 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pathological hypoxia-induced organ dysfunction contributes to the high mortality of sepsis. Because of the microcirculation dysfunction following severe sepsis, it is difficult for erythrocytes to transport oxygen to hypoxic tissues. Haemoglobin-based oxygen carriers (HBOCs) are capable of delivering oxygen to hypoxic tissues. The aim of this study is to observe the potential benefits of a novel bovine-derived, non-polymerized, cell-free HBOC solution, YQ23, on sepsis in rats. Cecum ligation and puncture was performed to induce sepsis in Sprague-Dawley rats. Effects of Lactate Ringer’s solution (LR), YQ23, and whole blood on oxygen delivery and consumption, mitochondrial function, organ protection and animal survival were observed. LR failed to restore oxygen delivery and the therapeutic effects were limited, whereas low dosage of YQ23 and whole blood significantly increased the tissue oxygen delivery and consumption, improved the mitochondrial function of heart, liver, kidney and intestine, prevented the vital organs injuries and improved the animal survival. The effects of 0.15 g·kg−1 YQ23 resembled that of the whole blood. In addition, YQ23 did not induce renal toxicity, severe oxidative effect and acute vasoconstriction. Thus, YQ23 is a safe and effective resuscitation fluid for sepsis.
Introduction
Sepsis is a life-threatening condition which has high morbidity and mortality [Citation1]. Although the bundles of approaches, including fluid resuscitation, antibiotics and vasoactive agents, etc. were developed [Citation2], the treatment effect is not as good as expected, however. Microcirculation dysfunction and tissues’ hypoxia play vital roles in organ damage following severe sepsis [Citation3,Citation4], and low oxygen delivery is related to patient death [Citation5]. Hypoxia can directly damage the cellular function and destroy the cellular structure, which is the major pathological basis of sepsis-related MODS and death. Thus, restoring tissue oxygen delivery may play pivotal roles in protecting organ function in these critical diseases.
Whole blood is an optimal fluid which not only expands blood volume but also increases the oxygen delivery. However, strict indications, safety concerns and blood shortage in urban areas restrain its application [Citation6]. Meanwhile, due to microcirculation stasis following sepsis, oxygen is difficult to be transported to tissues by erythrocytes [Citation7]. Thus, efforts have been made to develop oxygen carriers to solve this issue. Haemoglobin-based oxygen carriers (HBOCs) are developed and continuously attract attention for their oxygen-carrying capacity and availability in urgent applications. The modification on haemoglobin (Hb) reduces the potential adverse effects while the oxygen-carrying and -unloading capacities of Hb are well retained [Citation8]. Several studies demonstrated that HBOCs are beneficial in traumatic-hemorrhagic shock by improving tissue oxygen delivery [Citation9,Citation10]. In contrast with erythrocytes, cell-free haemoglobin could circulate more freely in microcirculation thanks to the small sizes and membrane-free structure. Thus, we assume that HBOCs could reverse pathological hypoxia in sepsis by increasing oxygen delivery and utilization.
Our previous study showed that YQ23, a novel bovine-derived, stabilized, non-polymerized, cross-linked, cell-free, tetrameric haemoglobin-based oxygen carrier, benefited traumatic hemorrhagic shock in rats and pigs without obvious adverse effects in a low dosage [Citation11]. The aim of this study is to explore whether YQ23 benefits the sepsis rats induced by cecum ligation and puncture (CLP) through increasing oxygen delivery and utilization and preventing organ injury in a low dosage.
Materials and methods
Ethical statement
The study was approved by the Laboratory Animal Welfare and Ethics Committee of Third Military Medical University according to the guidelines of the ethical use of animals. The investigation conformed to the Guide for the Care and Use of Laboratory Animals (the National Academies Press, eighth edition, 2011), and all rats were guaranteed to the least suffer.
YQ23 preparation
YQ23 is a bovine-derived, stabilized, non-polymerized, cross-linked, cell-free, tetrameric haemoglobin-based oxygen carrier which is provided by New B Innovation Limited, Hong Kong. The level of haemoglobin was >99.2% and the methemoglobin was <4.8%. The levels of dimeric haemoglobin, phospholipid, DNA impurities and protein impurities were undetectable or low. YQ23 was stored as a 5 g·dl−1 solution (protein amount). The pH range of the solution was 7.3–7.6, the p50 value was approximately 40 mmHg. The osmolality and viscosity (at 37 °C) were >250 mOsm/kg and 0.9 cP, respectively. According to our previous study, the YQ23 solution was further diluted into 0.15 g·dl−1, 0.5 g·dl−1, 0.75 g·dl−1 with Lactate Ringer’s solution (LR) before use to avoid potential side effects.
Experiment protocol
This experiment was performed on Sprague-Dawley rats (weight: 220–240 g) obtained from the Animal Center of Research Institute of Surgery. Rats were caged in a specific-pathogen-free caring-room at the temperature of 24 ± 1 °C and light cycled (6 a.m.–6 p.m.). The standard pellet diets were taken ad libitum.
The entire experimental design is illustrated in . In this study, rats were anaesthetized with sodium pentobarbital (50 mg·kg−1 [of body weight], i.p.) and subjected to cecum ligation and puncture (CLP) as described previously to induce sepsis [Citation12], the sham-operated rats only received cecum ligation without the puncture. After surgery, rats were settled back to cages and allowed for food and water ad libitum in a clean room. Twelve hours later, the rats were re-anaesthetized and when the mean arterial pressure (MAP) was ≤65 mmHg, the sepsis model was regarded as achieved and suitable for the subsequent experiment. The success rate of sepsis model in this study was 85.0%.
Figure 1. Schematics of the study design. (A) The design of the whole study; (B) The composition of fluid administrated in each group; (C) The protocol of fluid resuscitation. CLP: cecum ligation and puncture; LR: Lactate Ringer’s Solution.
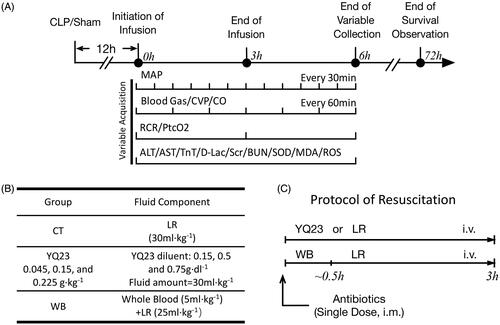
Sepsis rats were randomly assigned into five groups and resuscitated with different fluids (n = 8/group). Rats in the conventional therapy (CT) group were resuscitated with LR (30 ml·kg−1 [of body weight]) according to the guidelines [Citation2], rats in YQ23 groups received 30 ml·kg−1 (of body weight) YQ23 solution in three different concentrations, which means rats received 0.045, 0.15 and 0.225 g·kg−1 [of body weight] YQ23, respectively. Rats in whole blood (WB) group received fresh whole blood (5 ml·kg−1 [of body weight]) and LR (25 ml·kg−1 [of body weight]) separately. The duration of fluid resuscitation was 3 h. Besides, cefuroxime (50 mg·kg−1 [of body weight], i.m.) was given at the beginning of resuscitation in each group. Variables were collected during and after resuscitation, the duration of survival observation was 72 h and the data of the sham-operated rats were regarded as the control. After data were collected, the rats were subjected to euthanasia humanely with over-dosed sodium pentobarbital (100 mg·kg−1 [of body weight]).
Variable acquisition
The cardiac output (CO) was measured with thermodilution technique as described previously [Citation11]. Briefly, a thermodilution catheter was inserted into the aorta ascendens through the right carotid artery, and ice-bathed saline (0.3 ml) was injected through the right external jugular vein catheter. The CO was determined using a CO analyzer (Power Laboratory, AD Instruments, Sydney, Australia). MAP, heart rate and central venous pressure (CVP) were determined by a polygraph physiological recorder (SP844; Power Laboratory, AD Instruments, Australia). Cardiac index (CI) and stroke index (SI) were calculated with the following equations: CI = CO/S, S = 9.1× SI = CI/HR, in which S represents the body surface, and W represents the body weight. In addition, the systemic vascular resistance index (SVRI) was calculated with the equation: SVRI = 79.92 × (MAP-CVP)/CI.
Arterial and venous blood gases (PaO2, SaO2, SvO2, Lac, Hb) were determined with the blood gas analyzer (ABL 800 FLEX; Radiometer, Cop1enhagen, Denmark). To avoid additional blood loss in rats, an equal volume of blood obtained from donor rats was infused back right after sampling. Oxygen delivery (DO2) and consumption (VO2) were calculated with the following equations: DO2 = CI × 13.4 × [Hb] × SaO2; VO2 = CI × 13.4 × [Hb] × (SaO2 – SvO2). Transcutaneous partial pressure of oxygen (PtcO2) at right abdomen was determined by a laser Doppler system (Periflux System 5000; Perimed, Stockholm, Sweden).
The oxygen consumption rate and respiratory control rate (RCR) of heart, intestine, liver and kidney mitochondria were determined by a Clark electrode (Strathkelvin 782, North Lanarkshire, Scotland). Briefly, the heart, small intestine, liver and kidney were harvested to isolate mitochondria by differential centrifugation. Then, 1.2 ml experiment buffer (sucrose 0.25 mol·l−1, KCl 15 mmol·l−1, KH2PO4 15 mmol·l−1, Tris-HCl 50 mmol·l−1, EDTA-Na2 1 mmol·l−1, pH 7.4, 30 °C) and 0.6 mg mitochondria (protein amount) were put into the reaction chamber and equilibrated for 20 s, then the substrates (5 μmol sodium malate and 5 μmol sodium glutamate) were added (in order to use up intrinsic adenosine diphosphate [ADP]). After the reaction chamber is sealed up and the mitochondria were allowed for another 15s’ equilibration, then extrinsic ADP (5 μl, 400 nmol·l−1) was added to determine the oxygen consumption rate (OCR) of state 3 respiration. When ADP was used up again, the OCR of state 4 respiration was recorded subsequently. RCR is the ratio of the OCR of state 3 respiration rate and OCR of state 4 respiration [Citation13].
Renal function (blood urea nitrogen/BUN and serum creatinine/Scr), liver injury markers (aspartate aminotransferase/AST and alanine aminotransferase/ALT), and cardiac injury markers (troponin/TnT) were measured using a Biochemical Analyzer (DX800; Beckman Coulter, Fullerton, CA). Intestinal barrier injury was reflected by determining D-lactate level by D-Lac test kits (Nanjing Built Bio Co, Nanjing, China). Oxidative stress-related variables (Reactive oxygen species/ROS, superoxide dismutase/SOD and malondialdehyde/MDA) in the serum were measured with related testing kits, respectively (Nanjing Built Bio Co, Nanjing, China).
Another 80 rats were randomly assigned into five groups for 72 h-survival observation (n = 16/group), and at the end of observation, the rats survived were subjected to euthanasia.
Statistical analysis
Parametric data (blood gases, DO2, VO2, PtcO2, RCR, renal and cardiac function, liver, heart and intestinal injury markers, ROS, SOD, MDA, MAP and SVRI) were presented as the mean ± standard deviation (SD) of n observations. The statistical differences among groups were analyzed using two-factor variance analysis, followed by the post-hoc Tukey test (SPSS v15.0; SPSS Inc, Chicago, IL) for multiple comparisons between two groups. Survival time was presented as median with interquartile range and survival rate was analyzed by Kaplan–Meier survival analysis and the log-rank test. p < .05 was considered significant.
Results
YQ23 significantly increased the systemic oxygen supply and utilization in sepsis rats
Since the decreased blood oxygen saturation and pathological hypoxia of tissues are closely related to the severity of sepsis [Citation14], we compared the effects of different fluids on oxygen delivery and utilization in sepsis rats. Consistently with previous studies [Citation12,Citation15], the PaO2, SaO2 and PtcO2 were significantly decreased following sepsis. LR failed to improve these parameters, whereas the 0.045 g·kg−1, 0.15 g·kg−1 and 0.225 g·kg−1 of YQ23 significantly increased the PaO2, SaO2 and PtcO2 (, ). Among them, the effects of 0.15 g·kg−1 and 0.225 g·kg−1 YQ23 of YQ23 are almost as same as the WB.
Figure 2. Effects of YQ23 on oxygen delivery and consumption in sepsis treatment. Data represent the mean ± SD of eight observations. (A) Transcutaneous partial pressure of oxygen (PtcO2); (B) Oxygen delivery (DO2); (C) Oxygen consumption (VO2). #p < .05 vs. Sham, *p < .05, **p < .01 vs. CT at the same time point, $p < .05 and $$p < .01 vs. WB at the same time point. Sham: Sham Operated; S: Sepsis; CT: conventional therapy; 0.045 YQ23 = 0.045 g/kg; YQ23; 0.15 YQ23 = 0.15 g/kg YQ23; 0.225 YQ23 = 0.225 g/kg YQ23; WB = whole blood.
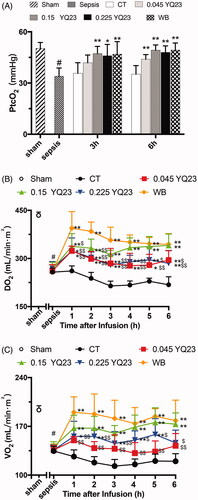
Table 1. Effects of YQ23 on blood gases in sepsis rats.
Oxygen delivery (DO2) and utilization (VO2) are the important predictors of mortality in critical diseases [Citation16]. Our study found that DO2 and VO2 were significantly decreased in sepsis rats. LR failed to elevate the DO2 and VO2. YQ23 and WB significantly increased the DO2 and VO2 at 1 h after infusion and 0.15 g·kg−1 of YQ23 had the best effect that was similar to WB ().
The lactic acid (Lac) is the product of anaerobic respiration and blood Lac is closely related to the outcome of critical diseases. Our study found Lac was significantly increased following sepsis, while CT, YQ23 and whole blood treatment did not decrease the blood Lac (), which might be explained by the delayed clearance of Lac or the mismatch between Lac clearance and microcirculation improvement [Citation17,Citation18]. These results illustrated that YQ23 was able to improve oxygen delivery and utilization and reduce cell hypoxia.
YQ23 restored mitochondrial function in sepsis rats
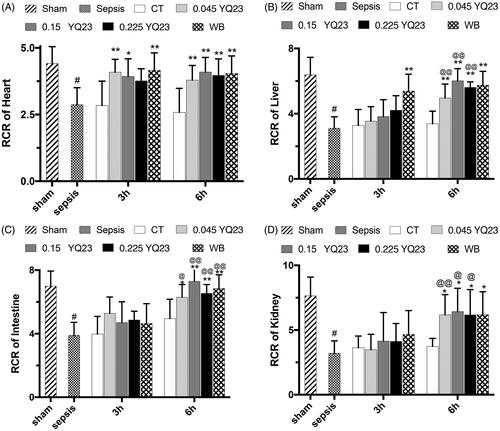
Mitochondria are vital organelles which are always affected by pathologic hypoxia, whose dysfunction is closely related to poor prognosis of sepsis. Early restoration of the mitochondrial function is correlated with better outcome [Citation19]. We observed the effects of YQ23 on the respiratory control rate (RCR) of vital organ mitochondria to know whether HBOC would rescue the mitochondria function. The results showed that YQ23 and whole blood could significantly restore the RCR of heart, liver, kidney and intestine mitochondria (). The 0.15 g·kg−1 of YQ23 had the best effect among the three dosages of YQ23.
Figure 3. Effect of YQ23 on respiratory control rate of vital organ mitochondria in sepsis rats. Data represent the mean ± SD of eight observations. (A) RCR of heart mitochondria; (B) RCR of liver mitochondria; (C) RCR of intestine mitochondria; (D) RCR of kidney mitochondria. #p < .05 vs. Sham; *p < .05 and **p < .01 vs. CT at the same time point; @p < .05 and @@p < .01 vs. 3 h in the same treatment group. Sham: Sham Operated; S: Sepsis; CT: conventional therapy; 0.045 YQ23 = 0.045 g/kg YQ23; 0.15 YQ23 = 0.15 g/kg YQ23; 0.225 YQ23 = 0.225 g/kg YQ23; WB = whole blood.
YQ23 improved the vital organ function in sepsis rats
The cardiac function determines the systemic circulation and tissue perfusion, the decreased cardiac function could aggravate hypoxia. In this study, LR has less influence on CO of sepsis rats. YQ23 significantly improved CO of sepsis rats, and CO in 0.15 g·kg−1 YQ23 group was increased by about 57.4% at 3 h after infusion and maintained at a high level (), and the effects were similar to WB. In order to further determine the heart performance of YQ23, CI and SI were employed. Consistently with CO, YQ23 significantly improved the CI and SI in sepsis rats ().
Figure 4. Effect of YQ23 on vital organ function and survival. Cardiac function of rats: (A) CO, (B) CI and (C) SI; vital organ injury markers: (D) AST, (E) ALT, (F) TnT, (G) D-Lac, Data represent the mean ± SD of eight observations. (H)–(I) Animal survival curve and survival time, data are presented as median with interquartile range (n = 16). #p < .05 vs. baseline, *p < .05 and **p < .01 vs. CT group at the same time point; @p < .05 and @@p < .01 vs. 3 h in the same treatment group. Sham: Sham Operated; B: Baseline; S: Sepsis; CT: conventional therapy; 0.045 YQ23 = 0.045 g/kg YQ23; 0.15 YQ23 = 0.15 g/kg YQ23; 0.225 YQ23 = 0.225 g/kg YQ23; WB = whole blood; MST = Median survival time.
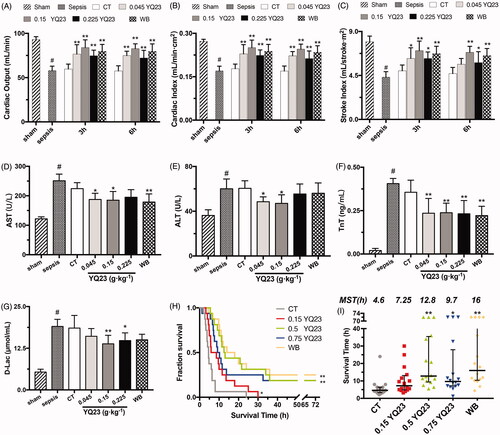
In order to investigate the organ-protective effects of YQ23, the AST, ALT, TnT and D-Lac were observed. The results showed that the heart, liver, kidney and intestinal barrier were significantly damaged after sepsis. CT did not influence the AST, ALT, TnT and D-Lac, whereas YQ23 and whole blood significantly reduced the ALT, AST, TnT and D-lac (). These results suggested that YQ23 could significantly restore the cardiac function and reduce vital organ injury.
YQ23 improved survival of sepsis rats
We observed the effects of YQ23 on animal survival to evaluate the overall effect of YQ23 in sepsis rats. The results showed that no rats survived over 72 h in the CT group, whereas 0.15 g·kg−1 and 0.225 g·kg−1 of YQ23 and whole blood significantly promoted the 72 h survival rate of sepsis rats (). The median survival time for 0.15 g·kg−1 and 0.225 g·kg−1 of YQ23 and WB group were 12.8 h, 9.7 h and 16 h, which were significantly longer than the CT group (4.6 h) ().
Side effects of YQ23 in sepsis rats
Effects of YQ23 on MAP and SVRI: CT resuscitation alone was not able to effectively increase the MAP of sepsis rats, whereas YQ23 and whole blood restored the MAP significantly (). Vasoconstriction is a common safety concern of HBOCs which could be reflected by SVRI. Our results showed that SVRI was significantly decreased in sepsis rats, which is consistent with previous reports [Citation20,Citation21]. YQ23 and whole blood resulted in a gradual and gentle increase of SVRI (), and there was no significant difference between YQ23 and WB group. This result suggested that YQ23 would not cause vasoconstriction.
Figure 5. Effects of YQ23 on vasoconstriction, renal injury and oxidizing injury in sepsis rats. Hemodynamics and vascular tension: (A) MAP, (B) SVRI; Renal function: (C) Scr, (D) BUN; Oxidizing parameters: (E) MDA, (F) SOD, (G) ROS. Each group contains eight observations. #p < .05 vs. sham; *p < .05 and **p < .01 vs. CT, $p < .05 and $$p < .01 vs. WB at the same time point. Sham: Sham Operated; S: Sepsis; CT: conventional therapy; 0.045 YQ23 = 0.045 g/kg YQ23; 0.15 YQ23 = 0.15 g/kg YQ23; 0.225 YQ23 = 0.225 g/kg YQ23; WB = whole blood.
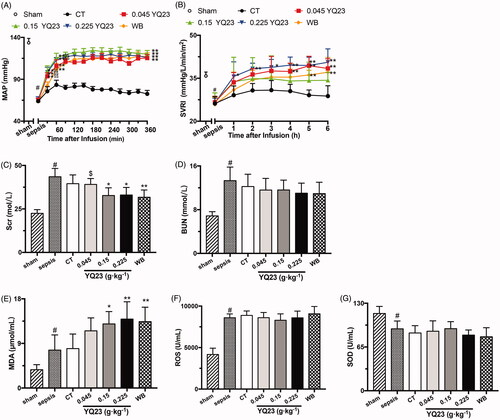
Effects of YQ23 on renal toxicity: Monomers and dimers of haemoglobin could cause damage in the kidney [Citation8]. We determined BUN and Scr to evaluate the side effects of YQ23 on the kidney. The results showed that YQ23 and whole blood administration significantly decreased the blood Scr of sepsis rats while did not influence the BUN (). Besides, low-molecular-weight haemoglobin was not detected in urine (data were not shown). These results suggested that YQ23 had no renal side effect.
Effects of YQ23 on oxidative stress: Hypoxia/re-oxygenation related oxidative stress is widely reported after hemorrhagic or septic shock [Citation22,Citation23]. Our results showed that YQ23 could increase blood MDA, the product of lipid oxidation () while did not affect the SOD and ROS, as compared with the WB (). These results suggested that YQ23 did not induce oxidation damage as compared to whole blood.
Discussion
Sepsis has long been considered as a public hazard which takes millions of lives each year in the world, systemic ischemia and pathological hypoxia are considered as the major basis of MODS following sepsis. Oxygen inhalation, mechanical ventilation, and fluid therapy are aimed at reversing hypoxia, however, effects are so limited. Blood transfusion is believed to be optimal access to improve oxygen delivery, but erythrocytes are not able to enter the capillaries in sepsis because of microcirculation dysfunction. HBOCs are beneficial for trauma, anaemia patients by transporting oxygen to capability [Citation24,Citation25]. Although some trials in the last decade reported increased risks of myocardial infarction, stroke or death during the administration, HBOCs are developing in zigzag, and there is still hope that they will be finally safe enough to save lives [Citation26].
In this study, we confirmed a novel bovine-derived, stabilized, cross-linked HBOC, YQ23 could increase the tissue oxygen delivery, improve the vital organ function and promote the animal survival in sepsis rats. The therapeutic effects increased dose-dependently in low YQ23 dosages while the beneficial effects did not continue to increase when dosage was >0.225 g·kg−1. This study suggested that the novel HBOC might be a promising functional resuscitation fluid in sepsis treatment.
Some HBOCs have been tested in trauma, shock animal or human patients. For instance, in a porcine model of cardiopulmonary bypass, pre-perfusion of HBOC-201 was related to improving the oxygen utilization, minimizing the anaerobic metabolism and lowering lactate acid level, as compared with whole blood [Citation27]. In a clinical study, HBOC-201 increased the brain tissue PaO2 in severe polytrauma patients [Citation28]. However, the massive application of HBOCs has been proved detrimental. Based on our previous study [Citation9], we presumed that HBOC in low dosage was capable of delivering oxygen and beneficial for sepsis rats. This study confirmed that the 0.15 g·kg−1 of YQ23 was beneficial by improving systemic oxygen metabolism, restoring mitochondrial function and preventing organ injury.
The vasoconstriction induced by NO scavenging by cell-free-haemoglobin is the priory safety concern of HBOCs, subsequential hemodynamic instability may induce acute hypertension, myocardial infarction and severe complications [Citation26,Citation28]. A diaspirin cross-linked haemoglobin increased perfusion and oxygen consumption in critically ill patients, but hypertension occurred [Citation29]. HBOC-201 is an advanced product which only induces minimal or modest alterations in systemic and pulmonary pressures. Nevertheless, regional organ blood flow was not influenced [Citation30]. In this study, low dosages of YQ23 were infused with microinfusion pumps to avoid acute MAP rises. Moreover, YQ23 was expected to restrict fluid in blood vessels with its colloidal characteristic. Results showed that MAP and SVRI in YQ23 treated groups were only slightly increased; this result confirmed the hypothesis and proved that small dosage of HBOC was able to stabilize the hemodynamics.
Dimeric haemoglobin (32kD) and methemoglobin are non-oxygen-carrying haemoglobin generated during HBOC storage which can damage the kidney. The stability of YQ23 guarantees that dimeric haemoglobin in this solution was undetectable and methemoglobin was only <4.8%. In addition, no haemoglobin was detected in urine, no significant differences were observed in kidney function among all five groups, which further confirmed the low renal toxicity of YQ23.
The hypoxia-reoxygenation injury is common and difficult to prevent. In our study, MDA levels were increased dose-dependently, which is comparable to whole blood group. To reduce the oxidative stress, the antioxidative and oxygen-carrying polyhemoglobin-superoxide dismutase-catalase-carbonic anhydrase (PolyHb-SOD-CAT-CA) was developed [Citation31]. However, the combination of blood (or blood substitute) and antioxidants such as Vitamin C might be an effective and applicable approach at present [Citation32].
Our study demonstrated that HBOC in low dosage benefited to sepsis rats without inducing severe adverse effects, suggesting HBOCs are promising therapeutic fluid for septic shock. But, there are some limitations: (1) the heterogeneity between CLP- and LPS-induced septic shock rats did exist despite practised operation; (2) the duration for collecting parameters was only 6 h after infusion, long-term influence of the YQ23 on vital organ function and its immunogenicity were not observed; (3) experiments were conducted only in rats, the effects of the YQ23 on large animal sepsis models remains to be studied; (4) cell metabolism was not observed despite mitochondrial function was restored.
In conclusion, a newly developed HBOC, YQ23, is capable of increasing tissue oxygen delivery and utilization, protecting heart, liver, intestine and promoting survival in CLP-induced rat sepsis model without a severe side effect in a low dosage. The HBOC may be a promising resuscitation fluid for severe sepsis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alvaro-Meca A, Jimenez-Sousa MA, Micheloud D, et al. Epidemiological trends of sepsis in the twenty-first century (2000-2013): an analysis of incidence, mortality, and associated costs in Spain. Popul Health Metr. 2018;16:4.
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377.
- De Backer D, Orbegozo Cortes D, Donadello K, et al. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence. 2014;5:73–79.
- Chan YL, Han ST, Li CH, et al. Transfusion of red blood cells to patients with sepsis. Int J Mol Sci. 2017;18:E1946.
- Soussi S, Deniau B, Ferry A, et al. Low cardiac index and stroke volume on admission are associated with poor outcome in critically ill burn patients: a retrospective cohort study. Ann Intensive Care. 2016;6:87.
- Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician. 2011;83:719–724.
- Charlton M, Sims M, Coats T, et al. The microcirculation and its measurement in sepsis. J Intensive Care Soc. 2017;18:221–227.
- Moradi S, Jahanian-Najafabadi A, Roudkenar MH. Artificial blood substitutes: first steps on the long route to clinical utility. Clin Med Insights Blood Disord. 2016;9:33–41.
- Li Y, Yan D, Hao S, et al. Polymerized human placenta hemoglobin improves resuscitative efficacy of hydroxyethyl starch in a rat model of hemorrhagic shock. Artif Cells Nanomed Biotechnol. 2015;43:174–179.
- Patel MB, Feinstein AJ, Saenz AD, et al. Prehospital HBOC-201 after traumatic brain injury and hemorrhagic shock in swine. J Trauma. 2006;61:46–56.
- Li T, Yang G, Zhu Y, et al. Beneficial effects of novel cross-linked hemoglobin YQ23 on hemorrhagic shock in rats and pigs. J Surg Res. 2017;210:213–222.
- Liu L, Wu H, Zang J, et al. 4-Phenylbutyric acid reveals good beneficial effects on vital organ function via anti-endoplasmic reticulum stress in septic rats. Crit Care Med. 2016;44:e689–e701.
- Wu Y, Zhu Y, Chen XY, et al. Diversity of vascular reactivity and the treatment response in diabetic, hypertensive, hyperlipidemic, and healthy rats subjected to hemorrhagic shock. Shock. 2016;45:174–183.
- Zhang Z, Ji X. Quadratic function between arterial partial oxygen pressure and mortality risk in sepsis patients: an interaction with simplified acute physiology score. Sci Rep. 2016;6:35133.
- Zhu Y, Wu H, Wu Y, et al. Beneficial effect of intermedin 1-53 in septic shock rats: contributions of rho kinase and BKCA pathway-mediated improvement in cardiac function. Shock. 2016;46:557–565.
- Rivers EP, Yataco AC, Jaehne AK, et al. Oxygen extraction and perfusion markers in severe sepsis and septic shock: diagnostic, therapeutic and outcome implications. Curr Opin Crit Care. 2015;21:381–387.
- Zafrani L, Ergin B, Kapucu A, et al. Blood transfusion improves renal oxygenation and renal function in sepsis-induced acute kidney injury in rats. Crit Care 2016;20:406.
- Puskarich MA, Shapiro NI, Massey MJ, et al. Lactate clearance in septic shock is not a surrogate for improved microcirculatory flow. Acad Emerg Med. 2016;23:690–693.
- Carre JE, Orban JC, Re L, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182:745–751.
- Russell JA, Rush B, Boyd J. Pathophysiology of Septic Shock. Crit Care Clin. 2018;34:43–61.
- Erdogan S, Bosnak M. Using terlipressin in a pediatric patient with septic shock resistant to catecholamines. North Clin Istanb. 2017;4:283–287.
- Sinning C, Westermann D, Clemmensen P. Oxidative stress in ischemia and reperfusion: current concepts, novel ideas and future perspectives. Biomark Med. 2017;11:11031–11040.
- Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551.
- Jahr JS, Moallempour M, Lim JC. HBOC-201, hemoglobin glutamer-250 (bovine), Hemopure (Biopure Corporation). Expert Opin Biol Ther. 2008;8:1425–1433.
- Davis JM, El-Haj N, Shah NN, et al. Use of the blood substitute HBOC-201 in critically ill patients during sickle crisis: a three-case series. Transfusion. 2018;58:132–137.
- Natanson C, Kern SJ, Lurie P, et al. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299:2304–2312.
- McNeil JD, Propper B, Walker J, et al. A bovine hemoglobin-based oxygen carrier as pump prime for cardiopulmonary bypass: reduced systemic lactic acidosis and improved cerebral oxygen metabolism during low flow in a porcine model. J Thorac Cardiovasc Surg. 2011;142:411–417.
- Freilich D, Pearce LB, Pitman A, et al. HBOC-201 vasoactivity in a phase III clinical trial in orthopedic surgery subjects–extrapolation of potential risk for acute trauma trials. J Trauma. 2009;66:365–376.
- Saxena R, Wijnhoud AD, Carton H, et al. Controlled safety study of a hemoglobin-based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke. 1999;30:993–996.
- Mongan PD, Moon-Massat PF, Rentko V, et al. Regional blood flow after serial normovolemic exchange transfusion with HBOC-201 (Hemopure) in anesthetized swine. J Trauma. 2009;67:51–60.
- Bian Y, Rong Z, Chang TM. Polyhemoglobin-superoxide dismutase-catalase-carbonic anhydrase: a novel biotechnology-based blood substitute that transports both oxygen and carbon dioxide and also acts as an antioxidant. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:127–136.
- Chen G, Duan Y, Liu J, et al. Antioxidant effects of vitamin C on hemoglobin-based oxygen carriers derived from human cord blood. Artif Cells Nanomed Biotechnol. 2016;44:56–61.
