Abstract
Aims
The study aimed to investigate the clinical characteristics of patients with pleural effusion (PE), and explore the effective indicators for definite diagnosis of tuberculous pleural effusion (TBPE).
Methods
The adult patients with the presence of PE were enrolled. All the patients received pleural fluid Mycobacterium tuberculosis DNA detection, ADA activity measure and blood T-SPOT.TB test. The clinical characteristics and examination results were recorded.
Results
A total of 77 PE patients, including 30 (38.96%) TBPE, 19 (24.67%) malignant PE, 6 (7.79%) empyema, 10 (12.99%) parapneumonic effusion and 12 (15.58%) miscellaneous causes, were enrolled. The diagnostic sensitivity and specificity of pleural fluid M. tuberculosis DNA detection were 33.3% and 100%, respectively. The diagnostic parameters of pleural fluid ADA for TBPE were as follows: sensitivity 50% and specificity 78.7%. In PE cases with pleural fluid lactate dehydrogenase (LDH) more than 500 U/L, the diagnostic values of DNA detection and ADA activity were enhanced, and DNA detection was superior to ADA activity. In addition, the ratio of blood T-STOP.TB A + B to lymphocyte was a potential diagnostic biomarker for TBPE with the sensitivity of 83.3% and the specificity of 66.0%.
Conclusion
The clinical significance of pleural fluid M. tuberculosis DNA detection is superior to ADA activity in the diagnosis of TBPE, especially in PE cases with LDH value more than 500 U/L. The ratio of blood T-STOP.TB A + B to lymphocyte is a potential indicator for definite diagnosis of TBPE, with high sensitivity.
Introduction
Pleural effusion (PE) is referred to the excessive accumulation of pleural fluid in pleural place, which is a frequently diagnosed complication in clinic [Citation1]. PE could be caused by various diseases, such as malignancy, cirrhosis, pneumonia, tuberculosis (TB), empyema, chylothorax, lupus erythematosus, etc. [Citation2]. Since the different etiologies of PE can cause different clinical manifestations, the differential diagnosis is key for the therapeutic strategies.
TB is a frequently diagnosed infectious disease, with high mortality and morbidity [Citation3]. In 2016, there were approximately 10.4 million TB cases, posting a great threat to human health [Citation4]. Tuberculous pleural effusions (TBPE) is characterized by the positive culture of Mycobacterium tuberculosis in pleural fluid, tissue or blood samples, accounting for about 40% PE cases in China [Citation5,Citation6]. For patients with TBPE, timely antituberculous therapy is important for clinical outcomes. The global standard for TBPE diagnosis is bacteria culture and histology, but the diagnostic sensitivity is limited [Citation7,Citation8]. Pleural biopsy is an effective method to make diagnosis, with high diagnostic efficacy. However, the procedure is invasive, complex and technical difficulty [Citation9]. In order to improve the early screening of TBPE, various novel biomarkers and assays are explored, including adenosine deaminase (ADA) activity, Interferon-gamma (IFN-γ) release assay (IGRA), M. tuberculosis DNA detection, etc. [Citation10,Citation11]. ADA activity detection is a method specific to an enzyme associated with T-lymphocyte activity, which is easy and fast to do, with less cost [Citation12]. However, the diagnostic accuracy of ADA may be limited due to the presence of false-positive/-negative results [Citation9,Citation13]. It may be used as an auxiliary tool for conventional method in early diagnosis of TBPE. IGRA measures the IFN-γ release by the specific T cells in response to M. tuberculosis infection [Citation14]. There are several commercial kits for this method, such as the enzyme-linked immunosorbent spot (ELISpot) assay (T-SPOT.TB; Oxford Immunotec Limited, United Kingdom), the enzyme-linked immunosorbent assay (ELISA) (QuantiFERON; Cellestis Limited, Australia), the tuberculin skin test (TST) using random effects models [Citation15]. Whereas, accumulating evidence have demonstrated that the diagnostic performance of IGRA is poor in early diagnosis of TBPE [Citation16,Citation17]. Mycobacterium tuberculosis DNA detection is a method to amplify the genetic DNA of M. tuberculosis through polymerase chain reaction (PCR) methods. Mycobacterium tuberculosis DNA detection is a rapid and accurate method for identification of M. tuberculosis infection [Citation18]. However, the low sensitivity may limit its wide application [Citation19]. Despite of various available biomarkers and methods, the definite diagnosis of TBPE still remains a great challenge. Novel and accurate diagnostic strategies are in urgent need for early diagnosis of TBPE.
In the current study, we investigated the clinical characteristics of the patients with PE in China. Moreover, we compared the performances of pleural fluid M. tuberculosis DNA detection, ADA activity and T-STOP.TB in definite detection of TBPE.
Materials and methods
Study subjects
The current prospective study was carried out in Daping Hospital from October 2016 to May 2018. The study procedures were approved by the Ethic Committee of the hospital. All the enrolled patients signed the informed contents.
The patients enrolled in our study should meet the following criteria: (1) adult group; (2) presenting with PE through chest ultrasonic examinations; (3) having available clinical records. The patients who had a coexisting systemic disease, immunodeficiency, autoimmune disease or hemothorax would be excluded from the current investigation. Additionally, the moribund patients were also excluded from our study. Finally, a total of 77 eligible patients were included in our study.
Sample collection and examinations
All the patients underwent thoracocentesis. A volume of 20 cc of pleural fluid and 4 ml of peripheral blood samples, as well as pleural tissues, were collected from each patient for routine biological, microbiological, molecular and histological examinations. The biochemical parameters, including total protein, glucose, chlorine, and lactate dehydrogenase (LDH) were recorded from the automated chemistry analyzer while manual microscopy was applied for cell count. The levels of C-reactive protein (CRP) were estimated using immunoturbidometric method which was performed using Beckman Coulter AU5800 Clinical Chemistry System.
PE samples were centrifuged at 3000 g for 10 min, and the sediments were cultured in mycobacterial growth indicator tube for 6 weeks (MGIT 960 system, Becton Dickinson, Sparks, MD). The positive culture was further confirmed by the presence of M. tuberculosis through real-time polymerase chain reaction (qRT-PCR) detecting IS6110 sequence.
T-SPOT TB test was performed using the T-SPOT TB assay (Oxford Immunotec Ltd., Abingdon, UK), and the procedures were performed according to the instructions of the manufacturer. T-SPOT.TB A was specific to ESAT-6 antigens while T-SPOT.TB B was specific to CFP10 antigen. Spot-forming cells (SFCs) were read from an automated ELISPOT reader (AID-ispot, Strassberg, Germany).
The activity of ADA was confirmed using an adenosine deaminase assay kit (Beijing Strong Biotechnologies, Beijing, China). The results with ADA value no less than 40 U/L were considered as positive for TB.
For M. tuberculosis DNA detection, the DNA sample was extracted from PE specimens using QIAamp Circulating Nucleic Acid Kit (Qiagen, Hilden, Germany). Then, M. tuberculosis fluorescent PCR kit was applied for amplification, and the operation was carried out following to the manufacture’s instructions.
Diagnosis
The final diagnosis was made based on the clinical manifestations, bacteriological, biochemical examinations and histopathological evaluation. The PE with positive M. tuberculosis culture or tuberculous infection confirmed by pleural biopsy was defined as TBPE. Malignant pleural effusion (MPE) was confirmed with the evidence of negative M. tuberculosis culture and histopathological or cytological diagnosis of tumors. Parapneumonic effusion (PPE) was caused by pneumonia which was confirmed according to the criteria of the American Thoracic Society (ATS) [Citation20]. Empyema cases were with negative M. tuberculosis culture, and definitely based on ATS guidelines [Citation21]. For the cases with unclear etiology of PE, the thoracoscopy and video-assisted thoracic surgery (VATS) were performed.
Statistical analysis
All data analysis was performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). The continuous data were shown as mean ± standard deviation (SD) and compared between two groups using student’s t-test if they were in normal distribution, otherwise, the Wilcoxon rank-sum test was used. The comparison of continuous data among three or more groups was performed using one-way ANOVA. Chi-square test was used for the analysis of categorical variables. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ration (NLR) and Youden index were calculated to estimate the diagnostic performance of the indicators. In addition, receiver operating characteristic (ROC) curve was plotted to evaluate the diagnostic value of continuous data for TBPE. The results with p values less than 0.05 were considered as statistical significance.
Results
Baseline characteristics of the study group
According to the selection criteria, a total of 77 patients with PE were enrolled in our study. The demographic and clinical characteristics of the patients were collected from their medical records and summarized in . There were 60 (85.7%) males and 17 (14.3%) females, and their average age was 55.80 ± 21.60 years. The mean ADA in pleural fluid was 47.29 ± 65.11 U/L. Total protein was 41.68 ± 14.83 g/L, chlorine was 106.04 ± 17.42 mmol/L, glucose was 5.41 ± 3.26 mmol/L, lactate dehydrogenase (LDH) was 2333.17 ± 7353.06 U/L in pleural fluid. Blood analysis demonstrated that LDH was 280.00 ± 265.13 U/L, total protein was 65.04 ± 9.12g/L, lymphocyte was 1.12 ± 0.69 × 109/L, CRP was 47.46 ± 49.56 mg/L, T-SPOT.TB A was 18.63 ± 20.94 and T-SPOT.TB B was 18.01 ± 22.21. The ratio of pleural fluid LDH to blood LDH was 11.93 ± 46.14, and the ratio of pleural fluid protein to blood protein was 0.63 ± 0.20. In addition, 30 patients (38.96%) were confirmed with TBPE, 19 patients (24.67%) were diagnosed with MPE, 6 patients (7.79%) were caused by empyema, 10 patients (12.99%) were confirmed with PPE, and the rest 12 patients (15.58%) were attributed to miscellaneous causes. According to pleural fluid M. tuberculosis DNA detection, 10 patients showed positive results, and all of them were confirmed as TBPE, accounting for 33.33% of TBPE cases. Compared with TBPE patients, other types of pleural effusion patients in age, Mycobacterium tuberculosisDNA detection, ADA level, total protein, glucose, LDH levels, CRP, T-SPOT.TB A , T-SPOT.TB B and pleural fluid LDH/blood LDH, pleural protein/blood protein had significant differences (p < 0.05, Table 1).
Table 1. The clinical characteristics of the study subjects.
Comparison of the baseline characteristics according to the etiology of PE
The comparison of the baseline parameters of the included patients according to their etiologies of PE are shown in . Age showed significant differences among the five groups, and TBPE patients were the youngest (p < .001). The pleural fluid ADA activity exhibited obviously different among the five groups (p < .001), and empyema cases had the highest level, followed by TBPE. The protein levels in pleural fluid and blood samples were also significantly different among the five groups (p < .001, and p = .007, respectively), and TBPE cases had the highest levels. Besides, the pleural fluid glucose, LDH, the ratios of pleural fluid LDH to blood LHD, and the pleural fluid protein to blood protein were remarkably different among the five groups (p < .05 for all). In addition, the five groups exhibited obvious differences in T-SPOT.TB assay (p = .002 and .004, respectively), and the TBPE group had the highest values.
The diagnostic performance of pleural fluid M. tuberculosis DNA detection for TBPE
In our study, 30 patients were diagnosed with TBPE, and 10 of them showed positive in pleural fluid M. tuberculosis DNA detection, moreover, none of the non-TBPE patients had positive results. The diagnostic sensitivity of pleural fluid M. tuberculosis DNA detection was 33.3% (95% CI: 17.9–52.9%), and the specificity was 100% (95% CI: 90.6–100%). The PPV and NPV were 100% (95%CI: 65.5–100%) and 70.1% (95%CI: 57.6–80.4%), respectively. The NLR was 0.667 (95%CI: 0.518–0.859), and Youden index was 0.333 ().
Table 2. Diagnostic performance of pleural fluid Mycobacterium tuberculosis DNA detection and ADA for TBPE.
We compared the clinical characteristics between M. tuberculosis DNA detection positive (n = 10) and negative (n = 20) TBPE patients. Analysis results demonstrated that TBPE patients with positive M. tuberculosis DNA detection showed significantly higher pleural fluid ADA (89.31 ± 76.36 vs. 36.04 ± 13.77, p = .005) and LDH levels (3068.65 ± 5788.31 vs. 445.45 ± 229.08, p = .049). In M. tuberculosis DNA detection negative group, 6 patients (30%) had LDH value more than 500 U/L while the number was 7 (70%) in M. tuberculosis DNA detection positive group (p = .037). The results might reveal that pleural fluid ADA and LDH might help the diagnostic accuracy of M. tuberculosis DNA detection for TBPE. Additionally, the other parameters showed similar distribution between the two studied groups (p > .05 for all) ().
Table 3. The comparison of baseline characteristics between pleural TB DNA positive and negative tuberculous pleural effusion.
The clinical significance of pleural fluid ADA for early detection of TBPE
The diagnostic significance of pleural fluid ADA for TBPE was also estimated in our study, and the results are shown in . In the study subjects, 25 patients (32.5%) with ADA positive results, and 52 patients (67.5%) with ADA negative results. In TBPE group, 15 patients (50%) with ADA positive while 15 patients (50%) with ADA negative. The diagnostic sensitivity and specificity were 50% (95%CI: 31.7–68.3%) and 78.7% (95%CI: 63.9–88.8%), respectively. The PPV was 60% (95%CI: 38.9–78.2%), NPV was 71.1% (95%CI: 56.7–82.4%), PLR was 2.35 (95%CI: 1.22–4.53) and NLR was 0.635 (95%CI: 0.439–0.919). Additionally, Youden index was 0.287.
According to the pleural fluid ADA activity, the TBPE patients were divided into positive (n = 15) and negative group (n = 15). The comparison of clinical characteristics between pleural fluid ADA detection positive and negative was also performed. Analysis results suggested that the positive group exhibited significantly higher level of ADA than the negative group (79.53 ± 61.69 vs. 28.06 ± 11.52, p = .004). Furthermore, the pleural fluid glucose level was decreased in positive group compared to the negative group (3.24 ± 2.20 vs. 4.99 ± 2.02, p = .031). In positive group, 9 (60%) patients had pleural fluid LDH level more than 500 U/L, and there were only 4 (26.7%) patients with LDH value more than 500 U/L in ADA negative group. Meanwhile, the two group did not show significant differences in age (p = .232), gender (p = .439), pleural fluid M. tuberculosis DNA detection (p = .121), total protein (p = .087), chlorine (p = .349), LDH (p = .152), LDH distribution (p = .065), as well as all the blood parameters (p > .05 for all) ().
Table 4. The comparison of baseline characteristics between pleural fluid ADA positive and negative tuberculous pleural effusion.
Diagnostic value of pleural fluid M. tuberculosis DNA detection and ADA for TBPE in PE cases with LDH more than 500 U/L
In our study, 31 patients (40.3%) with pleural fluid LDH value more than 500 U/L. The diagnostic performances of pleural fluid M. tuberculosis DNA detection and ADA were estimated for TBPE among PE cases with LDH value more than 500 U/L. For M. tuberculosis DNA detection, the diagnostic sensitivity was 53.8% (95%CI: 26.1–79.6%), specificity was 100% (95%CI: 78.1–100%), PPV was 100% (95%CI: 26.1–79.6%), NPV was 75% (95%CI: 52.9–89.4%) and NLR 0.461 (95%CI: 0.257–0.830). Youden index was 0.538. For DA detection, the diagnostic performance parameters were as follows: sensitivity was 69.2% (95%CI: 38.9–89.6%), specificity was 50% (95%CI: 26.8–73.2%), PPV was 50% (95%CI: 26.8–73.2%), NPV was 69.2% (95%CI: 38.9–89.6%), PLR was 1.384 (95%CI: 0.770–2.490), NLR was 0.615 (95%CI: 0.244–1.55) and Youden index was 0.192 (). The diagnostic value of pleural fluid M. tuberculosis DNA detection was superior to ADA detection in TBPE patients with LDH value more than 500 U/L.
Table 5. The diagnostic significance of pleural fluid Mycobacterium tuberculosis DNA detection and ADA for TBPE in PE cases with LDH more than 500 (U/L).
The diagnostic significance of T-SPOT.TB A + B/blood lymphocyte for TBPE
T-SPOT.TB assay was commonly used for diagnosis of TB through T-cell interferon-gamma release in blood sample [Citation22]. In our study, we found that the values of T-SPOT.TB assay were significantly higher in TBPE samples than that in other types of PE. Moreover, TBPE group had the lowest blood lymphocyte level. Thus, we estimated the diagnostic performance of T-SPOT.TB A + B/blood lymphocyte for TBPE using ROC. The curve demonstrated that the ratio of T-SPOT.TB A + B to lymphocyte could distinguish TBPE patients from other types of PE with the AUC (area under the curve) value of 0.766 (95%CI: 0.655–0.877) (). The cut-off value was 15.71, with the sensitivity of 83.3% (95%CI: 65.5–93.7%) and specificity of 66.0% (95%CI: 50.6–78.7%). The PPV was 61.0% (95%CI: 44.5–75.4%), NPV was 86.1% (95%CI: 69.7–94.8%), PLR was 2.448 (95%CI: 1.594–3.759), and NLR was 0.253 (95%CI: 0.111–0.575). The Youden index was 0.493 ().
Figure 1. The diagnostic value of blood T-SPOT.TB A + B/lymphocyte for TBPE. The AUC value was 0.766 (95%CI: 0.655–0.877), revealing that the ratio of blood T-SPOT.TB A + B to lymphocyte could distinguish TBPE from other types of PE.
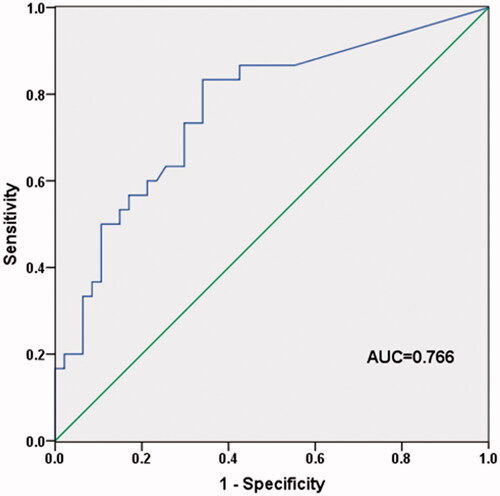
Table 6. Diagnostic performance of T-SPOT.TB a + B/lymphocyte for tuberculous pleural effusion.
Discussion
The differential diagnosis is critical for treatment and clinical outcomes of patients with PE. The common causes for PE include TB, malignancy, pneumonia, etc. In our study, a total of 77 adult patients with PE were included. 30 patients were confirmed with TBPE, accounting for 38.96% PE cases, and the percentage was in line with the reported data [Citation6]. MPE was observed in 24.67% PE cases, empyema cases accounted for 7.79%, 12.99% patients were diagnosed with PPE and the miscellaneous causes contributed to 15.58% PE in our study. We compared the clinical characteristics among the five groups. Analysis results demonstrated that the five groups exhibited significant differences in age, M. tuberculosis DNA detection, ADA activity, pleural fluid total protein, glucose and LDH, blood protein, T-SPOT.TB, as well as the ratios of LDH and protein between pleural fluid and blood samples. The patients with TBPE had the youngest age, higher ADA activity, and highest T-SPOT.TB.
Pleural fluid M. tuberculosis DNA detection is an effective method for TBPE diagnosis based on PCR method, which could provide direct evidence for M. tuberculosis infection. In our study, only 10 patients showed positive results in pleural fluid M. tuberculosis DNA detection, and all of them were TBPE patients. The diagnosis sensitivity was 33.3%, specificity was 100%, PPV was 100%, NPV was 70.%, NLR was 0.667 and the Youden index was 0.333. Porcel et al. had reported that the diagnostic sensitivity and specificity of M. tuberculosis DNA detection for TBPE diagnosis was 15% and 100%, respectively [Citation19]. The related study carried out by Zhang et al. reported that the positive rate of pleural fluid DNA detection was only 21% [Citation23]. The low sensitivity might be attributed to the instability of the dissociative DNA samples in PE, compared to the genetic DNA samples isolated from the cultured M. tuberculosis [Citation24]. In addition, we compared the clinical parameters of TBPE according to their M. tuberculosis DNA detection results. The two groups showed significant differences in ADA activity and LDH distribution. The TBPE patients with positive M. tuberculosis DNA detection showed higher ADA activity and LDH level.
The pleural fluid ADA is considered as an effective biomarker for TBPE. In our study, the cut-off value was definite as 40 U/L [Citation25,Citation26]. The diagnostic sensitivity, specificity, PPV, NPV, PLR, NLR and Youden index were 50%, 78.7%, 60%, 71.1%, 2.35, 0.635 and 0.287, respectively. The diagnostic value accuracy of ADA was poor for TBPE. The cut-off value of 40 U/L might be not appropriate for early diagnosis of TBPE. The study constructed by Chang et al. reported that with the diagnostic cut-off value of 26.5 U/L, pleural fluid ADA could be used for TBPE prediction with the sensitivity of 57.3%, specificity of 93.2% and the accuracy of 91.9% [Citation27]. The cut-off value of pleural fluid ADA for TBPE diagnosis was controversial, and the further investigations were required.
LDH is an important indicator for PE management [Citation28]. The elevated pleural fluid LDH could be observed in various types of PE [Citation29]. However, there were 14 TBPE patients with LDH less than 500 U/L in DNA detection negative group. Additionally, among TBPE patients with ADA negative, there were 12 cases with LDH value less than 500 U/L, accounting for 80%. Based on these results, we hypothesized that LDH value might influence the diagnostic accuracy of M. tuberculosis DNA detection and ADA activity for TBPE. We estimated the diagnostic accuracy of M. tuberculosis DNA detection and ADA activity for TBPE in PE patients with LDH more than 500 U/L. The diagnostic accuracy of pleural fluid M. tuberculosis DNA detection was significantly enhanced. Moreover, the diagnostic value of M. tuberculosis DNA detection was superior to ADA activity.
T-SPOT.TB is a commonly used method for rapid TB detection, but it has limited diagnostic accuracy [Citation16,Citation17]. In our study, the TBPE group exhibited significantly highest level of T-SPOT.TB A and B values, and lowest blood lymphocyte level, in comparison with other types of PE. The phenomenon might reveal the diagnostic capacity of T-SPOT.TB and lymphocyte in our study population. We estimated the diagnostic value of the ratio of T-SPOT.TB A + B to lymphocyte for TBPE diagnosis. ROC curve demonstrated that T-SPOT.TB A + B/lymphocyte could distinguish TBPE patients from other types of PE with the AUC value of 0.766. The cut-off value was 15.71, with the sensitivity of 83.3% and specificity of 66.0%. The diagnostic sensitivity of T-SPOT.TB A + B/lymphocyte was highest among all the three detected indicators in our study. The positive results of T-SPOT.TB A + B/lymphocyte might reveal the occurrence of TBPE, and antituberculous therapy was necessary. This might be the first study to explore the diagnostic value of the ratio of T-SPOT.TB A + B to lymphocyte for TBPE. However, due to the relatively small sample size, the results obtained in our study required further verification.
In conclusion, the diagnostic accuracy of pleural fluid M. tuberculosis DNA detection is superior to ADA activity for TBPE, especially among PE cases with pleural fluid LDH more than 500 U/L. The ratio of T-SPOT.TB A + B to lymphocyte may be a potential indicator for definite diagnosis of TBPE, with high sensitivity.
Acknowledgements
The authors wished to thank Guoqiang Cao for their assistance in microbiological-biochemical and molecular analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Fisk M, Branley H. Pleural effusion. Br J Hosp Med (Lond). 2013;74:C50–54.
- Light RW. Pleural effusions. Med Clin North Am. 2011;95:1055–1070.
- Zumla A, Chakaya J, Centis R, et al. Tuberculosis treatment and management–an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir Med. 2015;3:220–234.
- Glaziou P, Floyd K, Raviglione MC. Global epidemiology of tuberculosis. Semin Respir Crit Care Med. 2018;39:271–285.
- Villena Garrido V, Cases Viedma E, Fernandez Villar A, et al. Recommendations of diagnosis and treatment of pleural effusion. Update. Arch Bronconeumol. 2014;50:235–249.
- Wang XJ, Yang Y, Wang Z, et al. Efficacy and safety of diagnostic thoracoscopy in undiagnosed pleural effusions. Respiration. 2015;90:251–255.
- Zhai K, Lu Y, Shi HZ. Tuberculous pleural effusion. J Thorac Dis. 2016;8:E486–E494.
- Steingart KR, Flores LL, Dendukuri N, et al. Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med. 2011;8:e1001062.
- Wong CF. Early diagnosis of tuberculous pleural effusion: apart from pleural fluid adenosine deaminase, pleural biopsy still has a role. Hong Kong Med J. 2018;24:316–317.
- Liu F, Gao M, Zhang X, et al. Interferon-gamma release assay performance of pleural fluid and peripheral blood in pleural tuberculosis. PLoS One. 2013;8:e83857.
- Vorster MJ, Allwood BW, Diacon AH, et al. Tuberculous pleural effusions: advances and controversies. J Thorac Dis. 2015;7:981–991.
- Khow-Ean N, Booraphun S, Aekphachaisawat N, et al. Adenosine deaminase activity level as a tool for diagnosing tuberculous pleural effusion. Southeast Asian J Trop Med Public Health. 2013;44:655–659.
- Islam A, Hossain MA, Paul SK, et al. Role of adenosine deaminase in diagnosis of tubercular pleural effusion. Mymensingh Med J. 2014;23:24–27.
- Ates G, Yildiz T, Ortakoylu MG, et al. Adapted T cell interferon-gamma release assay for the diagnosis of pleural tuberculosis. Respiration. 2011;82:351–357.
- Fan L, Chen Z, Hao XH, et al. Interferon-gamma release assays for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. FEMS Immunol Med Microbiol. 2012;65:456–466.
- Aggarwal AN, Agarwal R, Gupta D, et al. Interferon gamma release assays for diagnosis of pleural tuberculosis: a systematic review and meta-analysis. J Clin Microbiol. 2015;53:2451–2459.
- Mollo B, Jouveshomme S, Philippart F, et al. Biological markers in the diagnosis of tuberculous pleural effusion. Ann Biol Clin (Paris). 2017;75:19–27.
- Tang TH, Ahmed SA, Musa M, et al. Rapid detection of Mycobacterium tuberculosis in clinical samples by multiplex polymerase chain reaction (mPCR). World J Microbiol Biotechnol. 2013;29:2389–2395.
- Porcel JM, Palma R, Valdes L, et al. Xpert® MTB/RIF in pleural fluid for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2013;17:1217–1219.
- Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy. Am J Respir Crit Care Med. 2001; 163:1730–1754.
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
- Losi M, Bossink A, Codecasa L, et al. Use of a T-cell interferon-gamma release assay for the diagnosis of tuberculous pleurisy. Eur Respir J. 2007;30:1173–1179.
- Shou J, Xie QM, Long ZL, et al. Diagnostic value of cell free Mycobacterium tuberculosis DNA in effusion pleural for detection of tuberculosis. Zhonghua Bing Li Xue Za Zhi. 2018;47:465–467.
- Che N, Yang X, Liu Z, et al. Rapid detection of cell-free Mycobacterium tuberculosis DNA in Tuberculous pleural effusion. J Clin Microbiol. 2017;55:1526–1532.
- Light RW. Update on tuberculous pleural effusion. Respirology. 2010;15:451–458.
- Lee J, Lee YD, Lim JK, et al. Predictive factors and treatment outcomes of tuberculous pleural effusion in patients with cancer and pleural effusion. Am J Med Sci. 2017;354:125–130.
- Chang KC, Chan MC, Leung WM, et al. Optimising the utility of pleural fluid adenosine deaminase for the diagnosis of adult tuberculous pleural effusion in Hong Kong. Hong Kong Med J. 2018;24:38–47.
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–513.
- Wang J, Liu J, Xie X, et al. The pleural fluid lactate dehydrogenase/adenosine deaminase ratio differentiates between tuberculous and parapneumonic pleural effusions. BMC Pulm Med. 2017;17:168.
