Abstract
The aim of this study is to investigate the effect of miR-148b on cell proliferation and migration of Schwann cells and explore its mechanism. The miR-148b group, miR-con group and the anti-miR-148b group, anti-miR-con group, si-con group, si-CALR group, Ctrl group, CALR group were transfected into Schwann cells by liposome method; the expression of miR-148b was detected by qRT-PCR; the cell viability was detected by MTT assay; the migration of cells was detected by Transwell method; WB assay was used to detect the protein expression of CALR. Firstly, we found that compared with miR-con group and si-con group, the proliferation and migration of miR-148b group and si-CALR group were significantly down-regulated (P < .05). Moreover, compared with anti-miR-con group and Ctrl group, anti-miR-148b group and CALR group cells proliferation and migration were significantly up-regulated (P < .05). In addition, miR-148b was targeted to CALR, and silencing CALR could reverse the inhibitory effect of miR-148b on Schwann cell proliferation and migration. In conclusion, miR-148b can regulate the proliferation and migration of Schwann cells. The mechanism may be related to the targeted negative regulation of CALR, which will provide a basis for targeted therapy of peripheral nerve injury.
Introduction
In today’s world, the number of traffic accidents is increasing year by year, resulting in increasing peripheral nerve injury. In addition, various natural disasters and surgery can cause peripheral nerve damage. When the nerve is damaged, a gap is formed, which leads to interruption of neural transmission, affecting the neuromuscular function and even atrophy [Citation1]. Although the peripheral nerves have stronger regeneration and recovery ability than the central nervous system, the current medical technology cannot meet the patient’s needs for the recovery of the damage. Schwann cells are the most important glial cells in the peripheral nerve. When the peripheral nerve is damaged, Schwann cells undergo phenotypic changes such as dedifferentiation and demyelination, and then fill the damaged nerve tissue and play a role in promoting axonal growth [Citation2,Citation3]. miRNA is a type of endogenous short-chain non-coding RNA of about 21 to 23 nucleotides that silences a homologous target gene by base pairing with the 3’ untranslated region (3’UTR) of the mRNA encoding the protein gene. The major precursor of miRNA (pri-miRNA) is formed by RNA polymerase II transcription [Citation4]. In animals, most pri-microRNAs are processed from endonuclease Drosha and its cofactor DGCR8 to pre-microRNAs containing 60 to 100 hairpins in the nucleus, which are then exported to the cytoplasm. Most pre-miRNAs are further cleaved by RNase III-like endonuclease Dicer1 to produce mature microRNAs. A large number of studies have reported that miRNAs are involved in the proliferation and migration of Schwann cells, such as miR-221/222, miR-9 [Citation5,Citation6]. However, little is known about the role and mechanism of miR-148b in Schwann cells. Calreticulin (CALR, CRT, cC1qR) is a highly conserved multifunctional protein in the endoplasmic reticulum. It has important biological regulatory functions through intracellular calcium regulation, molecular chaperone and tumors, wound healing, cardiogenesis, autoimmune diseases and neurological diseases [Citation7]. In this study, Schwann cells were taken as the research object to observe the effects of interfering with miR-148b and CALR on the proliferation and migration of Schwann cells, revealing that the mechanism is related to the targeting of miR-148b to CALR, which will provide a new direction for the recovery of peripheral nerve injury.
Materials and methods
Materials
Schwann cells were purchased from ATCC (Manassas, VA, USA), SDS-PAGE kit, ECL luminescent solution and RIPA protein lysate were purchased from Beyotime Biotechnology Compan (Jiangsu, China). Matrigel and Transwell chambers were purchased from Coming, USA; DMEM complete medium, fetal bovine serum, MTT, trypsin were purchased from GIBCO, USA; LipofectamineTM2000, miRNA extraction kit, and reverse transcriptase kit were purchased from Takara company (Dalian, Liaoning, China).
Cell culture
Schwann cells were cultured in DMEM complete medium containing 10% fetal bovine serum and cultured in 37 °C and 5% CO2 incubator. The medium was replaced every other day.
Cell transfection
MiR-148b mimics, miR-con, anti-miR-148b mimics, anti-miR-con, si-con, si-CALR, pcDNA 3.1 and pcDNA 3.1-CALR were transfected into Schwann cells according to the requirements of Lipofectamine TM2000 liposome instructions. They were labeled as miR-148b group, miR-con-group, anti-miR-148b group, anti-miR-con group, si-con group, si-CALR group, Ctrl group, CALR group respectively. After successful transfection, it was used in subsequent experiments.
qRT-PCR
Take appropriate amount of cells in the logarithmic growth phase 1.2.2, extract RNA according to the instructions of the RNA extraction kit (miRNA extraction kit), quantify, and then synthesize cDNA according to the instructions of the reverse transcription kit. Finally, the miR-148b test was carried out according to the instruction of qRT-PCR kit. The expression of miR-148b was calculated by 2−△△Ct.
MTT experiment
A suitable amount of 1.2.2 cells in each group were cultured in MTT solution of 20 μL 5 g/L for 3.5/4 h, then the supernatant was discarded. 150 μL DMSO was added into each pore to make the crystals dissolve. The cell absorbance (A) was measured at 490 nm wavelength. The activity of cells is directly proportional to the absorbance.
Transwell experiment
The cells of 1.2.2 groups were inoculated on a 6-well plate with density adjusted to 106 holes. When the fusion degree was 80%, serum-free medium was replaced for overnight culture. The cell density was adjusted to 105 cells/mL, 100 μL was added to the upper chamber, and 600 μL of the serum-containing medium was added to the lower chamber and cultured overnight. The chamber was removed, and the cells in the upper chamber were wiped with a cotton swab, washed with PBS, fixed with methanol for 30 min, stained with 0.1% crystal violet for 20 min, and washed with PBS. The number of migrated cells attached to the lower surface of the chamber was observed under a microscope, and 5 fields of view were randomly selected and averaged.
Lay the appropriate thickness of the matrix on the upper surface of the Transwell chamber, then operate according to the above operation method. Finally, the invasive cells attached to the lower surface of the chamber were observed under the microscope.
Western blot experiment
The cells of each group at logarithmic growth stage 1.2.2 were taken for quantitative analysis by BCA after RIPA lysis, and the supernatant was taken for protein sampling after denaturation and centrifugation. Electrophoresis-transmembrane-blocking-I anti-incubation-II anti-incubation-development exposure was performed according to the routine procedure of Western blot experiment. Image J analyzed the gray value of the target band and expressed the expression of the target protein CALR by the ratio of the gray value of the target band to the gray value of the beta-actin.
Dual luciferase reporter gene assay
The luciferase reporter vector (psiCHECK2-CALR-WT, psiCHECK2-CALR-MUT) was transfected into XX cells with miR-miR-148b mimics and miR-NC, respectively. After 5 h of culture, fresh medium was replaced and then continued to be cultured for 48 h. Follow the instructions in the instructions for the dual luciferase reporter assay kit instructions. The binding strength of miR-148b to CALR was measured by the ratio of the luminescence intensity of sea cucumber luciferase to the luminescence intensity of firefly luciferase.
Statistical analysis
All data in the experiment were analyzed using SPSS 21.0 software. Measurement data were expressed as mean ± standard deviation (x ± s). Data between groups were compared by one-way analysis of variance. Pairwise comparisons were performed using SNK-q test. P < .05 was considered statistically significant.
Results
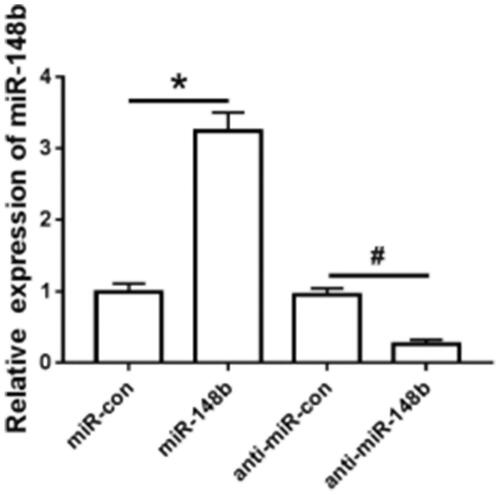
Detection of miR-148b interference efficiency in Schwann cells
The expression level of miR-148b in Schwann cells transfected with miR-148b mimics, anti-miR-148b, miR-con and anti-miR-con was detected by qRT-PCR. The expression level of miR-148b was significantly increased in the miR-148b group compared with the miR-con group; Compared with the anti-miR-con group, the expression level of miR-148b was significantly decreased in the anti-miR-148b group (), which was statistically significant (P < .05).
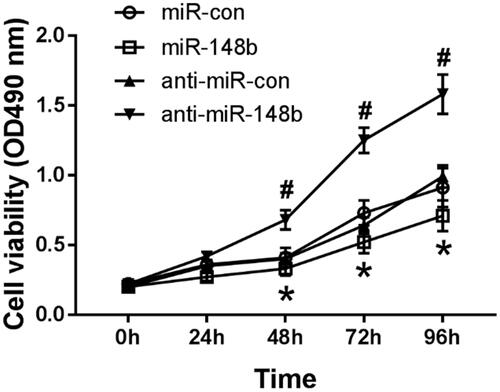
MiR-148b inhibits proliferation of Schwann cells
MTT assay was used to detect the activity of Schwann cells in each group interfering with miR-148b. Compared with the miR-con group, the activity of Schwann cells in miR-148b group decreased significantly. Compared with the anti-miR-con group, the cell activity of the anti-miR-148b group was significantly increased (), and both were statistically significant (P < .05). Therefore, miR-148b inhibits proliferation of Schwann cells.
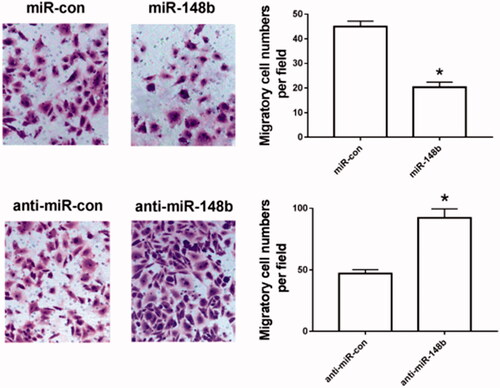
MiR-148b inhibits Schwann cell migration
Transwell assay was used to detect the migration of Schwann cells in each group interfering with miR-148b. Compared with the miR-con group, the migration of Schwann cells in the miR-148b group was significantly decreased. Compared with the anti-miR-con group, the cell migration of the anti-miR-148b group was significantly increased (), and both were statistically significant (P < .05). It can be seen that miR-148b inhibits Schwann cell migration.
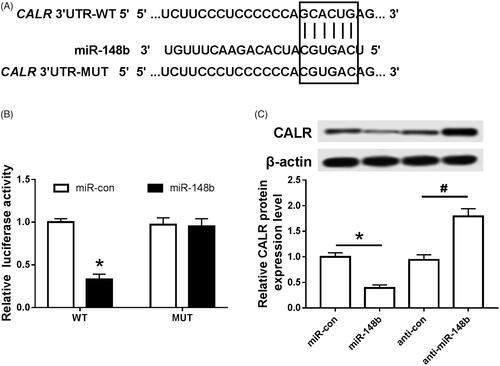
MiR-148b targets negative regulation CALR
Target scan was used to predict the possible binding of CALR to miR-148b. It was found that the binding of miR-148b to CALR was highly possible (). The fluorescence activity of cells in each group was detected by dual luciferase reporter gene assay. Compared with the miR-NC group, the fluorescence activity of the cells transfected with WT in the miR-148b group was significantly decreased without affecting the fluorescence activity of MUT cells, and the protein expression of CALR was significantly decreased (). Compared with the anti-miR-NC group, the expression of CALR protein in the anti-miR-148b group was significantly increased (), and both were statistically significant (P < .05). It can be seen that miR-148b targets negative regulation of CALR.
Figure 4. MiR-148b targeting CALR. (A) miR-148b targeting combined with the sequence information of the CALR mRNA 3’UTR; (B) the effect of miR-148b on the activity of Schwann cells luciferase; (C) the effect of miR-148b on the expression of CALR protein in Schwann cells; Compared with the miR-con group, *P < .05; compared with the anti-miR-con group, #P < .05.
Effect of interventional CALR on proliferation and migration of Schwann cells
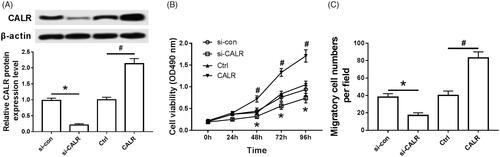
As shown in , the expression of CALR protein was significantly decreased in the si-CALR group compared with the si-con group (), the cell activity was significantly decreased (), and the cell migration amount was significantly decreased (); Compared with the ctrl group, CALR protein expression was significantly increased in the CALR group (), cell activity was significantly increased (), and cell migration was significantly increased (), both of which were statistically significant (P < .05).
Figure 5. Intervention of CALR affects proliferation and migration of Schwann cells. (A) Effect of intervention of CALR on the expression of CALR protein in Schwann cells; (B) Effect of intervention of CALR on Schwann cell activity; (C) Effect of intervention of CALR on migration in Schwann cells; Compared with si-con group, *P < .05; compared with the Ctrl group, #P < .05.
Effects of miR-148b and CALR intervention on proliferation and migration of Schwann cells
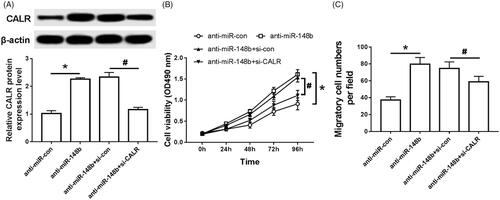
As shown in , compared with the anti-miR-con group, the expression of CALR protein in the anti-miR-148b group increased significantly (), cell activity increased significantly (), and cell migration increased significantly (). Compared with the anti-miR-148b + si-con group, the expression of CALR protein in the anti-miR-148b + si-CALR group was significantly decreased (), and the cell activity was significantly decreased (). The amount of cell migration was significantly reduced (), both of which were statistically significant (P < .05).
Figure 6. Effects of intervention of miR-148b and CALR on proliferation and migration of Schwann cells. (A) Effect of intervention of miR-148b and CALR on the expression of Schwann cell CALR protein; (B) Effect of intervention of miR-148b and CALR on Schwann cell activity; (C) Effect of intervention of miR-148b and CALR on Schwann cell migration; Compared with the anti-miR-con group, *P < .05; compared with the anti-miR-con + si-con group, #P < .05.
Discussion
Schwann cells in the peripheral nervous system are of great concern for the function of neurons, and their failure leads to destructive demyelinating disease [Citation8]. During development, Schwann cell precursors underwent a series of molecular and structural changes clearly defined in time, which eventually lead to the cessation of proliferation and the formation of highly complex myelin sheaths [Citation9]. Recent studies have demonstrated that microRNA (microRNA) plays a key role in the development of oligodendrocytes and Schwann cell precursors for myelin status [Citation10,Citation11]. miRNAs are widely involved in neurogenesis, including cell fate determination, nerve patterns, synaptic plasticity, activity-dependent regulation, and neurological diseases [Citation12]. Previous studies have confirmed that miRNA expression is different in different stages of normal myelin cell development. Some of these miRNAs play multiple roles in oligodendrocytes and SC cells, including cell proliferation, differentiation, and myelin homeostasis [Citation13]. Studies have shown that miR-219 and miR-338 are involved in repairing maturation and myelin damage of human oligodendrocyte, while miR-23 can target laminin B1 leading to demyelinating disease [Citation14]. Yu et al. [Citation15] used high-throughput sequencing to periodically detect miRNA expression during rat sciatic nerve injury and found that about 200 miRNAs showed significant abnormal expression. Further bioinformatics analysis revealed that these potential targets of miRNAs were involved in nerve regeneration, including neurogenesis, neuronal differentiation, vesicle-mediated transport, homophilic cell adhesion, and negative regulation of programmed cell death. It is suggested that the abnormal expression of miRNA may be helpful to study the molecular mechanism of nerve regeneration, which is a potential target for therapeutic intervention in nerve regeneration. Qian et al. [Citation16] reported that miR-148b-3p could target and negatively regulate the proliferation of Alcam, Cpd, Dedd, Nptx2, Phf20, Ptpn14 and Stard 13 in Schwann cells to repair nerve damage. In this study, MTT assay and Transwell assay were used to detect the proliferation and migration of Schwann cells overexpressing miR-148b and inhibiting miR-148b. Overexpression of miR-148b down-regulated Schwann cell proliferation and migration and inhibition of miR-148b was reversed. The role of inhibition of miR-148b has the effect of promoting the growth of Schwann cells, which is conducive to the protection of nerve damage; furthermore, dual luciferase reporter gene assay was used to verify that the expression of CRT was negatively regulated by miR-148b.
CALR mainly exists in the endoplasmic reticulum. Its main function is to regulate Ca2+ homeostasis, act as a molecular chaperone and stabilize MHC I peptide-loaded complex [Citation17,Citation18]. In recent years, a large number of studies have reported that CALR is closely related to neurological diseases. In the study of neuroblastoma, Shih et al. [Citation19] clarified that CRT can significantly stimulate the production of nerve growth factor NGF to promote neuronal differentiation, showing CRT-dependent regulation of NGF-induced neuronal differentiation, which is beneficial to the prognosis of neuroblastoma. Huang et al. [Citation20] isolated SCs from mouse sciatic nerve in Schwann cells and constructed SCs that overexpressed CALR and knocked down CALR. Then the expression of CALR was determined by qRT-PCR and Western analysis. MTT, Transwell and flow cytometry were used to measure cell proliferation, migration and apoptosis. It was found that overexpression of CALR could promote cell viability and migration of SCs. However, inhibition of apoptosis and knockdown of CALR showed the opposite effect, and the phosphorylation levels of AKT (Thr308 and Ser473), ERK and S6 in overexpressing CALR cells were up-regulated, while those in knocking down CALR cells were down-regulated, which revealed that overexpressing CALR promoted the proliferation and migration of SCs cells and inhibited apoptosis. The mechanism may be related to activation of PI3K/AKT and ERK/S6 pathway. This study examined the proliferation and migration of Schwann cells which overexpressed CALR and knocked down CALR. It was found that overexpressed CRT promoted the proliferation and migration of Schwann cells, and knockdown of CALR inhibited the proliferation and migration of Schwann cells, suggesting that CALR could promote the growth of Schwann cells and protect nerve damage, which was consistent with Huang’s research results. The in-depth study found that knockdown of CALR can partially reverse the inhibition of miR-148b on the proliferation and migration of Schwann cells.
In conclusion, miR-148b inhibits the proliferation and migration of Schwann cells, and its mechanism may be related to the targeted negative regulation of CALR, providing a new target for targeted treatment of neurological diseases.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hatzenbuehler J. Peripheral nerve injury. Curr Sports Med Rep. 2015;14:356–357.
- Beltran M, Puig I, Pena C, et al. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev. 2008;22:756–769.
- Tam OH, Aravin AA, Stein P, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 2008;453:534–538.
- Vishnoi A, Rani S. MiRNA biogenesis and regulation of diseases: an overview. Meth Mol Biol. 2017;1509:1–10.
- Yu B, Zhou S, Wang Y, et al. miR-221 and miR-222 promote Schwann cell proliferation and migration by targeting LASS2 after sciatic nerve injury. J Cell Sci. 2012;125:2675–2683.
- Zhou S, Gao R, Hu W, et al. MiR-9 inhibits Schwann cell migration by targeting Cthrc1 following sciatic nerve injury. J Cell Sci. 2014;127:967–976.
- Boelt SG, Norn C, Rasmussen M, et al. Mapping the Ca(2+) induced structural change in calreticulin. J Proteomics. 2016;142:138–148.
- Cobianchi S, Jaramillo J, Luvisetto S, et al. Botulinum neurotoxin A promotes functional recovery after peripheral nerve injury by increasing regeneration of myelinated fibers. Neuroscience 2017;359:82–91.
- Mogha A, Harty BL, Carlin D, et al. Gpr126/Adgrg6 has schwann cell autonomous and nonautonomous functions in peripheral nerve injury and repair. J Neurosci. 2016;36:12351–12367.
- He X, Yu Y, Awatramani R, et al. Unwrapping myelination by microRNAs. Neuroscientist. 2012;18:45–55.
- Vo NK, Cambronne XA, Goodman RH. MicroRNA pathways in neural development and plasticity. Curr Opin Neurobiol. 2010;20:457–465.
- Chang LW, Viader A, Varghese N, et al. An integrated approach to characterize transcription factor and microRNA regulatory networks involved in Schwann cell response to peripheral nerve injury. BMC Genomics. 2013;14:84.
- Zhan C, Ma CB, Yuan HM, et al. Macrophage-derived microvesicles promote proliferation and migration of Schwann cell on peripheral nerve repair. Biochem Biophys Res Commun. 2015;468:343–348.
- Lin ST, Fu YH. MiR-23 regulation of lamin B1 is crucial for oligodendrocyte development and myelination. Dis Model Mech. 2009;2:178–188.
- Yu B, Zhou S, Wang Y, et al. Profile of microRNAs following rat sciatic nerve injury by deep sequencing: implication for mechanisms of nerve regeneration. PLoS One. 2011;6:e24612.
- Qian TM, Zhao LL, Wang J, et al. miR-148b-3p promotes migration of Schwann cells by targeting cullin-associated and neddylation-dissociated 1. Neural Regen Res. 2016;11:1001–1005.
- Luo W, Yu Z. Calreticulin (CALR) mutation in myeloproliferative neoplasms (MPNs). Stem Cell Investig. 2015;2:16.
- Wijeyesakere SJ, Gagnon JK, Arora K, et al. Regulation of calreticulin-major histocompatibility complex (MHC) class I interactions by ATP. Proc Natl Acad Sci USA. 2015;112:E5608–E5617.
- Shih YY, Nakagawara A, Lee H, et al. Calreticulin mediates nerve growth factor-induced neuronal differentiation. J Mol Neurosci. 2012;47:571–581.
- Huang G, Sun Z, Wu J, et al. Calreticulin promotes proliferation and migration but inhibits apoptosis in Schwann cells. Med Sci Monit. 2016;22:4516–4522.