Abstract
A dual-layer biomimetic cartilage scaffold was prepared by mimicking the structural design, chemical cues and mechanical characteristics of mature articular cartilage. The surface layer was made from collagen (COL), chitosan (CS) and hyaluronic acid sodium (HAS). The transitional layer with microtubule array structure was prepared with COL, CS and silk fibroin (SF). The PLAG microspheres containing kartogenin (KGN) and the polylysine-heparin sodium nanoparticles containing TGF-β1 (TPHNs) were constructed for the surface, transitional layer, respectively. The SEM result showed that the dual-layer composite scaffold had a double structure similar to natural cartilage. The vitro biocompatibility experiment showed that the biomimetic cartilage scaffold with orientated porous structure was more conducive to the proliferation and adhesion of BMSCs. A rabbit KOA cartilage defect model was established and biomimetic cartilage scaffolds were implanted in the defect area. Compared with the surface layer and transitional layer scaffolds group, the results of dual-layer biomimetic cartilage scaffold group showed that the defects had been completely filled, the boundary between new cartilage and surrounding tissue was difficult to identify, and the morphology of cells in repair tissue was almost in accordance with the normal cartilage after 16 weeks. All those results indicated that the biomimetic cartilage scaffold could effectively repair the defect of KOA, which is related to the fact that the scaffold could guide the morphology, orientation, and proliferation and differentiation of BMSCs. This work could potentially lead to the development of multilayer scaffolds mimicking the zonal organization of articular cartilage.
Introduction
Cartilage defects were thought to be a challenge for tissue engineering, due to its avascular character and the fact that only one cell type (chondrocytes) is present [Citation1,Citation2]. Mature cartilage is composed of different zones or layers, which vary in extracellular matrix (ECM) components and the orientation of the constituents [Citation3,Citation4]. Each zone is maintained by a unique combination of cellular, biomolecular, mechanical, and physical factors. Enzymatic degradation of extracellular matrix, deficient new matrix formation, cell death and hypertrophic differentiation of cartilage cells would lead to arthritis Knee osteoarthritis (KOA) [Citation5,Citation6]. Hence, the treatment of KOA is still a critical and urgent problem in orthopaedic treatment worldwide. Current therapies such as autograft transfer or autologous chondrocyte transplantation rarely restore the tissue to its normal state [Citation7]. Fortunately, cartilage tissue engineering provides a promising alternative strategy [Citation8]. There is a need for engineered scaffolds that recreate the zonal organization of articular cartilage for treating full-thickness articular cartilage defects.
Natural or synthetic scaffolds [Citation9–12] have been shown to support the development of cartilage tissue, but due to their simplified structure, their orientation of the constituents and mechanical behaviour is different from that of mature hyaline cartilage [Citation13,Citation14]. Stratified scaffolds which mimic the bone cartilage not only emulated the graded mechanical properties of articular cartilage but also had excellent biocompatibility, biodegradability and cell affinity [Citation15]. Some kinds of natural materials and growth factors, such as collagen (COL), chitosan (CS), Kartogenin (KGN) and transforming growth factor-β (TGF-β), have been widely used in cartilage tissue engineering [Citation16].
COL is the major constituent of the ECM components in natural articular cartilage [Citation17]. In the outermost superficial zone collagen fibrils are oriented parallel to the articulating surface and in the middle and calcified zones, the collagen fibrils are random and perpendicular. COL has been widely used in cartilage tissue engineering due to its excellent biocompatibility, negligible immunogenicity, cell adhesion and biodegradability [Citation18,Citation19]. COL can also specifically interact with growth factors which promote cell ingrowth and remodelling [Citation20,Citation21]. However, COL use is commonly limited by its insufficient mechanical property and high release rate [Citation22–24]. Chitosan (CS) is a kind of natural and good quality polysaccharide similar to glycosaminoglycan (GAG) in structure, which has been reported to enhance cell adhesion, mesenchymal stromal cells (MSCs) proliferation and functional expression of osteoblasts [Citation16, Citation25]. Some recent studies suggested that the degradation of hyaluronic acid sodium (HAS) naturally contained in the articular structures is related to inflammatory factors, enzymes, immune cells and oxidants present in the KOA articulation [Citation26]. Intra-articular injection of HAS is current therapy for the treatment of osteoarthrosis [Citation27]. It is known that HA downregulates inflammatory factors and restores the rheological properties of the synovial fluid [Citation28]. As a natural biomaterial, SF has caught much attention in cartilage tissue engineering because of its desirable biocompatibility, immunogenicity, controllable biodegradability and high mechanical strength [Citation29]. However, it is reported that SF has many benefits for enhancing the properties of bioactive molecules in vitro and in vivo [Citation30,Citation31].
Polymer microspheres and nanoparticles have been widely used to encapsulate protein or growth factor to preserve their activity and delivery, such as PLGA microspheres and polylysine-heparin sodium (PLL-HS) nanoparticles have been combined with scaffolds in order to achieve controlled release [Citation32,Citation33]. Growth factors encapsulated with polymer microspheres have been demonstrated effective in rendering excellent local promotion of cell adhesion, proliferation and growth [Citation34,Citation35]. Kartogenin (KGN) as a non-protein small molecule chondrogenesis inducing agent was first reported by Johnson et al. in 2012 [Citation6]. Several studies of KGN on cartilage have been reported and the results showed that KGN can promote the healing of cartilage defect [Citation36]. Zonal organization of articular cartilage during development is formed by controlling the secretion and spatial distribution of transforming growth factor-β1 (TGF-β1). The outermost superficial zone and the middle zone are maintained by TGF-β1 signalling.
We here report a dual-layer biomimetic cartilage scaffold that mimics the distinct collagen orientation and ECM components of mature articular cartilage. The characterizations of morphology and mechanical property of the scaffold showed that the new scaffold had a structure similar to natural cartilage and possessed high mechanical strength. The chondroinductivity and cartilage repair ability of the dual-layer biomimetic cartilage scaffold were further studied in vitro and in vivo.
Materials and methods
Materials
Bovine collagen was supplied by Bote Biotech, Co., Ltd (Fuzhou, China). Bovine serum albumin (96%), CS (95% degree of deacetylation, 100–200 mPa.s viscosity), poly (vinyl alcohol) (PVA) 1788 low-viscosity, and HAS (95%, Mw 403.31) were purchased from the Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). PLGA (lactide to glycolide ratio of 75:25, Mw 38–54 kDa) and KGN (98%) were obtained from the Aladdin Industrial Corporation (Shanghai, China). SF was extracted from Bombyx mori with the method described by Zhang et al [Citation37]. Human TGF-beta1 was purchased from the PeproTech (Suzhou, China). CCK-8 kit was purchased from Best Bio (Shanghai, China). Genipin (≥98%) was purchased from Xi’an Kai Lai Biological Engineering Co., Ltd (Xi’an, China). M5 BCA protein assay kit was purchased from Mei5 Biotechnology, Co., Ltd (Beijing, China). All other chemical reagents were of analytical grade and obtained from commercial sources.
Extraction of silk fibroin (SF)
SF was prepared by a previous method [Citation38]. In brief, silkworm cocoons were boiled in a 0.5% Na2CO3 solution (wt) for 30 min. Then it was washed thoroughly with distilled water to remove the glue-like sericine proteins and wax. The degummed silk fiber collected above was dissolved in a CaCl2/H2O/CH3CH2OH solution (molar ratio of 1:8:2) at 80 °C for 1 h. The resulting mixture was dialyzed in distilled water with a cellulose membrane tube (molecular weight cutoff: 814 kDa) for 3 days. The concentration of the formed aqueous solution (1.343%, wt) was determined via measuring the dry weight of the resulting solution.
Fabrication of the surface layer scaffolds
The surface layer was made from COL, CS and HAS. The PLAG microspheres (MPs) containing KGN were loaded in the surface layer. The surface layer scaffold was prepared via freeze-drying method. We prepared five proportions of scaffolds (COL/CS/HAS/MPs, 0.5COL/CS/HAS/MPs, 0.1COL/CS/HAS/MPs, COL/CS/0.5HAS/MPs and COL/CS/0.1HAS/MPs). PLGA MPs were prepared using double emulsion-solvent evaporation method. The preparation of the surface layer scaffold has been described in detail in the previous paper [Citation38].
Fabrication of the transitional layer scaffold
The orientated microtubules scaffold consists of COL, CS, SF and polylysine-heparin sodium nanoparticles containing TGF-β1. We prepared four proportions of scaffolds (COL/CS/TPHNs, COL/CS/0.5SF/TPHNs, COL/CS/SF/TPHNs and COL/CS/3SF/TPHNs). The transitional layer scaffolds were fabricated using a modification of the unidirectional freeze-drying technique. The preparation of the surface layer scaffold has been described in detail in the previous paper [Citation39].
Analysis of the optimal ratio of the surface layer and the transitional layer scaffolds
A scaffold composed of different COL/CS/HAS salt and COL/CS/SF ratios were evaluated by determining porosity, swelling, loss rate in hot water, mechanical property and cell proliferation to obtain optimum conditions for manufacturing porous scaffolds. Please refer to our previous reports on specific experimental methods [Citation38,Citation39].
Fabrication of the dual-layer scaffold
The prepared COL/CS/0.1HAS/MPs scaffold was compressed and cut to obtain a 4 mm diameter surface layer scaffold. The prepared transition layer COL/CS/0.5SF/TPHNs scaffold adhered to the surface layer COL/CS/0.1HAS/MPs scaffold with a small amount of 1% SF. The dual-layer scaffold was dried in the ultra-clean platform.
Microstructure characterization
The morphology of the dual-layer scaffold was characterized using SEM (Philips-FEI XL30 ESEM-TMP, Eindhoven, the Netherlands). The samples were frozen in liquid nitrogen for 2 min and fractured into sections using a sharp blade. Then, the samples sprayed with gold were characterized using SEM.
Mechanical properties of the dual-layer scaffold
The mechanical property of the dual-layer scaffold was examined by universal material testing machine (Instron, Britain) at a cross-head speed of 1 mm/min. During the test, the samples were all compressed to 70% strain. The compressive strength and compressive elastic modulus were obtained from stress–strain data. Each measurement was repeated three times.
Isolation and culture of BMSCs
BMSCs were extracted from the bone marrow of a 4-week-old male SD rat. All the procedures were approved by Medical University of Fujian Institutional Animal Care and Use Committee. Briefly, the tibia and femur were removed and repeatedly flushed by a syringe containing 4 ml DMEM/F-12 with FBS (10%), penicillin (1%, 100 U/ml)-streptomycin (100 mg/ml) under aseptic environment. After that, BMSCs were placed in an incubator with 5% (v/v) carbon dioxide (CO2) at 37 °C. The medium was changed every 2 days, and the passage 3–4 cells were used for follow-up experiments.
Cell proliferation assessment
MTT assay was applied in this study to quantitatively assess the number of viable cells attached and grew on the dual-layer scaffold. The scaffold was sterilized by gamma rays, and the BMSCs (1 × 104 cells/mL) were co-cultured with scaffolds in 24-well plates. Then, MTT solution (5 mg/ml in PBS) was added to each well at different culture time points (1, 3, 5, 7 days) and incubated at 37 °C with 5% (v/v) CO2 for 4 h. Then, the DMSO (200 μL) was added to the top of each well to dissolve the formazan crystals. The absorbance of liquid in each well was measured at 490 nm using an enzyme-linked immunosorbent assay reader (DNM-9602, Beijing Perlong New Technology Co., Ltd., China).
Cell viability assessment
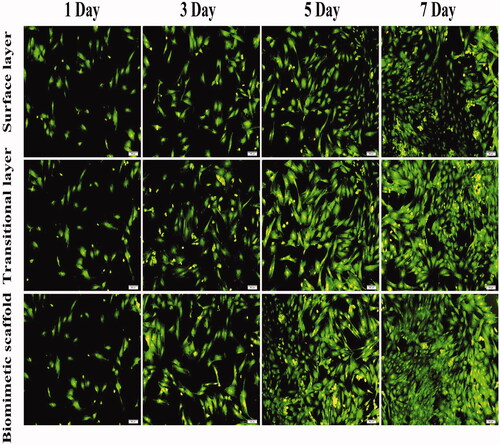
The viability of BMSCs cultured with the scaffold was evaluated by the AO/EB double staining. BMSCs suspension (1 × 104) cells/ml were cultured in a mixture of culture medium and half of leach liquor of scaffolds in 24-well plates and then respectively incubated for 3, 5 and 7 days. Medium was changed every 2 days. A mixture of AO (100 μg/ml) and EB (100 μg/ml) was then added under dark environment for 10 min staining, followed by washing with PBS. Finally, the pictures of strained cells were observed under an inverted fluorescence microscope (Olympus, IX71, Tokyo, Japan).
In vivo animal experiments
Establishment of animal model
All animal studies were obtained at Medical University of Fujian Institutional Animal Care and Use Committee (Fuzhou, China). All animal surgery protocols were approved by Medical University of Fujian Institutional Animal Care and Use Committee.
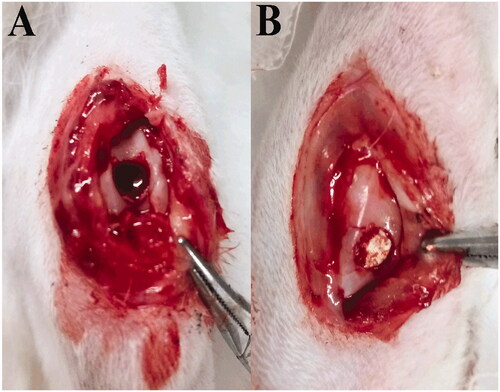
A total 16 male New Zealand white rabbits aged 16 weeks were used and randomly divided into four groups (the control group without any implants, the group containing the surface layer scaffolds, the group containing the transitional layer scaffolds, the group containing the dual-layer scaffolds). The anterior cruciate ligament of the knee joint of the rabbit was first cut off, and the KOA model was obtained after feeding for 1 month. One month later, the epidermis and muscle tissue of the knee joint were cut lengthwise in a sterile environment, and the surface of the knee joint was drilled with manual corneal trephine, and the animal model of KOA cartilage defect was obtained. In brief, the knee joint of rabbits were exposed through operation under general anaesthesia (). A full-thickness cylindrical defect (4 mm in diameter and 5 mm deep) was created by a trephine bur in the middle of patellar groove of the rabbit’s knees. The cartilage samples were harvested at 4, 8, 12, 16 weeks post-operation.
Histological analysis
The obtained articular cartilage samples were fixed with 4% paraformaldehyde solution for 7 days and then decalcified in 10% ethylenedi-aminetetraacetic acid solution for 2 months while embedded in paraffin. The samples were cut into 4-μm-thick section and stained with hematoxylin and eosin (H&E), alcian blue (A-B), toluidine blue (T-B) and safranin-o fast green (S-F). The biopsies were observed and imaged using a light microscope.
For evaluating the repair effect of cartilage tissue, histological sections were scored according to a modified histological grading score [Citation40]. The total scores range from 0 to 16, with 16 points referring to normal tissue. The samples were blindly scored by five independent observers according to the assess scale.
Statistical analysis
Results have been expressed as means and standard deviations. Statistical analysis was performed using one-way analysis of variance (ANOVA). The level of significance was defined at p < .05, and p > .05 was considered as statistically non-significant.
Results
Fabrication and characterization of the surface layer scaffold
Scaffolds composed of different COL/CS/HAS salt were evaluated by physical characterization and in vitro cell experiment. Previous research has shown that the optimal ratio of COL/CS/HAS salt porous scaffold was 1:1:0.1. The COL/CS/0.1HAS scaffold has suitable porosity (50%), swelling (650%), loss rate in hot water (12%) and mechanical properties (0.32 MPa). Results of in vitro fluorescence staining and cell proliferation suggested that COL/CS/0.1HAS/MPs had good biocompatibility and the capability to promote bone marrow stromal cell proliferation.
Fabrication and characterization of the transitional layer scaffold
All scaffolds had higher porosity, which ranged from 65% to 92%, and the porosity of COL/CS/0.5SF could reach 92% indicating it had a more optimal interconnected porous structure. With the increase of SF content, the water adsorption and swelling ratio decreased gradually. COL/CS/0.5SF scaffold exhibited the highest compressive strength (29.24 ± 0.10 KPa) amongst the four scaffolds, and there were significant differences. The COL/CS/0.5SF/TPHNs scaffold showed the optimal properties via the evaluation of the physicochemical characterization, mechanical properties and biocompatibility.
Fabrication and characterization of the dual-layer scaffold
SEM of the dual-layer scaffold
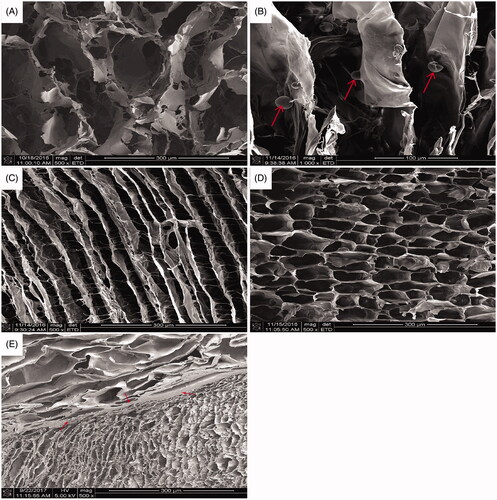
The SEM morphology of biomimetic oriented cartilage scaffold is shown in . The surface layer and the transition layer were cemented by 1% SF as shown. This microstructure was completely consistent with the original design of natural cartilage layer structure. The surface layer is a porous structure through each other, and the transitional layer is a directional microtubule array structure. It can be seen that the aperture of surface layer was significantly larger than that of the layer in transition. It is good for autologous BMSCs to move from the bone marrow cavity along the wall of the microtubule to the surface layer and induce the BMSCs to differentiate into chondrocytes.
Mechanical testing
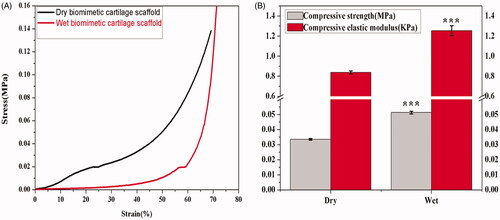
It is important for scaffold to withstand stress during culturing in vitro and as implants in vivo. Mechanical properties also influence specific cell functions within the engineered tissues. During the test, the samples were all compressed to 70% strain. As shown in , there were three stages in the linear stress–strain curves of dry and wet scaffolds, which were a linear elastic stage (strain < 5%.), a steady collapse plateau stage (15% < strain < 45%), and a sharply increasing densification stage (strain > 50%) , which matches the typical stress-strain curves of cellular solids under compression. showed compressive strength and elastic modulus of dry and wet biomimetic cartilage scaffolds. Compared with dry biomimetic cartilage scaffolds (0.033 MPa), wet biomimetic cartilage scaffolds (0.051 MPa) has higher compression strength and has extremely significant difference (p < .001). The compressive elastic modulus of wet biomimetic cartilage scaffolds is only 1.2 KPa, which is still a little different from normal cartilage (0.1 MPa).
Cell proliferation and viability
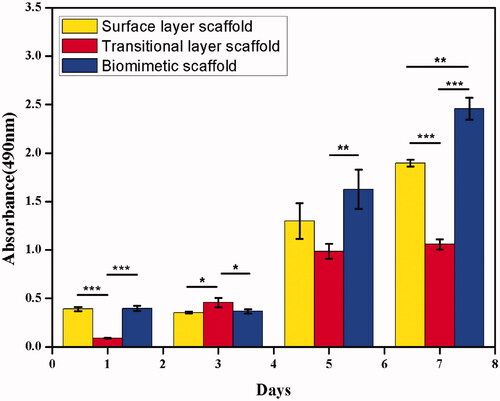
BMSCs were used to test the biocompatibility of biomimetic cartilage scaffolds. showed the proliferation of BMSCs were cultured in a mixture of culture medium and half of leach liquor of surface layer scaffolds, transitional layer scaffolds and biomimetic cartilage scaffolds. For three kinds of scaffolds, there have significant differences (p < .05) on the first day. This may be due to the relatively few cells adhering to the transitional layer. The cells grew faster in the transitional layer between 1 and 3 day. The scaffolds considerably increased on the 5th day because cells need an environmental adaptation period within the first 3 days. Compared with surface layer scaffolds and transitional layer scaffolds, cells exhibited best proliferation rate in biomimetic cartilage scaffolds on the 7 days. Proliferation results suggested that biomimetic cartilage scaffolds could enhance the cells proliferation.
BMSCs were used to test the biocompatibility of scaffolds through AO-EB double staining after these BMSCs were cultured in a mixture of culture medium and half of leach liquor of surface layer scaffolds, transitional layer scaffolds and biomimetic cartilage scaffolds for 7 days (). AO-EB double staining results showed that nearly all of BMSCs were bright green and showed better fusiform cell morphology, and the integrity of cell nucleus was maintained. The number of cells in biomimetic cartilage scaffolds was significantly more than the other two groups after 3 days.
Cartilage repair assessment
Macroscopic observation
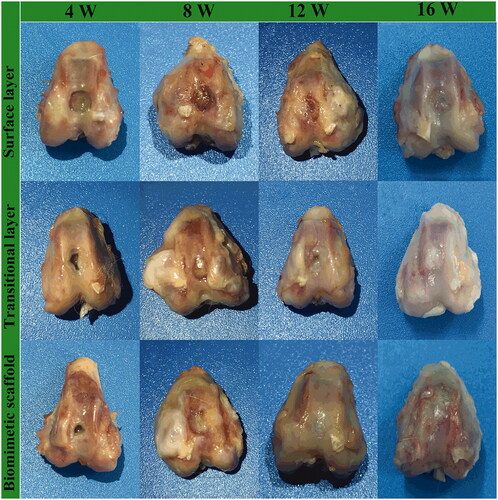
A cartilage defect model in rabbits was used to evaluate the repair capability for surface layer scaffolds, transitional layer scaffolds and biomimetic cartilage scaffolds (). At postoperative 4 weeks, the indentation in the articular cartilage defect is still present, but new tissue begins to appear in the transitional layer and biomimetic cartilage scaffolds groups. A few of the regeneration cartilage appeared after 8 weeks, but there were more osteophytes at the joint edge. The tissue transparency and surface smoothness in the biomimetic cartilage scaffold group were superior to other groups after 12 weeks. At postoperative 16 weeks, in gross observation, articular surface of biomimetic cartilage scaffold group was smooth. Obviously, compared with the other two groups, the bionic oriented cartilage scaffold group with two factors showed the best regeneration effect.
Histological staining analyses
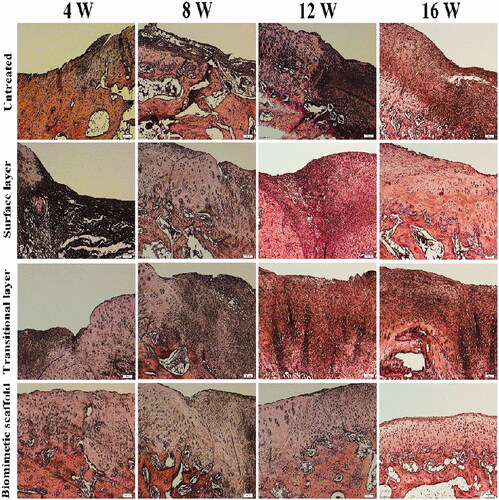
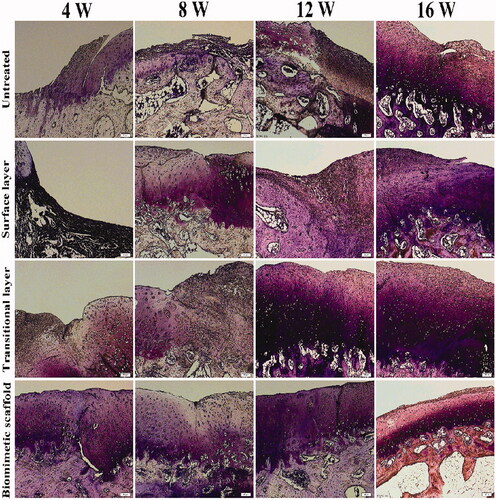
All of the New Zealand white rabbits survived without any obvious immunological or infectious complications during the experiment. The H&E staining of regenerated cartilages in four groups is shown in . H&E staining showed well-defined construct of chondrocytes and cell aggregation. Compared with 4, 8 weeks in vivo results, significant increase in cell number and higher staining intensity were observed in the biomimetic cartilage scaffold group of 12 weeks. The biomimetic cartilage scaffold group formed mature regenerated cartilage similar to the normal tissue with uniformly aligned chondrocytes after 16 weeks. The cartilage defects in the control group were still evident. A few of the regeneration cartilage appeared in surface layer scaffold group and transitional layer scaffold group, but the distribution of cells was irregular, and a significant difference between the biomimetic cartilage scaffold group and normal tissue still existed. There was no doubt that the biomimetic cartilage scaffold group showed the most satisfactory effect compared to all other groups.
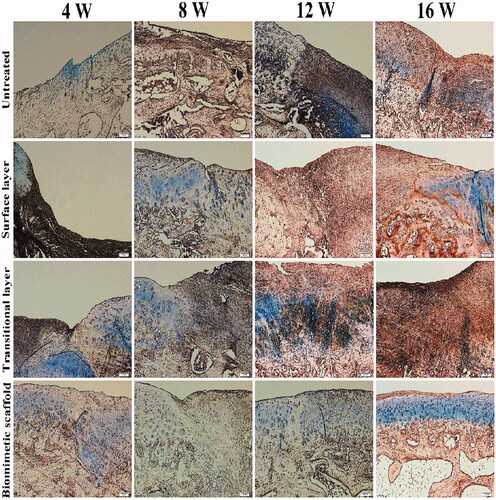
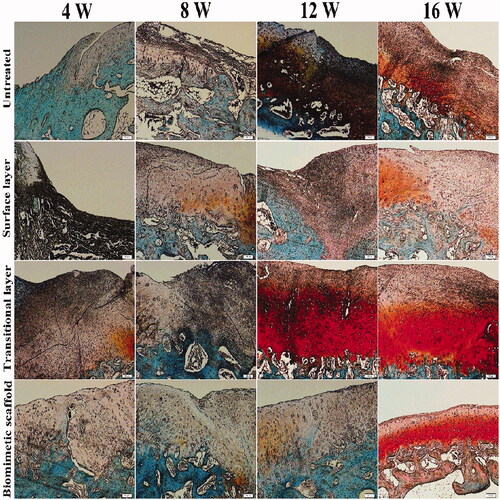
The blue dye shown in alcian blue staining detects all cartilaginous tissues (). A few of the proteoglycan composition appeared before 8 weeks, but the distribution of staining was irregular. Compared with other components, the proteoglycans in the biomimetic cartilage scaffold group were significantly and neatly distributed at 16 weeks. This result indicated that the biomimetic cartilage scaffold group exhibited the greatest amount of hyaline-like cartilage and had a stimulatory effect on cartilage defect repair.
In the results of toluidine blue staining, cartilage and osteoblasts were purple and blue. As shown in , it can be clearly seen that toluidine blue staining intensity will be higher with the increase of the content of glucosamine polysaccharide in the cartilage extracellular matrix. From 4 to 16 weeks, the staining results of either group were significantly deepened. Toluidine blue staining was the deepest and the surface was relatively smooth in the bionic oriented cartilage bracket group. After implantation for 16 weeks, in the biomimetic cartilage scaffold group, a layer of cartilage-like tissue was formed at the surface of defects, which was well connected to the subchondral bone.
The red dye of safranin O, colouring all nuclei in red, indicates the proportion of proteoglycan content in the cartilage (). An obvious defect appeared in the control group, and the Safranin O staining was pale in colour. The staining of the transitional layer showed that there were more chondrocytes on the surface, but there were longitudinal cracks and the surface was rough after implantation for 16 weeks. After implantation for 16 weeks, all components had some degree of repair. In comparison with the other group, the biomimetic cartilage scaffolds group had a stimulatory effect on cartilage defect repair.
Histologic evaluation
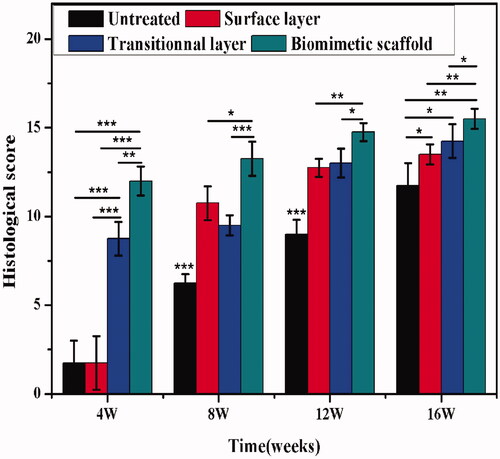
A quantitative scoring for the histological staining was further performed (). As for the evaluation of overall tissue, the biomimetic cartilage scaffold group showed the highest scores at the 4th-week post-implantation (p < .05). Among all the component scores, histological evaluation results of the biomimetic cartilage scaffold group were superior to other groups and had significant differences (p < .05). Therefore, these results suggested that the biomimetic cartilage scaffold group was able to support stability repair of cartilage defect.
Discussion
Biomimetic cartilage scaffolds have been extensively used for the treatment of cartilage damage. Previous studied indicated that natural or synthetic scaffolds [Citation9–12] could support the development of cartilage tissue. We prepared a dual-layer biomimetic cartilage scaffold by mimicking the structural design, chemical cues and mechanical characteristics of mature articular cartilage. To further enhance the chondroinductivity of the scaffold, KGN and TGF-β1 were used to functionalize the scaffold. COL/CS/0.1HAS/MPs scaffold was selected as the surface layer of the biomimetic cartilage scaffold, and COL/CS/0.5SF/TPHNs scaffold was selected as the transition layer of the biomimetic cartilage scaffolds.
The results of in vitro biocompatibility and AO/EB staining revealed that the biomimetic cartilage scaffold could significantly enhance cell proliferation. It indicates that its function was related to the synergistic effect of KGN and TGF-β1. Previous study indicated that growth factors promoted superior chondrogenesis [Citation6, Citation35]. The results of in vivo animal experiments suggested that the defects of the biomimetic cartilage scaffold group had been completely filled, the boundary between new cartilage and surrounding tissue was difficult to identify, and the morphology of cells in repair tissue was almost in accordance with the normal cartilage after 16 weeks. All those results indicated that the biomimetic cartilage scaffold guide the morphology, orientation, and proliferation and differentiation of BMSCs. The results were related to the physical and chemical properties of the materials and different structures and orientations of the composite. In this study, seed cells repaired with articular cartilage defects in the KOA model may be derived from endogenous BMSCs. First, the cells moved from the broken subchondral bone into the implantation of the transitional layer material. Then, it reaches the surface layer because of TGF-β1could promote adhesion and proliferation to endogenous BMSCs. Finally, the effect of KGN on the proliferation and differentiation of BMSCs could achieve the effective repair of new cartilage tissue in the defect area. Ultimately, the proposed treatment can be applied in cartilage repair engineering.
Conclusions
We prepared a dual-layer biomimetic cartilage scaffold to fabricate an excellent biomaterial for cartilage repair and ultimately treat KOA. Compared with the surface layer scaffold and the transition layer scaffold, the dual-layer biomimetic cartilage scaffold could optimally promote cell adherence and proliferation. We further studied the chondroinductive and cartilage regeneration ability of the biomimetic cartilage scaffold in vivo. We concluded that the biomimetic cartilage scaffold could achieve the effective repair of new cartilage tissue in the defect area. Thus, taking all these biological assays into account, the biomimetic cartilage scaffold could become a promising candidate for tissue engineering.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–921.
- Griffith LG, Naughton G. Tissue engineering-current challenges and expanding opportunities. Science. 2002;295:1009–1014.
- Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–1342.
- Athanasiou KA, Darling EM, Hu JC. Articular cartilage tissue engineering. Synth Lect Tissue Eng. 2009;1:1–182.
- Mathews S, Bhonde R, Gupta PK, et al. Novel biomimetic tripolymer scaffolds consisting of chitosan, collagen type 1, and hyaluronic acid for bone marrow-derived human mesenchymal stem cells-based bone tissue engineering. J Biomed Mater Res Part B Appl Biomater. 2014;102:1825–1834.
- Johnson K, Zhu S, Tremblay MS, et al. A stem cell-based approach to cartilage repair. Science. 2012;336:717–721.
- Mollon B, Kandel R, Chahal J, et al. The clinical status of cartilage tissue regeneration in humans. Osteoarthritis Cartilage. 2013;21:1824–1833.
- Nie L, Zhang G, Hou R, et al. Controllable promotion of chondrocyte adhesion and growth on PVA hydrogels by controlled release of TGF-β1 from porous PLGA microspheres. Colloids Surf B Biointerfaces. 2015;125:51–57.
- Liao I, Moutos FT, Estes BT, et al. Composite three-dimensional woven scaffolds with interpenetrating network hydrogels to create functional synthetic articular cartilage. Adv Funct Mater. 2013;23:5833–5839.
- Kim TG, Shin H, Lim DW. Biomimetic scaffolds for tissue engineering. Adv Funct Mater. 2012;22:2446–2468.
- Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues–state of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12:107–124.
- Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524.
- Keeney M, Lai JH, Yang F. Recent progress in cartilage tissue engineering. Curr Opin Biotechnol. 2011;22:734–740.
- Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543.
- Karpiak JV, Ner Y, Almutairi A. Density gradient multilayer polymerization for creating complex tissue. Adv Mater. 2012;24:1466–1470.
- Park H, Choi B, Hu J, et al. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomaterialia. 2013;9:4779–4786.
- Shingleton WD, Cawston TE, Hodges DJ, et al. Collagenase: a key enzyme in collagen turnover. Biochem Cell Biol. 1996;74:759–775.
- Ishihara M, Sato M, Hattori H, et al. Heparin-carrying polystyrene (HCPS)-bound collagen substratum to immobilize heparin-binding growth factors and to enhance cellular growth. J Biomed Mater Res. 2001;56:536–544.
- Yu X, Xu L, Cui FZ, et al. Clinical evaluation of mineralized collagen as a bone graft substitute for anterior cervical intersomatic fusion. J Biomat Tissue Eng. 2012;2:170–176.
- Wang K, Jing R, Song H, et al. Biocompatibility of graphene oxide. Nanoscale Res Lett. 2011;6:1–8.
- Zhang X, Yin J, Peng C, et al. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011;49:986–995.
- Xia ZM, Wei W. Biomimetic fabrication of collagen-apatite scaffolds for bone tissue regeneration. J Biomat Tissue Eng. 2013;3:369–384.
- Engelhardt EM, Micol LA, Houis S, et al. A collagen-poly(lactic acid-co-ɛ-caprolactone) hybrid scaffold for bladder tissue regeneration. Biomaterials. 2011;32:3969–3976.
- Yang GH, Kim M, Kim G. A hybrid PCL/collagen scaffold consisting of solid freeform-fabricated struts and EHD-direct-jetprocessed fibrous threads for tissue regeneration. J Colloid Interface Sci. 2015;450:159–167.
- Huang YX, Zhang XL, Wu AM, et al. An injectable nano-hydroxyapatite (n-HA)/glycol chitosan (G-CS)/hyaluronic acid (HyA) composite hydrogel for bone tissue engineering. Rsc Adv. 2016;6:33529–33536.
- Hinton R, Moody RL, Davis AW, et al. Osteoarthritis: diagnosis and therapeutic considerations. Am Fam Physician. 2002;65:841–848.
- Strauss EJ, Hart JA, Miller MD, et al. Hyaluronic acid viscosupplementation and osteoarthritis: current uses and future directions. Am J Sports Med. 2009;37:1636–1644.
- Jackson DW, Simon TM. Intra-articular distribution and residence time of Hylan A and B: a study in the goat knee. Osteoarthritis Cartilage. 2006;14:1248–1257.
- Tay LX, Ahmad RE, Dashtdar H, et al. Treatment outcomes of alginate-embedded allogenic mesenchymal stem cells versus autologous chondrocytes for the repair of focal articular cartilage defects in a rabbit model. Am J Sports Med. 2012;40:83–90.
- Zhang H, Li L, Dai F, et al. Preparation and characterization of silk fibroin as a biomaterial with potential for drug delivery. J Transl Med. 2012;10:117.
- Suganya S, Venugopal J, Ramakrishna BS, et al. Aloe vera/silk fibroin/hydroxyapatite incorporated electrospun nanofibrous scaffold for enhanced osteogenesis. J Biomat Tissue Eng. 2014;4:9–19.
- Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. J Control Release. 2003;90:261–280.
- Nishida A, Yamada M, Kanazawa T, et al. Sustained-release of protein from biodegradable sericin film, gel and sponge. Int J Pharm. 2011;407:44–52.
- Cun D, Jensen DK, Maltesen MJ, et al. High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles: quality by design optimization and characterization. Eur J Pharm Biopharm. 2011;77:26–35.
- Tan H, Marra KG. Injectable, biodegradable hydrogels for tissue engineering applications. Materials. 2010;3:1746–1767.
- Xu X, Shi D, Shen Y, et al. Full-thickness cartilage defects are repaired via a microfracture technique and intraarticular injection of the small-molecule compound kartogenin. Arthritis Res Ther. 2015;17:20.
- Zhang Q, Zhao Y, Yan S, et al. Preparation of uniaxial multichannel silk fibroin scaffolds for guiding primary neurons. Acta Biomater. 2012;8:2628–2638.
- Sun XM, Wang JH, Wang YY, et al. Collagen-based porous scaffolds containing PLGA microspheres for controlled kartogenin release in cartilage tissue engineering. Artif Cells Nanomed Biotechnol. 2018; 46:1957–1966.
- Sun XM, Wang JH, Wang YY, et al. Scaffold with orientated microtubule structure containing polylysine-heparin sodium nanoparticles for the controlled release of TGF-β1 in cartilage tissue engineering. ACS Appl Bio Mater. 2018;1:2030–2040.
- Maehara H, Sotome S, Yoshii T, et al. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/COL) and fibroblast growth factor-2 (FGF-2). J Orthop Res. 2010;28:677–686.