Abstract
Silver nanoparticles (AgNPs) have attracted wide interest due to its broad range of applications. This study aims to describe the biosynthesis of AgNPs using an Arctic anti-oxidative bacterium Arc7-R13 and to study its characteristics and antibacterial activity. The biosynthesis of AgNPs was verified using UV–Vis spectrum with the maximum absorption at 416 nm. The morphology of the silver nanoparticles was characterized by TEM and its characterization were investigated by EDX and FTIR. Phylogenetic analysis based on 16S rRNA gene sequence showed that strain Arc7-R13 was affiliated with genus Paracoccus. TEM analysis revealed that the AgNPs synthesized by strain Arc7-R13 were spherical and ellipsoidal in shape with size ranging from 2 to 25 nm. The optimal concentration of AgNO3 and temperature for the biosynthesis were 4 mmol/L and 37 °C, respectively. EDX analysis verified the presence of the element silver in the biosynthesized AgNPs. FTIR analysis revealed that the specific functional groups, OH, CH3 and C≡N, might be responsible for reduction and stabilization of AgNPs. Antimicrobial test showed that the AgNPs had strong antimicrobial activity against all kinds of strains investigated, including Gram-positive Bacillus subtilis, Staphylococcus aureus and Gram-negative Pseudomonas aeruginosa, Escherichia coli.
Introduction
Nanomaterial offers a connecting link between bulk materials and atomic structure [Citation1]. The chemical properties of nanoparticles result from the high percentage of atoms on the surface of the particle [Citation2] and this leads nanomaterials to exhibit improved properties when compared with bulk materials [Citation1]. Metal nanoparticles (MNPs) have been shown to have enormous applications in many fields such as catalysis [Citation3], ceramics [Citation4] and drug delivery [Citation5] because of their unique properties such as increased catalytic activity [Citation6]. Among MNPs, silver nanoparticles (AgNPs) have gained tremendous interests and are used in various biotechnology fields due to its remarkable properties such as high surface to volume ratio, catalytic properties as well as biological effects, including biomedicine [Citation7], agriculture, medicine and diagnostics [Citation1].
Silver nanoparticles have strong antibacterial activity against a wide range of pathogens [Citation8], so it has significant perspectives as bactericidal agents against pathogenic bacteria [Citation9,Citation10]. Kvitek et al. [Citation11] have indicated that the small AgNPs (less than 10 nm) could inhibit cell growth by entering the bacterial cytoplasm. Hwang et al. [Citation12] and Rai et al. [Citation13] found that AgNPs could damage the cell wall and membranes and diffuse into the bacteria to destroy DNA by intercalating between bases. Owing to their antimicrobial properties, AgNPs have been applied to treat bacterial infections associated with burns [Citation14].
The traditional methods of AgNPs synthesis by chemical and physical approaches are expensive [Citation15] and involve the use of chemical reducing agents such as sodium borohydride and trisodium citrate, which might bring about environmental risks [Citation16]. The quest for eco-friendly and economical methods of synthesis has led to the development of bio-inspired approaches. Usually, the silver nanoparticles are highly unstable and must add a capping agent to keep the stability [Citation17]. The various biomoleculars presenting in the bacterium culture supernatants such as enzymes and proteins can act as both reducing and capping agents [Citation18].
Klaus et al. [Citation19] have reported the synthesis of AgNPs by Pseudomonasstutzeri AG 259 at first. Joerger et al. [Citation20] have used Pseudomonas stutzeri (AG259) for silver nanoparticles synthesis with a size smaller than 200 nm. In 2008, biosynthesis of AgNPs by Bacillus licheniformis was reported and the study indicated the mechanism of silver nanoparticles synthesis involving the nitrate reductase [Citation21].
Recent literature reveal that the microorganisms from the different habitats can also synthesize the silver nanoparticles [Citation22]. Kumar et al. [Citation23] have indicated that hydrogenases and reductases could act as both reducing and capping agent to reduce the metal salts and deter the aggregation of the nanoparticles. Some bacteria can live in the extreme environmental conditions that are very cold, high-pressure or high-oxygen and in these extreme environments, the bacteria can possess a special mechanism to defense those stresses. Oxidative stress is one of the major challenges faced by Arctic bacteria and the bacteria can produce the antioxidant protein in the extreme environment [Citation24].
In the present study, the culture supernatants of Paracoccus sp. Arc7-R13 isolated from Arctic sediment was investigated for the synthesis of AgNPs. The synthesized AgNPs was characterized by ultraviolet-visible (UV-vis) spectrophotometer, transmission electron microscopy (TEM) and energy-dispersive spectroscopy (EDX). In addition, Fourier transform infrared spectroscopy (FTIR) was performed for identifying the functional group and crystal structure, respectively. To the best of our knowledge, this report highlights the AgNPs biosynthesis using supernatant of Paracoccus sp. from the Arctic and these nanoparticles exhibit higher antibacterial activity.
Materials and methods
Chemicals and bacterial strains
Bacillus subtilis ATCC6633, Staphylococcus aureus ATCC6538, Pseudomonas aeruginosa PAO1 and Escherichia coli CGMCC1.2340 were stored at -80 °C and used for antimicrobial activity assay. Silver nitrate (AgNO3) was purchased from Shanghai Chemical Reagent Factory (Shanghai, China). The yeast extract and tryptone were purchased from Solarbio Ltd (Beijing, China). Agar powder was purchased from Solarbio Ltd (Beijing, China). The hydrogen peroxide was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Strain isolating and culture conditions
Sediment samples were collected during the 7th Chinese National Arctic Expedition in 2016 and stored at 4 °C. The samples were put into the sterile tubes with marine ZoBell 2216E medium supplemented with H2O2 (peptone, 5 g/L; yeast extract, 1 g/L; H2O2, 10 mmol/L; natural seawater, 1 L), and transferred to shaking incubator at 15 °C and 150 r/min for 2 d. After that, the cultures were serially diluted and spread on the 2216E medium agar plates to get pure strains. Finally, the isolates were identified on the basis of 16S rRNA gene amplified by PCR-method [Citation25].
Biosynthesis of silver nanoparticles
To synthesize the AgNPs, strain Arc7-R13, having the highest oxygen tolerance ability among the 19 isolates obtained, was cultured in 300 ml of YP medium (peptone, 10 g/L; yeast extract, 5 g/L; ultra-pure water, 1 L) and incubated in shaking incubator at 15 °C and 150 r/min. After 48 h, the cultures were centrifuged at 12,000 r/min for 15 min. About 40 ml of cell-free supernatant were mixed with 40 ml AgNO3 (4 mmol/L). The mixtures were kept on shaking at 37 °C (150 r/min) for a period of 48 h under 1200 Lx visible light illumination. In addition, the cell-free supernatant without AgNO3 and the liquid media with AgNO3 were kept in the same conditions and set as control. The formation of AgNPs was initially confirmed due to the color changing from yellow to brown, as a result of the surface plasmon resonance phenomenon [Citation26].
Characterization of silver nanoparticles
The formation of silver nanoparticles was confirmed by UV-Vis spectrum on the spectrophotometer (UV-Vis) in range of 300–700 nm at a resolution of 1 nm using Shimadzu spectrophotometer (Model UV 2550, Shanghai, China). Spectroscopy was performed for 6 h with intervals period of 48 h from the beginning time of reaction. The maximum absorption spectrum of AgNPs was determined by using different final concentrations of AgNO3 solution (0.5–5 mmol/L) to check the optimum solution concentration. The optimal AgNO3 concentration for AgNPs synthesis was determined by mixing culture supernatant with different Identical volume AgNO3 solution (final concentration 0.5–5 mmol/L). The FTIR spectrum was recorded on Nicolet iS10 (Thermo Electron Scientific Instruments LLC, USA) over the range of 4000–500 cm−1. The morphological and size characterization of the synthesized AgNPs (4 mmol/L) were carried out using TEM. The presence of Ag in samples was confirmed by using EDX (S-3400N).
Antibacterial activity
The synthesized AgNPs were tested for the antimicrobial activity by standard agar-well diffusion method [Citation27]. B. subtilis, S. aureus, P. aeruginosa and E. coli were used as the test specimen. These four strains were grown overnight in LB medium and then spread the bacteria supernatant uniformly on LB plates by using a cotton swab. Four circular wells of 8 mm diameter were made using a sterile cork-borer and 100 µL different concentration of the synthesized AgNPs (2, 4, 8, 10, 14 and 16 mg/mL) was added to different wells, respectively. The plates were incubated at 37 °C for 24 h and then the zones of inhibition were observed and calculated.
Results and discussion
Screening and identification of bacteria
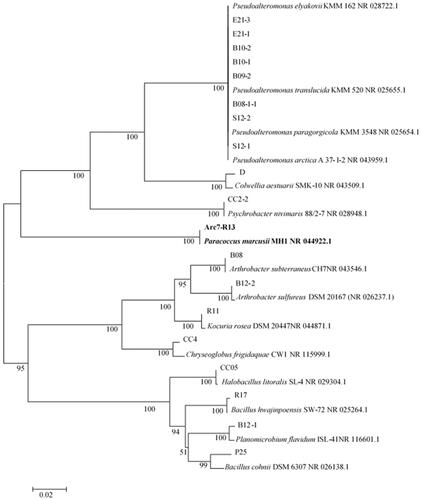
On the basis of the morphology of bacterial colony, 19 strains were isolated from marine ZoBell 2216E medium supplemented with H2O2. According to the 16S rRNA analysis, these 19 strains belonging to the genus of Pseudoalteromonas, Colwellia, Psychrobacter, Paracoccus, Arthrobacter, Kocuria, Halobacillus, Bacillus, Planomicrobium and Chryseoglobus, respectively (. During the screening study, a bacterial strain Arc7-R13 showed rapid growth on medium supplemented with H2O2, indicating that this strain Arc7-R13 had a high ability of tolerating H2O2. The almost complete 16S rRNA gene sequence of strain Arc7-R13 was analyzed to NCBI and showed the highest similarity (100%) with Paracoccus marcusii strain MH1 (NR_044922.1). The phylogenetic trees also indicated that strain Arc7-R13 was affiliated with the genus Paracoccus. (.
Biosynthesis of AgNPs
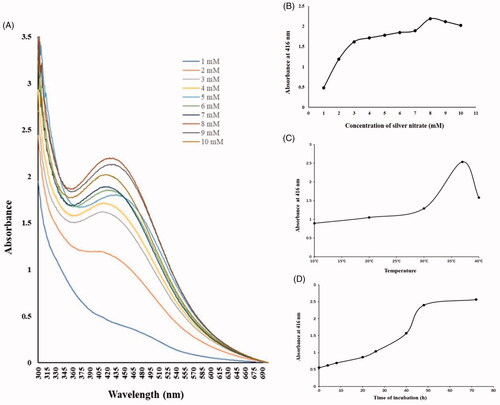
Microbially synthesized AgNPs were brown in color [Citation26] and the color change from yellow to brown preliminarily confirmed the formation of silver nanoparticles (. Also, the AgNPs synthesis was further verified by a characteristic peak at approximately 416 nm because of the surface plasmon resonance by using UV-vis spectral scanning. The synthesized AgNPs by using supernatant of Paracoccus sp. showed a characteristic peak at approximately 416 nm (), which confirms the AgNPs were accumulated in the supernatant and AgNO3 mixture solution. The size and shape of microbial synthesis nanoparticles were depended on the temperature, incubation period [Citation28] and the species of bacteria [Citation29]. In the beginning, an increase in the peak at 416 nm with the increasing of AgNO3 concentration and incubation temperature, the highest absorption peak was detected at 8 mmol/L concentration of AgNO3 and solution 37 °C, respectively (). It also showed that the absorbance at 416 nm reached the maximum value after 48 h of the mixed reaction of supernatant and AgNO3 4 mmol/L solution ().
Characterization of silver nanoparticles
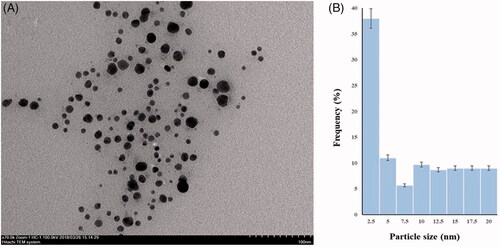
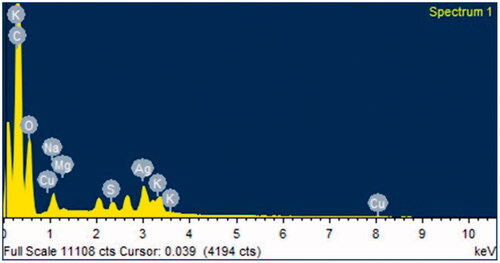
The AgNPs synthesized by strain Arc7-R13 were spherical and ellipsoidal in shape observed with TEM (). The size of AgNPs ranged from 2 nm to 25 nm with the maximum percentage of total AgNPs having the size of 2 nm–5 nm (). To the best of our knowledge, this is the first time that biosynthesis of AgNPs has so small particle size. The size and shape of nanoparticles can influence the antimicrobial activity of AgNPs [Citation30]. Smaller particles can exhibit more antimicrobial activity than the larger particles due to the smaller particles having a larger surface area [Citation31,Citation32]. According to the previous experimental results, the average size of biosynthetic AgNPs were 16.82 nm [Citation1], 70 nm [Citation17] and 30–80 nm [Citation14]. Therefore, the results of this experiment might provide a smaller, better performance AgNPs in antimicrobial applications. The result of energy-dispersive X-ray (EDX) confirmed the presence of metallic silver and account for 5.31% of total AgNPs (.
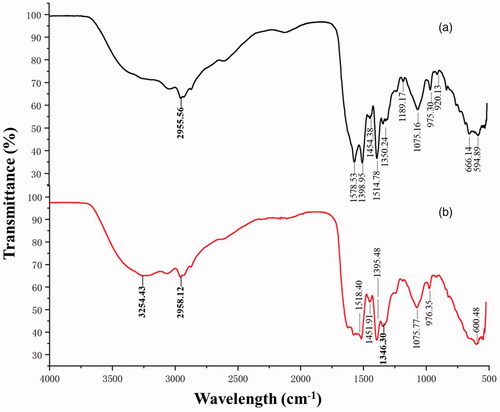
The comparison results of the comparison spectrum between the supernatant and synthesized AgNPs showed the potential functional groups responsible for the reduction of silver nitrate to AgNPs (. Compared with the FTIR results of the supernatant, the strong peaks at (2958.12 and 2955.56) and (1346.30 and 1350.24) cm−1 and the peak at 3254.43 cm−1 were changed or disappeared in that of AgNO3 treated supernatant. The disappearance of strong peaks at 3254.43 cm−1 might be due to the OH stretching of alcohols and phenols [Citation33]. The weak peaks changing from 2958.12 to 2955.56 cm−1 indicate CH3 stretch of alkanes [Citation33]. The aromatic groups (C≡N) was observed at 1346.30 cm−1 [Citation34]. The result of FTIR indicated that biomolecules containing functional groups OH, CH3 and C≡N played an important role as reducing and stabilizing agents of AgNPs in the supernatant of strain Arc7-R13.
Antibacterial activity
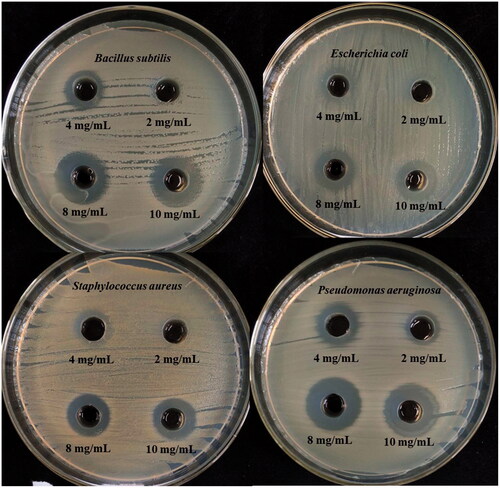
Several studies have demonstrated that silver nanoparticles have strong inhibitory activity against Gram-negative and Gram-positive bacteria [Citation35,Citation36]. In this study, the potential antibacterial activity of AgNPs was shown in . The inhibition zones were observed around the AgNPs and the diameter of the inhibition zone increased with the increasing AgNPs concentrations. When the concentration of AgNPs was 300 µg/mL, the inhibition zone appears for the first time, reached 2 mg/mL, a significant inhibition zone appeared. At a concentration of 16 mg/mL, the diameter of the zone of inhibition reaches a maximum of 16 mm.
Figure 7. Antimicrobial activities of synthesized AgNPs against S. aureus, P. aeruginosa, E. coli, and B. subtilis.
In the earlier studies, the mechanisms of the AgNPs exerting the antibacterial effects were proposed in different literatures. Krishnaraj et al. [Citation37] claimed AgNPs as a function of conductivity to change the membrane, leading to cell death. Furthermore, the AgNPs can break the double-stranded DNA molecules and release silver ions to react with other biomolecules, thus killing the cell [Citation38,Citation39]. Alsalhi et al. [Citation40] demonstrated that the biosynthesized AgNPs could be against infectious bacteria. This experiment further effectively proved that AgNPs had outstanding bacteriostatic activity.
Conclusion
We report a simple and green method of synthesizing silver nanoparticles by using supernatant of Paracoccus sp Arc7-R13. In this study, it revealed that many biomolecules and potential function groups in the supernatant were responsible for the formation of the AgNPs. The synthesized nanoparticles were spherical and ellipsoidal in shape with size ranging from 2 nm to 25 nm, among which the AgNPs with the size of 2–5 nm accounted for the majority. The most suitable reaction temperature and silver nitrate concentration were 37 °C and 4 mmol/L, respectively. In the process of reducing silver nitrate to AgNPs, the group of OH, CH3 and C≡N might play an important role. The bio-molecules of supernatant were responsible for reducing Ag ions and capping of biosynthesizing AgNPs. We also demonstrated that the AgNPs exhibited obvious antibacterial ability against Gram-positive and Gram-negative bacteria investigated, which might offer a new candidate in medical application. Overall, our study may provide new ideas for the biosynthesis of nanoparticles and the use of Arctic microbial resources.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kulkarni RR, Shaiwale NS, Deobagkar DN, et al. Synthesis and extracellular accumulation of silver nanoparticles by employing radiation-resistant Deinococcus radiodurans, their characterization, and determination of bioactivity. Int J Nanomedicine. 2015;10:963–974.
- Perni S, Hakala V, Prokopovich P. Biogenic synthesis of antimicrobial silver nanoparticles capped with l-cysteine. Colloid Surface A. 2014;460:219–224.
- Dang TD, Banerjee AN, Cheney MA, et al. Bio-silica coated with amorphous manganese oxide as an efficient catalyst for rapid degradation of organic pollutant. Colloid Surface B. 2013;106:151–157.
- Mondragon R, Enrique Julia J, Barba A, et al. Influence of the particle size on the microstructure and mechanical properties of grains containing mixtures of nanoparticles and microparticles: levitator tests and pilot-scaled validation. J Eur Ceram Soc. 2013;33:1271–1280.
- Park H, Tsutsumi H, Mihara H. Cell penetration and cell-selective drug delivery using alpha-helix peptides conjugated with gold nanoparticles. Biomaterials. 2013;34:4872–4879.
- Ahmed MJ, Murtaza G, Mehmood A, et al. Green synthesis of silver nanoparticles using leaves extract of Skimmia laureola: characterization and antibacterial activity. Mater Lett. 2015;153:10–13.
- Singh R, Shedbalkar UU, Wadhwani SA, et al. Bacteriagenic silver nanoparticles: synthesis, mechanism, and applications. Appl Microbiol Biotechnol. 2015;99:4579–4593.
- Jain J, Arora S, Rajwade JM, et al. Silver nanoparticles in therapeutics: development of an antimicrobial gel formulation for topical use. Mol Pharm. 2009;6:1388–1401.
- Rajamanickam K, Sudha SS, Francis M, et al. Microalgae associated Brevundimonas sp. MSK 4 as the nanoparticle synthesizing unit to produce antimicrobial silver nanoparticles. Spectrochim Acta A. 2013;113:10–14.
- Arunachalam KD, Annamalai SK. Chrysopogon zizanioides aqueous extract mediated synthesis, characterization of crystalline silver and gold nanoparticles for biomedical applications. Int J Nanomed. 2013;2013:2375–2384.
- Kvitek L, Panacek A, Prucek R, et al. Antibacterial activity and toxicity of silver–nanosilver versus ionic silver. J Phys: Conf Ser. 2011;304:012029.
- Hwang ET, Lee JH, Chae YJ, et al. Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small. 2008;4:746–750.
- Rai MK, Deshmukh SD, Ingle AP, et al. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol. 2012;112:841–852.
- Hina S, Juan D, Tae-Hoo Y. Green and rapid synthesis of silver nanoparticles using Borago officinalis leaf extract: anticancer and antibacterial activities. Artif Cells Nanomed Biotechnol. 2017;45:1310–1316.
- Tripathy A, Raichur AM, Chandrasekaran N, et al. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J Nanopart Res. 2010;12:237–246.
- Binupriya AR, Sathishkumar M, Yun SI. Myco-crystallization of silver ions to nanosized particles by live and dead cell fltrates of Aspergillus oryzae var. viridis and its bactericidal activity toward Staphylococcus aureus KCCM 12256. Ind Eng Chem Res. 2010;49:852–858.
- Raja S, Ramesh V, Thivaharan V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab J Chem. 2017;10:253–261.
- Krishnaraj C, Muthukumaran P, Ramachandran R, et al. Acalypha indica Linn: biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDAMB-231, human breast cancer cells. Biotechnol Rep. 2014;4:42–49.
- Klaus T, Joerger R, Olsson E, et al. Silver-based crystalline nanoparticles, microbially fabricated. P Natl Acad SCI USA. 1999;96:13611–13614.
- Joerger R, Klaus T, Granqvist CG. Biologically produced silver-carbon composite materials for optically functional thin film coatings. Adv Mater. 2000;12:407–409.
- Kalimuthu K, Babu RS, Venkataraman D, et al. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloid Surfaces B. 2008;65:150–153.
- Janardhanan A, Roshmi T, Soniya EV, et al. Biosynthesis of silver nanoparticles by a Bacillus sp. of marine origin. Mat Scie Poland. 2013;31:73.
- Kumar V, Gundampati RK, Singh DK, et al. Photoinduced green synthesis of silver nanoparticles with highly effective antibacterial and hydrogen peroxide sensing properties. J Photochem Photobiol B Biol. 2016;162:374–385.
- Lin XZ, Wang Z, Li Y, et al. Genome-wide transcriptional response of the Arctic bacterium Pseudoalteromonas sp. A2 to oxidative stress induced by hydrogen peroxide. Acta Oceanol Sin. 2016;35:73–80.
- Weisburg WG, Barns SM, Pelletier DA, et al. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703.
- Eustis S, Sayed MA. Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev. 2006;35:209–217.
- Nathan P, Law EJ, Murphy DF, et al. A laboratory method for selection of topical antimicrobial agents to treat infected burn wounds. Burns. 1978;4:177–187.
- Gurunathan S, Kalishwaralal K, Vaidyanathan R, et al. Biosynthesis, purifcation and characterization of silver nanoparticles using Escherichia coli. Colloid Surface B. 2009;74:328–335.
- Shivaji S, Madhu S, Shashi S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem. 2011;46:1800–1807.
- Iman R, Maryam G, Saeed B. Biosynthesis of silver nanoparticles using leaf extract of Satureja hortensis treated with NaCl and its antibacterial properties. Micropor Mesopor Mat. 2018;264:240–247.
- Kulthong K, Srisung S, Boonpavanitchakul K, et al. Determination of silver nanoparticle release from antibacterial fabrics into artificial sweat. Part Fibre Toxicol. 2010;7:8.
- Feng Q, Wu J, Chen G, et al. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662–668.
- Vanaja M, Gnanajobitha G, Paulkumar K, et al. Phytosynthesis of silver nanoparticles by Cissus quadrangularis: influence of physicochemical factors. J Nanostruct Chem. 2013;3:1–8.
- Rajasekar P, Priyadharshini S, Rajarajeshwari T, et al. Bio-inspired synthesis of silver nanoparticles using Andrographis paniculata whole plant extract and their antimicrobial activity over pathogenic microbes. Int J Res Biomed Biotech. 2013;3:47–52.
- Geethalakshmi R, Sarada DVL. Gold and silver nanoparticles from Trianthema decandra: synthesis, characterization, and antimicrobial properties. Int J Nanomed. 2012;7:5375–5384.
- Dhanalakshmi M. Silver nanoparticles and its antibacterial activity. Int J Pharm Biol Arch. 2013;4:819–826.
- Krishnaraj C, Jagan EG, Rajasekar S, et al. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloid Surfaces B. 2010;76:50–56.
- Tamboli DP, Lee DS. Mechanistic antimicrobial approach of extracellularly synthesized silver nanoparticles against gram positive and gram negative bacteria. J Hazard Mater. 2013;260:878–884.
- Składanowski M, Golinska P, Rudnicka K, et al. Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med Microbiol Immunol. 2016;205:603–613.
- Alsalhi MS, Devanesan S, Alfuraydi AA, et al. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. IJN. 2016;11:4439–4449.