Abstract
Proteinuria is one of the most important clinical features of nephrotic syndrome (NS). Injury of podocyte has been proved to contribute to the occurrence of proteinuria. This study explored the effects of geniposide (GEN) on lipopolysaccharide (LPS)-caused murine kidney podocyte MPC5 apoptosis and autophagy. Viability and apoptosis of MPC5 cells were respectively detected with the help of CCK-8 assay and Guava Nexin assay. 3-Methyladenine (3-MA) was used as an autophagy inhibitor, while rapamycin as autophagy activator. Si-Beclin-1 was transfected in MPC5 cells to down-regulate the expression of Beclin-1. We found that LPS stimulation significantly caused MPC5 cell viability reduction, apoptosis and autophagy (P < .05 or P < .01). GEN treatment remarkably alleviated the LPS-caused MPC5 cell viability reduction and apoptosis, but promoted cell autophagy (P < .05). Moreover, 3-MA incubation or si-Beclin-1 transfection notably weakened the effects of GEN on LPS-caused MPC5 cell apoptosis and autophagy (P < .05), while rapamycin had opposite effects (P < .05). Furthermore, GEN activated Ras/Raf/MEK/ERK pathway in LPS-treated MPC5 cells (P < .05). In conclusion, this research verified the protective effects of GEN on podocytes damage. GEN alleviates LPS-caused apoptosis of murine kidney podocytes by activating Ras/Raf/MEK/ERK-mediated cell autophagy.
LPS causes podocyte MPC5 apoptosis and autophagy.
GEN alleviates LPS-caused MPC5 cell apoptosis, but promotes cell autophagy.
3-MA or si-Beclin-1 weakens the effects of GEN on LPS-treated MPC5 cells.
Rapamycin strengthens the effects of GEN on LPS-treated MPC5 cells.
GEN activates Ras/Raf/MEK/ERK pathway in LPS-treated MPC5 cells.
Highlights:
Introduction
Nephrotic syndrome (NS) is a common kidney disease characterized by peripheral edema, heavy proteinuria, hypoalbuminemia and hyperlipidemia [Citation1]. Intrinsic glomerular disorders or secondary damage to the glomerulus caused by systemic diseases, infections or drugs are the main reasons for the occurrence of NS [Citation2,Citation3]. In the clinic, patients with NS are usually treated by sodium restriction, fluid restriction, loos diuretics, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy in combination with special psychological and diet nursing [Citation1]. Pathology research found that dysfunction of the glomerular filtration barrier is evident in patients with NS [Citation4]. Glomerular filtration barrier consists of endothelial cells, glomerular basement membrane and visceral epithelial cells known as podocytes [Citation5]. Among them, podocytes have been proved to play critical roles in maintaining the integrity of the glomerular filtration barrier and preventing leakage of urine proteins [Citation5]. Moreover, podocytes are also involved in the regulation of other components synthesis of the filtration barrier [Citation6]. Thus, it is worth believing that searching for effective medicines that can protect podocytes from damage will be helpful for effective therapy of NS.
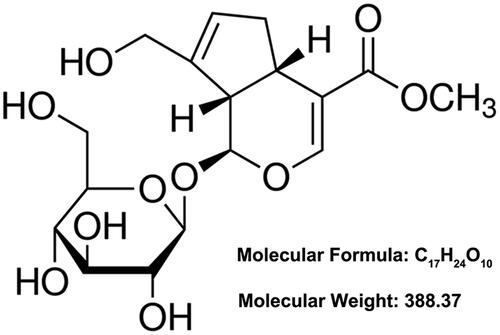
Geniposide (GEN, CAS number: 24512–63-8) is a plant-derived iridoid glycoside firstly isolated from the dry fruits of Gardenia jasminoides Ellis (also named as “Zhizi” in China) [Citation7]. In view of its chemical structure (), this compound is also considered as a glycoside consisting of one molecule of genipin and one molecule of glucose [Citation8]. The pharmacological activities of GEN include neuroprotection [Citation9], hepatic-protection [Citation10], anti-inflammation [Citation11], anti-oxidation [Citation12], anti-tumour [Citation13], anti-asthma [Citation14] and anti-diabetic [Citation15]. Experimental research from Xu et al. reported that GEN could ameliorate colitis in rats by inhibiting inflammatory cytokines release and restoring impaired intestinal barrier function [Citation11]. Another review about Chinese herbal medicines indicated that GEN could protect renal intrinsic cells, including podocytes, renal tubular epithelial cells and renal collecting duct epithelial cells, and relieve kidney disease progression [Citation16]. However, until now, the specific regulatory mechanism about the effects of GEN on NS still remains unclear. More studies are still needed to further explore the effects of GEN on podocytes damage in NS.
Lipopolysaccharide (LPS) is a compound of the cellular wall of Gram-negative bacteria [Citation17]. Previous studies demonstrated that LPS-caused podocyte damage could be used as an in vitro cell model of proteinuria to analyze the pathogenesis of NS and testing new medicine for NS [Citation18,Citation19]. In the current research, we explored the effects of GEN on podocyte damage caused by LPS and analyzed the possible internal mechanism related to cell apoptosis and cell autophagy. Our findings will provide a theoretical and experimental basis for further understanding of the beneficial effects of GEN on NS.
Materials and methods
Cell culture and treatment
Murine kidney podocyte cell line MPC5 (Tong-Pai Bio-Tech Co., Ltd, Shanghai, China) was cultured in RPMI-1640 medium (Gibco, Life Technologies, Carlsbad, CA, USA) supplemented with 10 U/ml r-interferon (Sigma-Aldrich, St Louis, MO, USA) and 10% (v/v) fetal bovine serum (FBS, Gibco) in 75 cm2 flask at 33 °C with 5% CO2. Flasks were pre-treated with 50 μg/ml type I collagen (Sigma-Aldrich) for 1 h at 37 °C.
LPS, 3-Methyladenine (3-MA) and rapamycin were all purchased from Sigma-Aldrich (catalog numbers: L2630, M9281 and V900930). MPC5 cells were treated by LPS (1 μg/ml in ultrapure water) for 12 h to simulate damage. 3-MA (5 mM in ultrapure water) and rapamycin (10 μM in dimethyl sulfoxide, DMSO) were used as autophagy inhibitor or activator added to culture medium simultaneously with the LPS and/or GEN treatment.
GEN (purity > 99.9%) was obtained from National Institute for the Control of Pharmaceutical and Biological Products (Jilin, China) and dissolved into phosphate buffered saline (PBS, Beyotime Biotechnology, Shanghai, China). MPC5 cells were treated by 100, 200 or 300 μg/ml GEN in this research.
Cell transfection
Si-Beclin-1 and its negative control (si-NC) were designed and synthesized by GenePharma Corporation (Shanghai, China). Cell transfection was performed with the help of Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) as previously described [Citation20]. Transfection efficiency was confirmed by using western blotting.
Cell viability assay
The viability of MPC5 cells was assessed using cell counting kit-8 (CCK-8) assay (Beyotime Biotechnology) as described previously [Citation21]. Briefly, transfected or non-transfected MPC5 cells were suspended in RPMI-1640 medium and seeded into 96-well culture plate with a density of 1 × 104 cells/well overnight. Then, LPS, GEN, 3-MA and/or rapamycin were added into the culture medium for 12 h. Subsequently, 10 μl CCK-8 kit solution was supplemented into each well of the plate for 1 h. The absorbance of each well at 450 nm was measured with the help of Micro-plate reader (Bio-Tek Instruments, Winooski, VT, USA). Results were expressed as a percentage of the control.
Cell apoptosis assay
Apoptosis of MPC5 cells was assessed using Guava Nexin assay (Guava Technologies, Hayward, CA, USA) [Citation22]. Briefly, transfected or non-transfected MPC5 cells were suspended in RPMI-1640 medium and seeded into 24-well culture plate with a density of 6.25 × 104 cells/well overnight. Then, LPS, GEN, 3-MA and/or rapamycin were added into the culture medium for 12 h. Subsequently, cells in each group were harvested, washed with PBS for twice and stained using kit solution for 25 min at room temperature in the dark. Followed by washing with PBS for twice, the rate of apoptotic cells in each group was measured using Guava EasyCyte flow cytometer (Guava Technologies, Hayward, CA, USA).
Western blotting
After different treatment or transfection, total proteins in MPC5 cells were isolated using RIPA Lysis buffer (Beyotime Biotechnology) containing with protease inhibitors (Roche, Basel, Switzerland). The concentration of total proteins was measured with the help of BCA Protein assay (Thermo Fisher Scientific). Western blotting was conducted as previously described [Citation23]. The following antibodies were used: Caspase 3 (#9662), Caspase 9 (#9508), LC3 (#4108), Ras (#3965), Raf (#4432), Mitogen-activated protein kinase (MEK, #4694), p-MEK (#9154), Extracellular signal-regulated kinases (ERK, #4695), p-ERK (#4370), β-actin (#3700), Anti-Rabbit IgG (H + L) DyLightTM 680 Conjugate (#5366) and Anti-Mouse IgG (H + L) DyLightTM 680 Conjugate (#5470, Cell Signaling Technology, Beverly, MA, USA). The signals of proteins were captured using OdysseyClx Infrared imaging system (Licor biosystems, Lincoln, NE, USA). Intensities of bands were analyzed using Odyssey software (Licor biosystems).
Statistical analysis
Each experiment in this study was repeated three times. Results were presented as the mean ± standard deviation (SD). For western blotting, the most representative images were shown. GraphPad 6.0 software (San Diego, CA, USA) was used for statistical analysis. P-values between the two groups were calculated using Student’s t-test and P-values between more than three groups were calculated with the help of one-way analysis of variance (ANOVA). A significant difference was judged to exist at a level of P < .05.
Results
LPS caused MPC5 cell apoptosis and autophagy
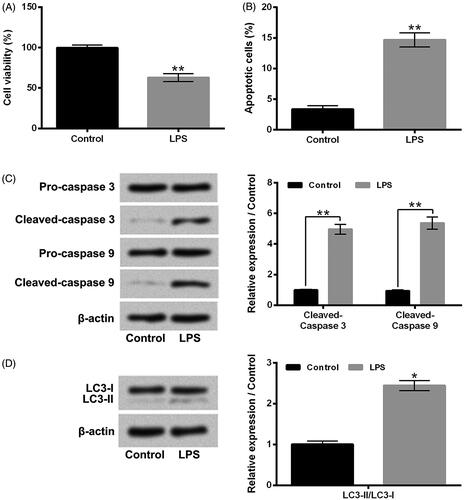
Firstly, the viability, apoptosis, and autophagy of MPC5 cells after LPS stimulation were analyzed. Results in shows that LPS stimulation significantly reduced the viability of MPC5 cells (P < .01). The rate of apoptotic MPC5 cells was obviously increased in LPS stimulation group, compared to control group (, P < .01). Moreover, the protein expression levels of pro-apoptotic proteins, Cleaved-caspase 3 and Cleaved-caspase 9, in MPC5 cells, were both remarkably enhanced after LPS stimulation (, P < .01). Moreover, the results of presents that the protein expression rate of LC3-II/LC3-I in MPC5 cells was also increased after LPS stimulation (P < .05). These above findings suggested that LPS could inhibit MPC5 cell viability and cause cell apoptosis and autophagy.
Figure 2. LPS caused MPC5 cell apoptosis and autophagy. After 1 μg/ml LPS stimulation for 12 h, (A) the viability of MPC5 cells was detected by CCK-8 assay; (B) the apoptosis of MPC5 cells was measured by Guava Nexin assay; (C and D) the protein expression levels of Pro-caspase 3, Cleaved-caspase 3, Pro-caspase 9, Cleaved-caspase 9, LC3-I and LC3-II in MPC5 cells were assessed by western blotting. LPS: Lipopolysaccharide. N = 3. *P < .05; **P < .01.
GEN alleviated LPS-caused MPC5 cell apoptosis, but promoted cell autophagy
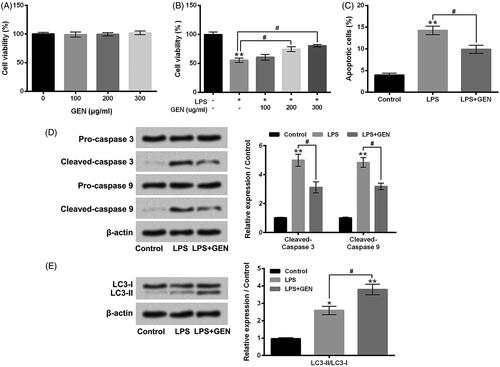
Next, the effects of GEN on LPS-caused MPC5 cell viability reduction, apoptosis and autophagy were explored. displays that 100–300 μg/ml GEN treatment had no significant effects on MPC5 cell viability. Nevertheless, LPS-caused MPC5 cell viability inhibition was notably alleviated by 200 or 300 μg/ml GEN treatment (, P < .05). The enhancement of MPC5 cell apoptosis caused by LPS was also attenuated by 300 μg/ml GEN treatment (, P < .05). In addition, Relative to LPS group, the protein expression levels of pro-apoptotic proteins, Cleaved-caspase 3 and Cleaved-caspase 9, in MPC5 cells were both decreased in LPS + GEN group (, P < .05). More interestingly, illustrates that 300 μg/ml GEN treatment noticeably promoted the LPS-caused increase of protein expression rate of LC3-II/LC3-I in MPC5 cells (P < .05). These above findings indicated that GEN could exert protective effects on LPS-caused MPC5 cell apoptosis, but promote cell autophagy.
Figure 3. GEN alleviated LPS-caused MPC5 cell apoptosis, but promoted cell autophagy. (A) After 100–300 μg/ml GEN treatment for 12 h, the viability of MPC5 cells was detected by CCK-8 assay. (B) After 1 μg/ml LPS and/or 100–300 μg/ml GEN treatment for 12 h, the viability of MPC5 cells was detected by CCK-8 assay. After 1 μg/ml LPS and/or 300 μg/ml GEN treatment for 12 h, (C) the apoptosis of MPC5 cells was measured by Guava Nexin assay; (D and E) the protein expression levels of Pro-caspase 3, Cleaved-caspase 3, Pro-caspase 9, Cleaved-caspase 9, LC3-I and LC3-II in MPC5 cells were assessed by western blotting. LPS: Lipopolysaccharide; GEN: Geniposide. N = 3. *P < .05 or **P < .01 vs. Control group; #P < .05 vs. LPS group.
3-MA mitigated the effects of GEN on LPS-caused MPC5 cell apoptosis and autophagy
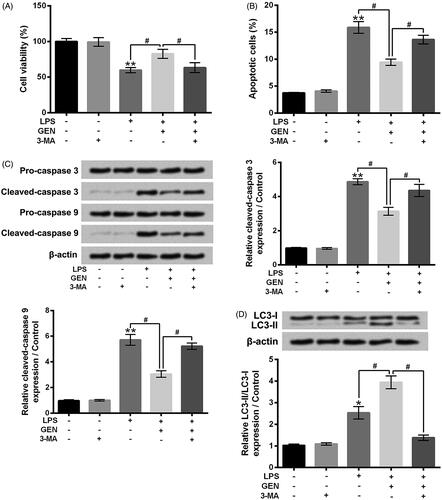
3-MA was used to inhibit autophagy of MPC5 cells. Then, MPC5 cell viability, apoptosis and autophagy after LPS and/or GEN treatment were assessed. Results in shows that 3-MA incubation dramatically reversed the effect of GEN on LPS-caused MPC5 cell viability inhibition (P < .05). The effect of GEN on LPS-caused MPC5 cell apoptosis was also mitigated by 3-MA incubation (, P < .05). Compared to LPS + GEN group, the protein expression levels of Cleaved-caspase 3 and Cleaved-caspase 9 in MPC5 cells were both increased in LPS + GEN + 3-MA group (, P < .05). Besides, the protein expression rate of LC3-II/LC3-I in MPC5 cells was distinctly reduced in LPS + GEN + 3-MA group, relative to LPS + GEN group (, P < .05). Taken together, these above findings suggested that inhibition of cell autophagy could mitigate the effects of GEN on LPS-caused cell viability inhibition and cell apoptosis.
Figure 4. 3-MA mitigated the effects of GEN on LPS-caused MPC5 cell apoptosis and autophagy. After 1 μg/ml LPS and/or 300 μg/ml GEN or 5 mM 3-MA treatment for 12 h, (A) the viability of MPC5 cells was detected by CCK-8 assay; (B) the apoptosis of MPC5 cells was measured by Guava Nexin assay; (C and D) the protein expression levels of Pro-caspase 3, Cleaved-caspase 3, Pro-caspase 9, Cleaved-caspase 9, LC3-I and LC3-II in MPC5 cells were assessed by western blotting. LPS: Lipopolysaccharide; GEN: Geniposide; 3-MA: 3-Methyladenine. N = 3. *P < .05 or **P < .01 vs. Control group; #P < .05 vs. LPS or LPS + GEN group.
Rapamycin strengthened the effects of GEN on LPS-caused MPC cell apoptosis and autophagy
To further explore the roles of cell autophagy, rapamycin was used to activate cell autophagy. presents that rapamycin incubation significantly enhanced the effect of GEN on LPS-caused MPC5 cell viability inhibition (P < .05). The effect of GEN on LPS-caused MPC5 cell apoptosis was also promoted by rapamycin incubation (, P < .05). Compared to LPS + GEN group, the protein expression levels of Cleaved-caspase 3 and Cleaved-caspase 9 in MPC5 cells were both decreased in LPS + GEN + rapamycin group (, P < .05). Moreover, the protein expression rate of LC3-II/LC3-I in MPC5 cells was increased in LPS + GEN + rapamycin group, relative to LPS + GEN group (, P < .05). These above findings further suggested that activation of cell autophagy might play critical roles in the effects of GEN on LPS-treated MPC5 cells.
Figure 5. Rapamycin strengthened the effects of GEN on LPS-caused MPC cell apoptosis and autophagy. After 1 μg/ml LPS and/or 300 μg/ml GEN or 10 μM rapamycin treatment for 12 h, (A) the viability of MPC5 cells was detected by CCK-8 assay; (B) the apoptosis of MPC5 cells was measured by Guava Nexin assay; (C and D) the protein expression levels of Pro-caspase 3, Cleaved-caspase 3, Pro-caspase 9, Cleaved-caspase 9, LC3-I and LC3-II in MPC5 cells were assessed by western blotting. LPS: Lipopolysaccharide; GEN: Geniposide. N = 3. *P < .05 or **P < .01 vs. Control group; #P < .05 vs. LPS or LPS + GEN group.
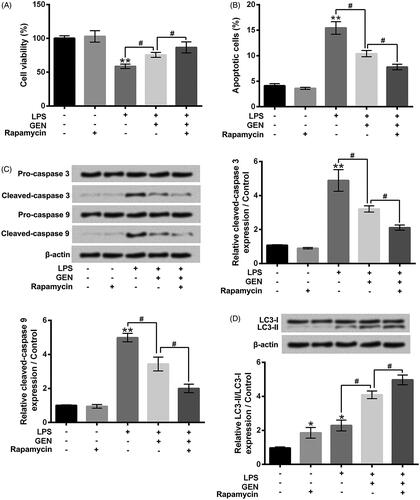
Silence of Beclin-1 weakened the effects of GEN on LPS-treated MPC5 cells
Si-Beclin-1 was transfected into MPC5 cells to down-regulate the expression level of Beclin-1 (). Similar to the effects of 3-MA, si-Beclin-1 transfection obviously alleviated the protective effects of GEN on LPS-caused MPC5 cell viability inhibition and apoptosis (, P < .05). Furthermore, compared to LPS + GEN group, the protein expression levels of Cleaved-caspase 3 and Cleaved-caspase 9 were increased, as well as the expression rate of LC3-II/LC3-I was decreased in LPS + GEN + rapamycin group (, P < .05). These results suggested that Beclin-1 might participate in the protective effects of GEN on LPS-treated MPC5 cells.
Figure 6. Knockdown of Beclin-1 weakened the effects of GEN on LPS-treated MPC5 cells. (A) After si-NC or si-Belcin-1 transfection, the protein expression level of Beclin-1 in MPC5 cells was detected by western blotting. After 1 μg/ml LPS and/or 300 μg/ml GEN treatment or si-Belcin-1 transfection (B) the viability of MPC5 cells was detected by CCK-8 assay; (C) the apoptosis of MPC5 cells was measured by Guava Nexin assay; (D and E) the protein expression levels of Pro-caspase 3, Cleaved-caspase 3, Pro-caspase 9, Cleaved-caspase 9, LC3-I and LC3-II in MPC5 cells were assessed by western blotting. LPS: Lipopolysaccharide; GEN: Geniposide. N = 3. *P < .05 or **P < .01 vs. Control group; #P < .05 vs. LPS or LPS + GEN group.
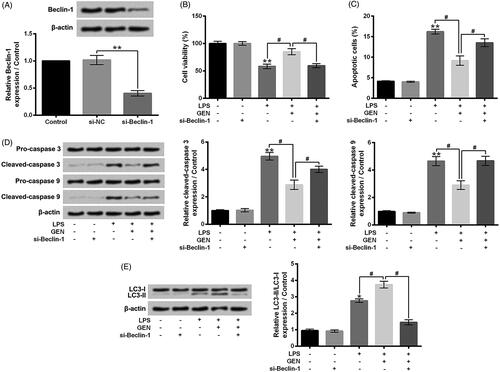
GEN activated Ras/Raf/MEK/ERK pathway in LPS-treated MPC5 cells
Ras/Raf/MEK/ERK pathway is a key autophagy-regulatory pathway in cells [Citation24]. Finally, the activation of Ras/Raf/MEK/ERK pathway in MPC5 cells after LPS and/or GEN treatment was investigated. As displayed in , LPS stimulation remarkably activated Ras/Raf/MEK/ERK pathway in MPC5 cells by up-regulating the expression levels (rates) of Ras, Raf, p-MEK/MEK and p-ERK/ERK (P < .05). Moreover, GEN treatment notably enhanced the activation of Ras/Raf/MEK/ERK pathway in MPC5 cells via further up-regulating the expression levels (rates) of Ras, Raf, p-MEK/MEK and p-ERK/ERK (P < .05). These findings indicated that GEN promoted MPC5 cell autophagy might be through activating Ras/Raf/MEK/ERK pathway.
Figure 7. GEN activated Ras/Raf/MEK/ERK pathway in LPS-treated MPC5 cells. After 1 μg/ml LPS and/or 300 μg/ml GEN treatment for 12 h, the protein expression levels of Ras, Raf, MEK, p-MEK, ERK and p-ERK in MPC5 cells were assessed by western blotting. LPS: Lipopolysaccharide; GEN: Geniposide. MEK: Mitogen-activated protein kinase; ERK: Extracellular signal-regulated kinases. N = 3. *P < .05 vs. Control group; #P < .05 vs. LPS group.
Discussion
Proteinuria is one of the most important clinical features of NS [Citation3]. The previous study has proved that the injury of podocytes participates in the occurrence of proteinuria [Citation25]. In this research, murine kidney podocytes MPC5 were stimulated by LPS to cause damage and we found that GEN could alleviate LPS-caused MPC5 cell apoptosis, but promote cell autophagy. Moreover, we confirmed that inhibition of cell autophagy could mitigate the effects of GEN on LPS-caused MPC5 cell apoptosis, while activation of cell autophagy had opposite effects. Furthermore, we revealed that GEN promoted MPC5 cell autophagy might be through activating Ras/Raf/MEK/ERK pathway.
Podocytes is a highly specialized and terminally differentiated cell with a limited mitotic ability [Citation26]. Injury of podocyte, especially reduction of podocyte number by apoptosis, has been considered as an early phenomenon contributing to the initiation of glomerular lesions [Citation27]. Experimental research from He et al. reported that LPS could cause podocytes injury by promoting an inflammatory response and oxidative response [Citation19]. In the present study, LPS stimulation significantly reduced murine kidney podocytes MPC5 viability. The apoptosis and autophagy of MPC5 cells were both increased after LPS stimulation. These results suggested that podocyte damage model caused by LPS was established successfully and could be used to test the effects of GEN.
As a plant-derived iridoid glycoside, GEN has been reported to possess wide ranges of biological beneficial activities [Citation8,Citation10–12]. Previous studies proved that GEN could protect renal intrinsic cells, including podocytes, and relieve kidney diseases progression [Citation16]. In consistent with the previous studies, we revealed that GEN treatment remarkably alleviated the LPS-caused MPC5 cell viability inhibition and apoptosis. More importantly, we discovered that the autophagy of MPC5 cells was distinctly increased after GEN treatment. Considering that apoptosis and autophagy constitute the two highly regulated cellular biological courses through which injured/aged cells or organelles are eliminated [Citation28]. Yadav et al. proved that in some settings, autophagy was a pattern of stress adaptation that could suppress apoptosis and protect cells from death [Citation29]. Furthermore, Tan et al. demonstrated that high-level autophagy played a key role in maintaining a stable state of podocytes [Citation30]. We could propose that GEN exerted protective effects on LPS-caused podocyte MPC5 apoptosis might be through promoting cell autophagy.
3-MA is a widely used autophagy inhibitor that can block autophagosome formation in cells [Citation31]. On the contrary, Lee et al. reported that rapamycin was widely used autophagy activator than could simulate cell starvation [Citation32]. Moreover, Beclin-1 is the critical autophagy promoting protein that participates in the formation of autophagosome [Citation33]. To further confirm the role of cell autophagy in the protective activity of GEN on LPS-caused podocyte MPC5 apoptosis, we added 3-MA or rapamycin, or silence Beclin-1 in our experiments. We found that 3-MA incubation or Beclin-1 silence weakened the effects of GEN on LPS-caused MP5C cell apoptosis and autophagy, while rapamycin incubation strengthened the effects of GEN on LPS-caused MP5C cell apoptosis and autophagy. These findings further indicated that GEN exerted protective effects on LPS-treated podocyte MPC5 by promoting cell autophagy and reducing cell apoptosis.
The previous study has reported that Ras/Raf/MEK/ERK pathway plays critical roles in both cell apoptosis and cell autophagy [Citation34]. Its effect is dependent on the cell type and the stimulus [Citation35]. In this study, we found that LPS stimulation dramatically activated Ras/Raf/MEK/ERK pathway in MPC5 cells. GEN treatment notably promoted the activation of Ras/Raf/MEK/ERK pathway in MPC5 cells caused by LPS. Considering that Corcelle et al. demonstrated that sustained activation of Ras/Raf/MEK/ERK pathway could result in the enhancement of cell autophagy [Citation36]. We propose that GEN promoted podocyte MPC5 cell autophagy might be via activating Ras/Raf/MEK/ERK pathway.
To sum up, this research verified the protective effects of GEN on podocytes damage. We revealed that GEN could alleviate LPS-caused apoptosis of murine kidney podocytes by activating Ras/Raf/MEK/ERK-mediated cell autophagy. The findings of our study offer new experimental evidence for further understanding of the beneficial effects of GEN on podocytes damage in NS. We propose that GEN may be as a novel preventive and therapeutic medicine for glomerular lesions in NS, in spite of further safety evaluation and in vivo experiments are still needed.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kodner C. Diagnosis and management of nephrotic syndrome in adults. Am Fam Physician. 2016;93:479–485.
- Al-Azzawi HF, Obi OC, Safi J, et al. Nephrotic syndrome-induced thromboembolism in adults. Int J Crit Illn Inj Sci. 2016;6:85.
- Seeger H, Fehr T. Nephrotic syndrome in adult patients–etiology and complications. Praxis. 2016;105:259–267.
- Rheault MN, Gbadegesin RA. The genetics of nephrotic syndrome. J Pediatr Genet. 2016;5:15–24.
- Inoue K, Ishibe S. Podocyte endocytosis in the regulation of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2015;309:F398–F405.
- Lewko B. Treatment of nephrotic syndrome: immuno- or rather podocyte therapy? Postepy Hig Med Dosw. 2016;70:459–470.
- Qi Q, Mao Y, Tian Y, et al. Geniposide inhibited endothelial-mesenchymal transition via the mTOR signaling pathway in a bleomycin-induced scleroderma mouse model. Am J Translation Res. 2017;9:1025–1036.
- Shan M, Yu S, Yan H, et al. A review on the phytochemistry, pharmacology, pharmacokinetics and toxicology of geniposide, a natural product. Molecules (Basel, Switzerland). 2017;22:1689.
- Huang B, Chen P, Huang L, et al. Geniposide attenuates post-ischaemic neurovascular damage via GluN2A/AKT/ERK-dependent mechanism. Cell Physiol Biochem. 2017;43:705–716.
- Rong YP, Huang HT, Liu JS, et al. Protective effects of geniposide on hepatic ischemia/reperfusion injury. Transplant Proc. 2017;49:1455–1460.
- Yu B, Shen Y, Qiao J, et al. Geniposide attenuates Staphylococcus aureus-induced pneumonia in mice by inhibiting NF-kappaB activation. Microbial Pathogen. 2017;112:117–121.
- Liu J, Yin F, Zheng X, et al. Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochem Int. 2007;51:361–369.
- Huang H, Zhang X, Huang Z, et al. Geniposide reverses multidrug resistance in vitro and in vivo by inhibiting the efflux function and expression of P-glycoprotein. Exper Therap Med. 2017;13:437–442.
- Deng Y, Guan M, Xie X, et al. Geniposide inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. Int Immunopharmacol. 2013;17:561–567.
- Zhang Y, Ding Y, Zhong X, et al. Geniposide acutely stimulates insulin secretion in pancreatic beta-cells by regulating GLP-1 receptor/cAMP signaling and ion channels. Mol Cell Endocrinol. 2016;430:89–96.
- Wu W, Yang JJ, Wan YG, et al. Pathogenesis and treatment of insulin resistance in chronic kidney disease and interventional effects of Chinese herbal medicine. China J Chinese Materia Medica. 2017;42:49–55.
- Boitsova EB, Morgun AV, Osipova ED, et al. The inhibitory effect of LPS on the expression of GPR81 lactate receptor in blood-brain barrier model in vitro. J Neuroinflamm. 2018;15:196.
- Hsu MF, Bettaieb A, Ito Y, et al. Protein tyrosine phosphatase Shp2 deficiency in podocytes attenuates lipopolysaccharide-induced proteinuria. Scientific Rep. 2017;7:461.
- He P, Kawamura H, Takemoto M, et al. Combination of cilostazol and probucol protected podocytes from lipopolysaccharide-induced injury by both anti-inflammatory and anti-oxidative mechanisms. J Nephrol. 2017;30:531–541.
- Zhou Y, Wu D, Tao J, et al. MicroRNA-133 inhibits cell proliferation, migration and invasion by targeting epidermal growth factor receptor and its downstream effector proteins in bladder cancer. Scand J Urol. 2013;47:423–432.
- Shi Z, Wu D, Yao JP, et al. Protection against oxygen-glucose deprivation/reperfusion injury in cortical neurons by combining omega-3 polyunsaturated acid with lyciumbarbarum polysaccharide. Nutrients. 2016;8:41.
- Hickey TE, Majam G, Guerry P. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytholethal distending toxin. Infect Immun. 2005;73:5194–5197.
- Li R, Yin F, Guo YY, et al. Knockdown of ANRIL aggravates H2O2-induced injury in PC-12 cells by targeting microRNA-125a. Biomed Pharmacother. 2017;92:952–961.
- Ouyang L, Chen Y, Wang XY, et al. Polygonatum odoratum lectin induces apoptosis and autophagy via targeting EGFR-mediated Ras-Raf-MEK-ERK pathway in human MCF-7 breast cancer cells. Phytomed: Int J Phytother Phytopharmacol. 2014;21:1658–1665.
- Raij L, Tian R, Wong JS, et al. Podocyte injury: the role of proteinuria, urinary plasminogen, and oxidative stress. Am J Physiol Renal Physiol. 2016;311:F1308–f1317.
- Wada T, Pippin JW, Marshall CB, et al. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. JASN. 2005;16:2615–2625.
- Menini S, Iacobini C, Oddi G, et al. Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia. 2007;50:2591–2599.
- Booth LA, Tavallai S, Hamed HA, et al. The role of cell signalling in the crosstalk between autophagy and apoptosis. Cellular Signalling. 2014;26:549–555.
- Yadav A, Vallabu S, Arora S, et al. ANG II promotes autophagy in podocytes. Am J Physiol Cell Physiol. 2010;299:C488–C496.
- Tan X, Chen Y, Liang X, et al. Lipopolysaccharide-induced podocyte injury is mediated by suppression of autophagy. Mol Med Rep. 2016;14:811–818.
- Shin JH, Bae DJ, Kim ES, et al. Autophagy regulates formation of primary Cilia in mefloquine-treated cells. Biomol Therap. 2015;23:327–332.
- Lee JS, Lee GM. Rapamycin treatment inhibits CHO cell death in a serum-free suspension culture by autophagy induction. Biotechnol Bioeng. 2012;109:3093–3102.
- Wang J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy. 2008;4:947–948.
- Sooro MA, Zhang N, Zhang P. Targeting EGFR-mediated autophagy as a potential strategy for cancer therapy. Int J Canc. 2018;143:2116–2125.
- McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochimica et Biophysica Acta. 2007;1773:1263–1284.
- Corcelle E, Nebout M, Bekri S, et al. Disruption of autophagy at the maturation step by the carcinogen lindane is associated with the sustained mitogen-activated protein kinase/extracellular signal-regulated kinase activity. Cancer Res. 2006;66:6861–6870.