an evolutionarily conserved protein and involved in the processing, translation, and degradation of the mRNAs of canonical histones, including H1, H2A, H2B, H3 and H4 [Citation24]. Besides, SLBP is a cell cycle-regulated protein that creates mature histone mRNA by 3’ end cleavage during G1 to S-phase progression [Citation25,Citation26]. Diverse studies have proposed that post-translational histone modification, the deposition of histone variants, and histone chaperones can be also involved in the development of cancers [Citation27–29]. For example, altered expression of many H2A variants is associated with cancer [Citation30]. To our knowledge, the effect of SLBP on cancer diseases has not been investigated. Whether miR-384 indirectly influenced histone modification by regulating SLBP expression should be explored in future studies.
MEK/ERK and PI3K/AKT pathways are thought to be important oncogenic pathways in human cancers, including osteosarcoma [Citation31,Citation32]. MEK/ERK pathway is associated with epithelial-mesenchymal transition and tumour metastasis of osteosarcoma [Citation33,Citation34]. PI3K/AKT is associated with apoptosis, growth and migration of osteosarcoma cells [Citation35,Citation36]. Growth of MG63 cells can be inhibited by down-regulation of PI3K/AKT [Citation37]. We, for the first time, proposed that suppression of miR-384 promoted the development of MG63 cells by activating MEK/ERK and PI3K/AKT pathways through regulating SLBP expression.
Overall, this study found miR-384 acted as a tumour suppressor in osteosarcoma cells. Low expression level of miR-384 promoted the growth and metastasis of MG63 cells via directly targeting SLBP. Restoration of miR-384 expression might provide a novel therapeutic approach to the inhibition of osteosarcoma growth and reduction of osteosarcoma metastasis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004. Cancer. 2009;115:1531–1543.
- Price CH. Osteogenic sarcoma: an analysis of the age and sex incidence. Br J Cancer. 1955;9:558–574.
- Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: National Cancer Data Base Report. Clin Orthopaed Relat Res. 2007;459:40–47.
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. In: Jaffe N, Bruland OS, Bielack S, editors. Pediatric and adolescent osteosarcoma. Boston, MA: Springer US; 2010. p. 3–13.
- Thompson LD. Osteosarcoma. Ear Nose Throat J. 2013; 92:288, 290.
- Jiang R, Zhang C, Liu G, et al. MicroRNA-101 inhibits proliferation, migration and invasion in osteosarcoma cells by targeting ROCK1. Am J Cancer Res. 2017;7:88–97.
- Wang Y, Zhang Y, Yang T, et al. Long non-coding RNA MALAT1 for promoting metastasis and proliferation by acting as a ceRNA of miR-144-3p in osteosarcoma cells. Oncotarget. 2017;8:59417–59434.
- Fontana L, Pelosi E, Greco P, et al. MicroRNAs 17-5p-20a-106a control monocytopoiesis through AML1 targeting and M-CSF receptor upregulation. Nat Cell Biol. 2007;9:775–787.
- Valencia-Sanchez MA, Liu J, Hannon GJ, et al. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515. 24.
- Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269.
- Osaki M, Takeshita F, Sugimoto Y, et al. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol Ther. 2011;19:1123–1130.
- Duan Z, Choy E, Harmon D, et al. MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol Cancer Ther. 2011;10:1337–1345.
- Ziyan W, Shuhua Y, Xiufang W, et al. MicroRNA-21 is involved in osteosarcoma cell invasion and migration. Med Oncol. 2011;28:1469–1474.
- Zhao G, Cai C, Yang T, et al. MicroRNA-221 induces cell survival and cisplatin resistance through PI3K/Akt pathway in human osteosarcoma. PLoS One. 2013;8:e53906.
- Song H, Rao Y, Zhang G, et al. MicroRNA-384 inhibits the growth and invasion of renal cell carcinoma cells by targeting astrocyte elevated gene 1. Oncol Res. 2018;28:457–466.
- Fan N, Zhang J, Cheng C, et al. MicroRNA-384 represses the growth and invasion of non-small-cell lung cancer by targeting astrocyte elevated gene-1/Wnt signaling. Biomed Pharmacother. 2017;95:1331–1337.
- Wang YX, Chen YR, Liu SS, et al. MiR-384 inhibits human colorectal cancer metastasis by targeting KRAS and CDC42. Oncotarget. 2016;7:84826–84838.
- Lai YY, Shen F, Cai WS, et al. MiR-384 regulated IRS1 expression and suppressed cell proliferation of human hepatocellular carcinoma. Tumor Biol. 2016;37:14165–14171.
- Chou AJ, Geller DS, Gorlick R. Therapy for osteosarcoma: where do we go from here? Paediatr Drugs. 2008;10:315–327.
- Miao J, Wu S, Peng Z, et al. MicroRNAs in osteosarcoma: diagnostic and therapeutic aspects. Tumor Biol. 2013;34:2093–2098.
- Chen Z, Yu C, Zhan L, et al. LncRNA CRNDE promotes hepatic carcinoma cell proliferation, migration and invasion by suppressing miR-384. Am J Cancer Res. 2016;6:2299–2309.
- Zhang B, Pan X, Cobb GP, et al. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12.
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233.
- Marzluff WF, Wagner EJ, Duronio RJ. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nat Rev Genet. 2008;9:843–854.
- Whitfield ML, Zheng LX, Baldwin A, et al. Stem-loop binding protein, the protein that binds the 3' end of histone mRNA, is cell cycle regulated by both translational and posttranslational mechanisms. Mol Cell Biol. 2000;20:4188–4198.
- Black D, Bird MA, Hayden M, et al. TNF alpha-induced hepatocyte apoptosis is associated with alterations of the cell cycle and decreased stem loop binding protein. Surgery. 2004;135:619–628.
- Gonzalez-Ramirez I, Soto-Reyes E, Sanchez PY, et al. Histones and long non-coding RNAs: the new insights of epigenetic deregulation involved in oral cancer. Oral Oncol. 2014;50:691–695.
- Seligson DB, Horvath S, Shi T, et al. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262–1266.
- Seligson DB, Horvath S, McBrian MA, et al. Global levels of histone modifications predict prognosis in different cancers. Am J Pathol. 2009;174:1619–1628.
- Monteiro FL, Baptista T, Amado F, et al. Expression and functionality of histone H2A variants in cancer. Oncotarget. 2014;5:3428–3443.
- Han G, Wang Y, Bi W. C-Myc overexpression promotes osteosarcoma cell invasion via activation of MEK-ERK pathway. Oncol Res. 2012;20:149–156.
- Zhang J, Yu X-H, Yan Y-G, et al. PI3K/Akt signaling in osteosarcoma. Clin Chim Acta. 2015;444:182–192.
- Hou C-H, Lin F-L, Hou S-M, et al. Cyr61 promotes epithelial-mesenchymal transition and tumor metastasis of osteosarcoma by Raf-1/MEK/ERK/Elk-1/TWIST-1 signaling pathway. Mol Cancer. 2014;13:236.
- Yu Y, Luk F, Yang JL, et al. Ras/Raf/MEK/ERK pathway is associated with lung metastasis of osteosarcoma in an orthotopic mouse model. Anticancer Res. 2011;31:1147–1152.
- Jin S, Pang R-P, Shen J-N, et al. Grifolin induces apoptosis via inhibition of PI3K/AKT signalling pathway in human osteosarcoma cells. Apoptosis. 2007;12:1317–1326.
- Li B, Yang Y, Jiang S, et al. Adenovirus-mediated overexpression of BMP-9 inhibits human osteosarcoma cell growth and migration through downregulation of the PI3K/AKT pathway. Int J Oncol. 2012;41:1809–1819.
- Liu B, Shi Z-l, Feng J, et al. Celecoxib, a cyclooxygenase-2 inhibitor, induces apoptosis in human osteosarcoma cell line MG-63 via down-regulation of PI3K/Akt. Cell Biol Int. 2008;32:494–501.