Abstract
Long noncoding RNA (lncRNA) MEG3 has been widely reported to be decreased in a growing list of primary human tumours and play a key role in tumour suppression. However, there are few reports about MEG3 expression and function in oesophagal squamous cell carcinoma (ESCC). Here, we found that MEG3 expression was significantly downregulated in tumour tissues, and its low expression was associated with large tumour size, lymph node metastasis and advanced clinical stage in ESCC patients. Univariate and multivariate analyses revealed low expression of MEG3 as an independent predictor for disease-free survival and overall survival. Cell experiments showed that MEG3 inhibited ESCC cell proliferation, migration and invasion. Subsequently, miR-4261 was identified and confirmed to be the target of MEG3, and MEG3 functions, at least in part, by targeting miR-4261. Additionally, Dickkopf-2 (DKK2), a Wnt/β-catenin signalling inhibitor, was identified to be a target of miR-4261. MEG3 interacted with miR-4261, derepressed DKK2 and blocked the Wnt/β-catenin signalling, thereby inhibiting tumourigenesis and progression in ESCC. In vivo experiments also confirmed this conclusion. Our study for the first time elaborated the critical role of MEG3-miR-4261-DKK2-Wnt/β-catenin signalling axis in ESCC, and MEG3 could represent a novel diagnostic and prognostic biomarker and therapeutic target in ESCC.
Introduction
Oesophagal carcinoma (EC), one of the most fatal types of digestive tract malignancy, poses a grave threat to life and health of mankind. Oesophagal adenocarcinoma (EA) and oesophagal squamous cell carcinoma (ESCC) are the most common histopathological types of EC. However, in China and other East Asia countries, up to 90% of cases are ESCC [Citation1]. Due to no early signs or symptoms in the ESCC, the early diagnosis of ESCC is extremely difficult, whereas advanced ESCC patients, trending to present with dysphagia, have already developed plentiful local invasion or regional lymph node metastasis [Citation2]. Although encouraging progress in multidisciplinary treatments has been achieved in recent years, the prognosis of ESCC patients remains unsatisfactory, with a 5-year survival rate of less than 40% [Citation3–5]. Thus, novel biomarkers involved in ESCC progression are urgently needed for the early diagnosis, prognosis prediction and treatment evaluation for ESCC.
Long non-coding RNAs (lncRNAs) are a kind of evolutionarily conserved non-coding RNAs with more than 200 nucleotides in length and have no protein-coding capacity. Recently, growing evidence points to the critical roles of lncRNAs in tumour initiation, progression and metastasis, including ESCC. For example, lncRNA HOTAIR and NEAT1 promote ESCC progression and correlate with poor prognosis [Citation6,Citation7]; lncRNA MALAT1 promotes ESCC cell proliferation and migration [Citation8]; lncRNA CCAT2 is associated with advanced clinical stage and poor prognosis in ESCC [Citation9]; and lncRNA 91 H promotes the initiation and progression of ESCC by inhibiting IGF2 expression [Citation10]. LncRNA MEG3 as one of first identified lncRNAs is widely reported to be decreased in a growing list of primary tumours and exerts very important role in tumour suppression. However, there are few reports about the expression level of MEG3 in ESCC, and its biological function in ESCC progression.
Here, we determined the expression level of MEG3 in ESCC tissues and analyzed its clinical significance. We also investigated the role of MEG3 during ESCC progression using in vitro and in vivo assays, and the molecular mechanisms whereby MEG3 contributed to the phenotypes of ESCC cells.
Material and methods
Tissue samples and cell lines
A total of 58 ESCC and matched adjacent normal tissues were obtained from patients who underwent surgery resection during 2009–2012 at the First Affiliated Hospital of Zhengzhou University. No patient received preoperative local or systemic therapy. All tissue samples were stored in liquid nitrogen. Permission to use the samples for research purposes was obtained and approved by the First Affiliated Hospital ethics committee. All participants provided written informed consent.
Five ESCC cell lines (EC109, EC9706, KYSE150, KYSE450, and KYSE510) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and a normal oesophagal epithelial cell (Het-1A) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were grown in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen) in a 5% CO2, 37 °C incubator.
Quantitative real-time PCR (qRT-PCR)
RNA was isolated from tissues or cells using TRIzol reagent (Invitrogen) following the manufacturer's protocol and was reversed transcribed to cDNA using M-MLV reverse transcriptase (Invitrogen). The expression of MEG3 mRNA was quantified using SYBR® Premix Ex Taq™ II kit (TaKaRa, China) on a 7500 Real-Time Sequence Detection System (Applied Biosystems, Foster City, CA, USA), and GAPDH was used as a loading control. miR-4261 was extracted using the PureLink™ miRNA Isolation Kit (Invitrogen) and quantified with TaqMan MicroRNA Assay Kit (Applied Biosystems). U6 snRNA was used as a loading control. Fold change in gene expression was calculated by using the 2−ΔΔCt method.
Cell transfection
Plasmid complementary DNA MEG3 cDNA (pcDNA-MEG3) was constructed by introducing MEG3 cDNA sequence into the pcDNA3.1 expression vector (Invitrogen). The miR-4261 mimic, miR-4261 inhibitor, siRNA targeting MEG3 (si-MEG3), si-DKK2, and their respective controls were purchased from GenePharma (Shanghai, China). All transfection reactions were carried out with Lipofectamine 2000 Reagent (Life Technologies, Carlsbad, CA, USA).
Cell proliferation assay
Cells (1.5 × 103 cells/well) were seeded on 96-well plates, and the proliferation of cells was determined every 24 h using Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) following the manufacturer's instructions. Cell proliferation was assessed by measuring the absorbance (450 nm) of reduced WST-8 at the indicated time points.
Migration assay
Cells (1 × 105 cells/well) were plated in 12-well plates and allowed to grow to approximately 90% confluence. Then, similar sized scratches were created on the cell monolayer using a sterile 200-μl plastic pipette tip. The scratched monolayer was rinsed in PBS three times to remove any free-floating cells and debris. Then, FBS-free medium was added, and the culture plates were incubated at 37 °C. Cell migration into the scratch area was observed with inverted microscopy. To visualize migrated cells and scratch healing, images were taken at 0 and 48 h. The migration ability was measured by quantifying the difference between the scratched width of 0 and 48 h.
Invasion assay
Cell invasion ability was determined with Matrigel-coated invasion chamber (8 μm, Costar, Corning, NY, USA). Briefly, 2 × 104 cells were resuspended in FBS-free medium and transferred to the top chamber of the Matrigel-coated insert. Culture medium with 10% FBS was placed in the bottom chamber. After a-24 h incubation at 37 °C, the non-invading cells were wiped from the upper membrane surface with a cotton tip, and the invasive cells that had migrated to the lower membrane surface were fixed with methanol and stained with 0.1% crystal violet. Invaded cells were counted in five randomly selected fields under a light microscope (Zeiss, Germany).
Luciferase reporter assay
To analyze the interaction between MEG3 and miR-4261, two luciferase reporters containing the MEG3 with wild-type (WT) or mutant (MUT) binding sites of miR-4261 were constructed. Due to that MEG3 contained two predicted binding sites of miR-4261, MEG3 mutant sequence was constructed by mutation of the two presumptive binding sites. Cells were cotransfected with the luciferase construct and miR-4261 mimic or mimic control. At 48 h post-transfection, luciferase activity was measured with the dual Luciferase reporter assay system (Promega, Madison, WI, USA).
Similarly, to analyze the interaction between miR-4261 and Dickkopf-2 (DKK2), two luciferase reporters containing the DKK2 3’UTR with WT or MUT binding sites of miR-4261 were constructed, and cells were cotransfected with the luciferase construct and miR-4261 mimic or mimic control. Transfection after 48 h, luciferase activity was determined.
In addition, TOPFlash luciferase assays were performed to evaluate the activity of Wnt/β-catenin signalling. Cells were transfected with TOPFlash or negative control FOPFlash (Biotime Biotechnology, China) together with inhibitor control, miR-4261 inhibitor, si-MEG3 or/and si-DKK2 by Lipofectamine 2000 Reagent. After 48 h transfection, cells were lysed and luciferase activity was measured with the dual Luciferase reporter assay system. The luciferase activity of each sample was normalized to that of Renilla.
Western blot assay
Cellular proteins were extracted from tissues or cultured cells using the Ready Prep Protein Extraction Kit (Bio-Rad, Hercules, CA, USA). Protein concentration was determined using the BCA protein assay kit (Pierce, Rockford, IL, USA) according to the manufacturer’s protocol. After mixing with loading buffer, the protein solutions were separated on 10% SDS-PAGE and transferred to PVDF membranes (BD Biosciences, San Diego, CA, USA). After blocking with 5% nonfat dried milk for 1 h, the membranes were probed with the specific primary antibodies at 4 °C overnight. Then, the membranes were washed with Tween-20/tris-buffered saline (TTBS), followed by incubation with HRP-conjugated secondary antibodies (Boster, Wuhan, China) for 1 h at room temperature. The blots were developed using ECL Western blotting detection kit (Amersham Biosciences, Uppsala, Sweden), and quantified using the Quantity One image-analysis software, v4.6.2 (Bio-Rad). The following primary antibodies were used: anti-DKK2 (Biovision Research, Mountain View, CA, USA), anti-β-catenin (Transduction Laboratories, San Diego, CA, USA), anti-Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-c-myc (Santa Cruz Biotechnology), and anti-β-actin (Santa Cruz Biotechnology).
Tumour xenografts in nude mice
Athymic female BALB/c mice aged 4 weeks were obtained from Slac Laboratory Animal Co. Ltd. (Shanghai, China) and were housed and maintained in laminar airflow chambers under specific pathogen-free (SPF) conditions. KYSE150 cells transfected with pcDNA-control, pcDNA-MEG3 or pcDNA-MEG3 + miR-4261 mimic were injected subcutaneously into a single side of the posterior flank of each mouse (1 × 107 cells/0.1 ml). Tumour size was monitored weekly, and tumour volume was calculated using the formula: length × width2/2. Five weeks after injection, all mice were sacrificed, and the tumours were excised, imaged, weighed and used for western blot detection. The experimental protocols were approved by the Animal Experimentation Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Statistical analysis
The data were expressed as mean ± standard deviation (SD). All of the statistical analyses were performed with SPSS 17.0 software (SPSS, Chicago, IL, USA). Student's t-test, Fisher’s exact test or χ2 test was performed for the comparisons between groups. Survival curves were plotted in accordance with the Kaplan–Meier method and were analyzed using log-rank test. Univariate and multivariate analyses were used according to Cox proportional hazard regression model. p Values less than 0.05 were considered statistically significant.
Results
MEG3 is downregulated in ESCC tissues and cell lines
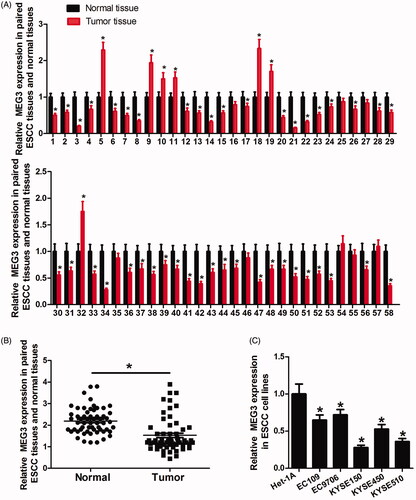
MEG3 expression was determined in 58 pairs of clinic ESCC tissue and matched adjacent normal tissue samples. The results showed that 43 cases (74.1%) had significantly decreased MEG3 expression in tumour tissues compared with normal tissues, whereas only seven cases (12%) upregulated MEG3 expression in ESCC tissues (). In summary, MEG3 was downregulated in ESCC tissues (). Moreover, MEG3 expression in ESCC cell lines was also determined. The results showed that MEG3 expression was obviously reduced in ESCC cells compared with normal oesophagal epithelial cells (Het-1A) ().
Figure 1. Relative MEG3 expression in ESCC tissues and cell lines assessed by qRT-PCR. (A) 43 cases (74.1%) have significantly decreased MEG3 expression in tumour tissues in comparison with normal tissues. (B) Difference in MEG3 expression between tumour tissues and normal tissues. (C) Expression level of MEG3 in ESCC cell lines (EC109, EC9706, KYSE150, KYSE450 and KYSE510) and the normal epithelial cell line Het-1A. *p < .05.
Downregulated MEG3 is associated with tumour progression and poor prognosis of ESCC patients
For the clinicopathological correlation analysis, ESCC patients were divided into a high MEG3 expression group (n = 29) and a low expression group (n = 29) using the median value of MEG3 as the cutoff. As shown in , MEG3 downregulation was related to tumour size, lymph node metastasis and clinical stage but not related to patient’s gender, age, tumour differentiation and location. Next, Kaplan–Meier survival analyses and log-rank tests were carried out to further investigate the correlation between MEG3 expression and the prognosis of ESCC patients. The results showed that the low MEG3 expression group exhibited significantly shorter disease-free survival (DFS) and overall survival (OS) than high expression group (). In addition, Cox regression analyses were performed to further evaluate the prognostic value of MEG3 expression in ESCC patients. Both univariate and multivariate analyses showed that MEG3 expression and lymph node metastasis were significantly correlated with DFS and OS of ESCC patients, suggesting that MEG3 could be an independent prognostic factor for ESCC ( and ).
Figure 2. The correlation between MEG3 expression and the DFS or OS of ESCC patients. Kaplan–Meier analysis of disease-free survival (DFS, A) or overall survival (OS, B) is performed according to MEG3 expression level.
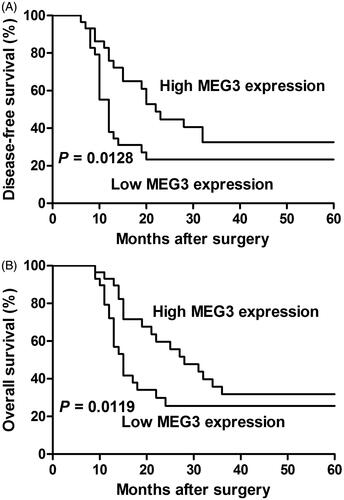
Table 1. Correlation of the expression of MEG3 with clinicopathologic features in ESCC patients.
Table 2. Univariate and multivariate Cox regression analyses of disease-free survival in ESCC patients.
Table 3. Univariate and multivariate cox regression analyses of overall survival in ESCC patients.
MEG3 inhibits ESCC cell proliferation, migration and invasion in vitro
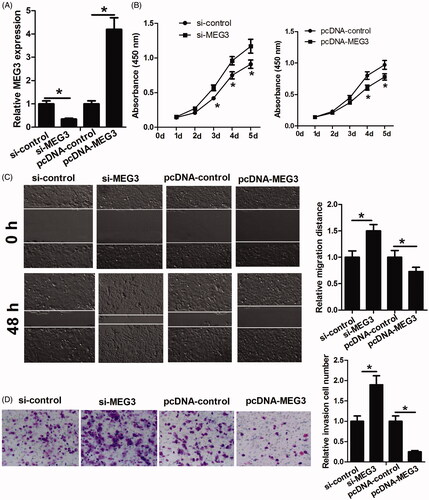
To assess the role of MEG3 in ESCC progression, KYSE150 cells were transfected with si-control, si-MEG3, pcDNA-control or pcDNA-MEG3, and then were used to perform qRT-PCR, CKK-8, migration and invasion assays. The results of qRT-PCR assay showed that si-MEG3 transfection significantly reduced MEG3 level compared with si-control transfection, and pcDNA-MEG3 transfection significantly increased MEG3 level compared with pcDNA-control transfection, which confirmed that these transfection reactions were successful (). CKK-8 assay showed that MEG3 downregulation markedly promoted KYSE150 cell proliferation while MEG3 upregulation inhibited KYSE150 cell proliferation (). Migration and invasion assays showed that the decrease of MEG3 significantly improved the migratory and invasive abilities of KYSE150 cells while the increase of MEG3 obviously inhibited KYSE150 cell migration and invasion (). Taken together, these data suggested that MEG3 inhibits KYSE150 cell proliferation, migration and invasion.
Figure 3. The functional analysis of MEG3 in ESCC cells. (A) MEG3 level is detected in KYSE150 cells after transfection with si-control, si-MEG3, pcDNA-control or pcDNA-MEG3 by RT-qPCR. (B) CCK-8 assay is performed to determine the proliferation of KYSE150 cells transfected with si-control, si-MEG3, pcDNA-control or pcDNA-MEG3. (C and D) Scratch healing assay and transwell assay are performed to determine the migration and invasion abilities of KYSE150 cells transfected with si-control, si-MEG3, pcDNA-control or pcDNA-MEG3. *p < .05.
miR-4261 is a target of MEG3
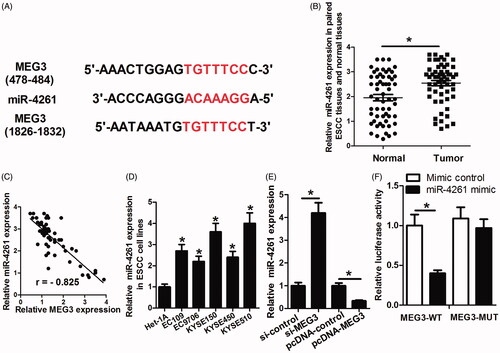
Bioinformatics analysis of miRNA recognition sequences on MEG3 by the online software miRDB showed the presence of two conserved binding sites for miR-4261 (). miR-4261 expression level in ESCC tissues was determined. The results showed that miR-4261 expression level was significantly increased in ESCC tissues in comparison with normal tissues and was negatively correlated with MEG3 expression in ESCC tissues (). miR-4261 level in ESCC cell lines was also determined. As shown in , miR-4261 level was significantly increased in ESCC cell lines compared with Het-1A cells. The decrease of MEG3 enhanced miR-4261 expression, and the increase of MEG3 reduced miR-4261 expression in KYSE150 cells (). Furthermore, we performed luciferase reporter assay and found that the luciferase activity of wild-type reporter was obviously suppressed when transfection with miR-4261 mimic compared to mimic control (). Collectively, these data indicated that miR-4261 is a target of MEG3, and MEG3 can directly regulate miR-4261 expression.
Figure 4. miR-4261 is a target of MEG3. (A) The putative miR-4261 binding sites in the MEG3 sequence. (B) Difference in miR-4261 expression between tumour tissues and normal tissues. (C) miR-4261 is negatively correlated with MEG3 expression in ESCC tissues. (D) Expression level of miR-4261 in ESCC cell lines and Het-1A. (E) The effects of MEG3 decrease or increase on the miR-4261 expression. (F) Luciferase reporter assay shows that in the miR-4261 mimic group, the luciferase activity driven by MEG3-WT is significantly reduced in comparison with that in the mimic control, while the reduced luciferase activity by miR-4261 is abolished through mutation of the presumptive binding sites in MEG3. *p < .05.
miR-4261 promotes ESCC cell proliferation, migration and invasion in vitro
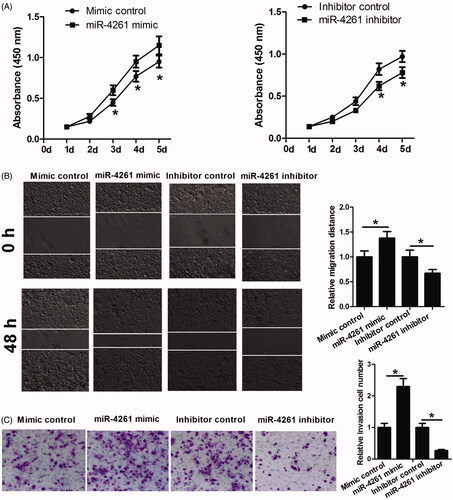
To further investigate whether MEG3 functions by targeting miR-4261, KYSE150 cells were transfected with miR-4261 mimic, miR-4261 inhibitor or their respective controls, and then were used to perform cell proliferation, migration and invasion assays. As shown in , the increase of miR-4261 promoted cell proliferation, migration and invasion while the decrease of miR-4261 had opposite effects. Collectively, these data indicated that MEG3 exerts anti-tumour effect on ESCC, at least in part, by targeting miR-4261.
Figure 5. The functional analysis of miR-4261 in ESCC cells. (A) CCK-8 assay is performed to determine the proliferation of KYSE150 cells transfected with miR-4261 mimic, miR-4261 inhibitor or their respective controls. (B and C) Scratch healing assay and transwell assay are performed to determine the migration and invasion abilities of KYSE150 cells transfected with miR-4261 mimic, miR-4261 inhibitor or their respective controls. *p < .05.
MEG3 inhibits growth of ESCC tumours and wnt/β-catenin signalling in vivo
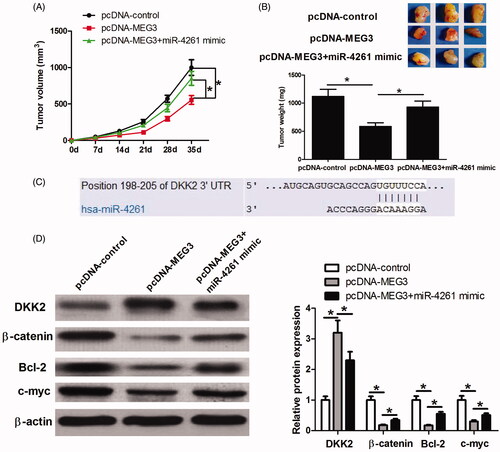
To further confirm that MEG3 exerts anti-tumour effects by targeting miR-4261, KYSE150 cells were transfected with pcDNA-control, pcDNA-MEG3 or pcDNA-MEG3 + miR-4261 mimic, and then were inoculated into nude mice. As shown in , tumour volume and weight in the pcDNA-MEG3 group were significantly less compared with those in the pcDNA-control group while the tumour volume and weight in the pcDNA-MEG3 + miR-4261 mimic group were significantly larger than those in the pcDNA-MEG3 group. These results further confirmed that MEG3 plays an anti-cancer role in ESCC by targeting miR-4261. In addition, we found that DKK2, a Wnt/β-catenin signalling inhibitor, was a potential target of miR-4261 by performing TargetScan database prediction (). This finding tempted us to speculate that MEG3 may function by sponging miR-4261, derepressing DKK2 and blocking the Wnt/β-catenin signalling. Thus, we took some tumour tissues for detecting DKK2, and Wnt/β-catenin signalling-related proteins β-catenin, Bcl-2 and c-myc expression via western blot assay. The results showed that the pcDNA-MEG3 treatment significantly increased DKK2 expression, and obviously decreased β-catenin, Bcl-2, and c-myc expression compared with the pcDNA-control treatment while the combined treatment of pcDNA-MEG3 and miR-4261 mimic significantly relieved the effects of pcDNA-MEG3 treatment on DKK2, β-catenin, Bcl-2, and c-myc expression (). These results indicated that DKK2 and Wnt/β-catenin signalling might be involved in the anti-cancer activity of MEG3-miR-4261 axis.
Figure 6. Effects of MEG3 and miR-4261 expression on tumour growth, DKK2 expression and the Wnt/β-catenin signaling in vivo. (A) The tumour volume is calculated once every 7 days after injection of KYSE150 cells stably transfected with pcDNA-control, pcDNA-MEG3, or pcDNA-MEG3 + miR-4261 mimic. (B) Mice are sacrificed, and the tumours are isolated, imaged and weighted after five weeks. (C) The putative miR-4261 binding sites in the DKK2 3’UTR sequence. (D) Western blot assay is performed to determine DKK2, β-catenin, Bcl-2, and c-myc expression in the tumours. *p < .05.
MEG3-miR-4261 axis regulates DKK2 and wnt/β-catenin signalling in vitro
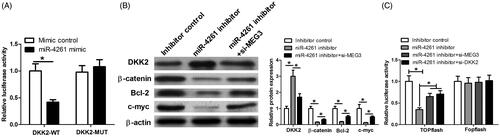
To further confirm that MEG3 functions by sponging miR-4261, derepressing DKK2 and blocking the Wnt/β-catenin signalling, the luciferase reporter assay was performed in KYSE150 cells to analyze the interaction between miR-4261 and DKK2. The results showed that miR-4261 mimic transfection significantly reduced the luciferase activity of reporter containing DKK2-WT compared with mimic control transfection, which confirmed the direct binding between miR-4261 and DKK2 (). In addition, we detected the effects of MEG3 and miR-4261 on DKK2, β-catenin, Bcl-2 and c-myc expression. We transfected KYSE150 cells with inhibitor control, miR-4261 inhibitor or miR-4261 inhibitor + si-MEG3. As shown in , the decrease of miR-4261 enhanced DKK2 expression, and reduced β-catenin, Bcl-2 and c-myc expression while MEG3 knockdown relieved the effects caused by the decrease of miR-4261. We also performed TOPflash assay to investigate the effect of MEG3-miR-4261-DKK2 axis on Wnt/β-catenin signalling. As shown in , the decrease of miR-4261 significantly inhibited the luciferase activity of Wnt/β-catenin signalling while the effect was relieved by MEG3 or DKK2 knockdown. Collectively, these data suggested that MEG3 inhibits tumourigenesis and progression in ESCC by sponging miR-4261, derepressing DKK2 and blocking the Wnt/β-catenin signalling.
Figure 7. The effect of MEG3-miR-4261 axis on DKK2 expression and Wnt/β-catenin signaling in vitro. (A) Luciferase reporter assay shows that miR-4261 mimic transfection significantly reduces the luciferase activity of reporter containing DKK2-WT compared with mimic control. (B) Western blot assay is performed to determine DKK2, β-catenin, Bcl-2, and c-myc expression in KYSE150 cells transfected with inhibitor control, miR-4261 inhibitor, or miR-4261 inhibitor + si-MEG3. (C) TOPflash assay was performed to evaluate the effect of MEG3, miR-4261 and DKK2 on Wnt/β-catenin signalling activity in KYSE150 cells.*p < .05.
Discussion
MEG3 has been found to be a tumour suppressor, decreased in various types of human tumours and correlated with tumour progression and prognosis. For example, downregulated MEG3 is related to poor prognosis in hepatocellular carcinoma and osteosarcoma [Citation11,Citation12]; downregulated MEG3 promotes autophagy and cell proliferation in bladder cancer [Citation13]; MEG3 inhibits cell growth and promotes apoptosis in lung cancer, prostate cancer and cervical cancer [Citation14–16]; and the downregulation of MEG3 caused by hypermethylation of the MEG3 promoter promotes cell proliferation in cervical cancer [Citation17]. However, less is known about MEG3 in ESCC.
In this study, the expression level of MEG3 in ESCC tissues was determined, and the clinical significance of MEG3 expression was analyzed. Results showed that MEG3 was downregulated in ESCC tissues, and a lower level of MEG3 was related to tumour size, lymph node metastasis, clinical stage and poor prognosis of ESCC patients. In vitro and in vivo experiments confirmed the inhibiting effects of MEG3 on ESCC cell proliferation, migration and invasion. These findings suggested MEG3 as a novel tumour suppressor in human ESCC.
LncRNAs and microRNAs (miRNAs, usually 18–25 nucleotides in length) are two classes of important non-coding RNAs. Extensive documentation has confirmed that many miRNAs regulate tumour initiation, progression and metastasis by regulating gene expression via translation inhibition, mRNA degradation or both. For example, miR-301a promotes pancreatic cancer cell proliferation by directly targeting bim; miR-17–92 cluster modulates cell proliferation and collagen synthesis by targeting TGFB pathway in mouse palatal mesenchymal cells; miR-506 inhibits cervical cancer progression by targeting Gli3; and the decrease of miR-125b-1 facilitates the progression of head and neck cancer via dysregulation of TACSTD2 and MAPK pathway [Citation18–21]. For lncRNAs, although many lncRNAs are dysregulated and exert crucial function in various cancers, most of them have not yet been studied in mechanistic details. Recently, growing evidence indicates that lncRNAs function by interacting with the miRNAs. For example, lncRNA CCAT1 contributes to gallbladder cancer development through reduction of miR-218-5p expression; lncRNA HOTAIR exerts its oncogenic activity via negative modulation of miRNA-130a in gallbladder cancer; lncRNA UCA1 promotes hepatocellular carcinoma progression via suppression of miR-216b and activation of FGFR1/ERK signalling; lncRNA HOST2 promotes epithelial ovarian cancer cell proliferation, migration and invasion by inhibiting microRNA let-7b; lncRNA ATB modulates cell biological behaviours in osteosarcoma by targeting miR-200s; and MALAT1 facilitates lung adenocarcinoma progression by suppressing miR-204 [Citation22–27]. These findings prompted us to determine whether MEG3 functions by interacting with miRNAs and derepression of microRNA targets. We performed bioinformatics analysis of miRNA recognition sequences on MEG3 by miRDB and identified miR-4261 that can bind to complementary sequences in MEG3. miR-4261, a newly identified miRNA, has recently been reported to be overexpressed in colorectal cancer and promote colorectal cancer cell proliferation, G1/S phase transition of cell cycle and migration [Citation28]. However, the involvement of miR-4261 in ESCC has not been reported. We first detected miR-4261 expression in ESCC tissues and cell lines and found that miR-4261 expression was markedly upregulated in ESCC tissues and cell lines and was negatively correlated with MEG3 expression in ESCC tissues. MEG3 could also regulate miR-4261 expression in ESCC cells. Luciferase reporter assay further confirmed that MEG3 can directly target miR-4261 in ESCC cells. Function assays showed that miR-4261 promoted ESCC cell proliferation, migration and invasion. Taken together, these data indicated that MEG3 functions, at least in part, by sponging miR-4261. Animal experiments also confirmed this conclusion. Recently, Dong et al. [Citation29] reported that MEG3 regulates YES2 cell (one ESCC cell line) proliferation and invasion by sponging miR-9. Here, we determined miR-9 expression in KYSE150 cells and found that there was no significant difference between the expression of miR-9 in Het-1A cells and in KYSE150 cells (data not shown), why miR-9 was not chosen for further study.
Wnt/β-catenin signalling is persistently activated in multiple types of cancer, including ESCC, and regulates a broad range of cellular processes, such as proliferation, invasion, migration, apoptosis, differentiation and other signalling pathways [Citation30–32]. Blockade of the Wnt/β-catenin pathway is known to be a potential therapeutic option for the treatment of various types of cancer. DKKs are a family of secreted proteins that comprise four members (DKK1, 2, 3 and 4), which act as inhibitors of Wnt/β-catenin signalling [Citation33–35]. Among the DKK family, DKK2 is a putative Wnt/β-catenin signalling inhibitor and downregulated in multiple types of cancer, including ovarian cancer, renal cancer, colorectal cancer, glioma and oesophagal adenocarcinoma [Citation34,Citation36–39]. Recently, some miRNAs have been reported to activate the Wnt/β-catenin signalling and promote the initiation and progression of cancer by targeting DKK2. For example, miR-221 enhances chemoresistance of oesophagal adenocarcinoma by targeting DKK2 and activating the Wnt/β-catenin signalling; miR-21 promotes oral cancer invasion via the Wnt/β-Catenin Pathway by targeting DKK2; and miR-222 promotes tumourigenesis via targeting DKK2 and activating the Wnt/β-catenin signalling in glioma [Citation38–40]. In this study, we found that DKK2 was a target of miR-4261, and the Wnt/β-catenin signalling could be regulated by MEG3-miR-4261 axis in vitro and in vivo. These data indicated that MEG3 sponges miR-4261, derepresses DKK2 and blocks the Wnt/β-catenin signalling, thereby inhibiting tumourigenesis and progression in ESCC.
In summary, our study first suggested that lncRNA MEG3 was downregulated in ESCC tissues and associated with tumour progression and poor prognosis. Moreover, in vitro and in vivo experiments suggested the inhibitory effects of MEG3 on cell proliferation, migration and invasion by sponging miR-4261, upregulating DKK2 and blocking the Wnt/β-catenin signalling. As a result, MEG3 could become a novel promising candidate for the diagnosis, prognosis prediction and therapy for ESCC in the future.
Disclosure statement
The authors declare that they have no conflicts of interest with the contents of this article.
References
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252.
- Miyazaki T, Kato H, Fukuchi M, et al. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer. 2003;103:657–663.
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–5067.
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg. 2010;251:46–50.
- Toh Y, Egashira A, Yamamoto M. Epigenetic alterations and their clinical implications in esophageal squamous cell carcinoma. Gen Thorac Cardiovasc Surg. 2013;61:262–269.
- Chen FJ, Sun M, Li SQ, et al. Upregulation of the long non-coding rna hotair promotes esophageal squamous cell carcinoma metastasis and poor prognosis. Mol Carcinog. 2013;52:908–915.
- Chen X, Kong J, Ma Z, et al. Up regulation of the long non-coding RNA NEAT1 promotes esophageal squamous cell carcinoma cell progression and correlates with poor prognosis. Am J Cancer Res. 2015;5:2808.
- Ren K, Li Y, Lu H, et al. Long noncoding RNA HOTAIR controls cell cycle by functioning as a competing endogenous RNA in esophageal squamous cell carcinoma. Transl Oncol. 2016;9:489–497.
- Zhang X, Xu Y, He C, et al. Elevated expression of CCAT2 is associated with poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 2015;111:834–839.
- Gao T, He B, Pan Y, et al. Long non-coding RNA 91H contributes to the occurrence and progression of esophageal squamous cell carcinoma by inhibiting IGF2 expression. Mol Carcinog. 2015;54:359–367.
- Zhuo H, Tang J, Lin Z, et al. The aberrant expression of MEG3 regulated by UHRF1 predicts the prognosis of hepatocellular carcinoma. Mol Carcinog. 2016;55:209–219.
- Tian Z-Z, Guo X-J, Zhao Y-M, et al. Decreased expression of long non-coding RNA MEG3 acts as a potential predictor biomarker in progression and poor prognosis of osteosarcoma. Int J Clin Exp Pathol. 2015;8:15138.
- Ying L, Huang Y, Chen H, et al. Downregulated MEG3 activates autophagy and increases cell proliferation in bladder cancer. Mol Biosyst. 2013;9:407–411.
- Lu K-h, Li W, Liu X-h, et al. Long non-coding RNA MEG3 inhibits NSCLC cells proliferation and induces apoptosis by affecting p53 expression. BMC Cancer. 2013;13:461
- Luo G, Wang M, Wu X, et al. Long non-coding RNA MEG3 inhibits cell proliferation and induces apoptosis in prostate cancer. Cell Physiol Biochem. 2015;37:2209–2220.
- Zhang J, Yao T, Wang Y, et al. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol Ther. 2016;17:104–113.
- Zhang J, Lin Z, Gao Y, et al. Downregulation of long noncoding RNA MEG3 is associated with poor prognosis and promoter hypermethylation in cervical cancer. J Exp Clin Cancer Res. 2017;36:5.
- Wen S, Lin Y, Yu Y, et al. miR-506 acts as a tumor suppressor by directly targeting the hedgehog pathway transcription factor Gli3 in human cervical cancer. Oncogene. 2015;34:717–725.
- Nakanishi H, Taccioli C, Palatini J, et al. Loss of miR-125b-1 contributes to head and neck cancer development by dysregulating TACSTD2 and MAPK pathway. Oncogene. 2014;33:702–712.
- Chen Z, Chen L, Dai H, et al. miR-301a promotes pancreatic cancer cell proliferation by directly inhibiting Bim expression. J Cell Biochem. 2012;113:3229–3235.
- Li L, Shi J, Zhu G, et al. MiR-17-92 cluster regulates cell proliferation and collagen synthesis by targeting TGFB pathway in mouse palatal mesenchymal cells. J Cell Biochem. 2012;113:1235–1244.
- Ma M, Chu B, Zhang Y, et al. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015;6:e1583.
- Ma M-z, Li C-x, Zhang Y, et al. Long non-coding RNA HOTAIR, a c-Myc activated driver of malignancy, negatively regulates miRNA-130a in gallbladder cancer. Mol Cancer. 2014;13:156.
- Wang F, Ying H-Q, He B-S, et al. Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Oncotarget. 2015;6:7899.
- Gao Y, Meng H, Liu S, et al. LncRNA-HOST2 regulates cell biological behaviors in epithelial ovarian cancer through a mechanism involving microRNA let-7b. Hum Mol Genet. 2015;24:841–852.
- Han F, Wang C, Wang Y, et al. Long noncoding RNA ATB promotes osteosarcoma cell proliferation, migration and invasion by suppressing miR-200s. Am J Cancer Res. 2017;7:770.
- Li J, Wang J, Chen Y, et al. LncRNA MALAT1 exerts oncogenic functions in lung adenocarcinoma by targeting miR-204. Am J Cancer Res. 2016;6:1099.
- Jiao G, Huang Q, Hu M, et al. Therapeutic suppression of miR-4261 attenuates colorectal cancer by targeting MCC. Mol Ther Nucl Acids. 2017;8:36–45.
- Dong Z, Zhang A, Liu S, et al. Aberrant methylation-mediated silencing of lncRNA MEG3 functions as a ceRNA in esophageal cancer. Mol Cancer Res. 2017;15:800–810.
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480.
- MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26.
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205.
- Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179–183.
- Zhu J, Zhang S, Gu L, et al. Epigenetic silencing of DKK2 and Wnt signal pathway components in human ovarian carcinoma. Carcinogenesis. 2012;33:2334–2343.
- Krupnik VE, Sharp JD, Jiang C, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313.
- Hirata H, Hinoda Y, Nakajima K, et al. Wnt antagonist gene DKK2 is epigenetically silenced and inhibits renal cancer progression through apoptotic and cell cycle pathways. Clin Cancer Res. 2009;15:5678–5687.
- Silva A-L, Dawson SN, Arends MJ, et al. Boosting Wnt activity during colorectal cancer progression through selective hypermethylation of Wnt signaling antagonists. BMC Cancer. 2014;14:891.
- Li Q, Shen K, Zhao Y, et al. MicroRNA-222 promotes tumorigenesis via targeting DKK2 and activating the Wnt/β-catenin signaling pathway. FEBS Lett. 2013;587:1742–1748.
- Wang Y, Zhao Y, Herbst A, et al. miR-221 mediates chemoresistance of esophageal adenocarcinoma by direct targeting of DKK2 expression. Ann Surg. 2016;264:804–814.
- Kawakita A, Yanamoto S, Yamada S-i, et al. MicroRNA-21 promotes oral cancer invasion via the Wnt/β-catenin pathway by targeting DKK2. Pathol Oncol Res. 2014;20:253–261.
