Abstract
The combination of composite nerve materials prepared using degradable polymer materials with biological or physical factors has received extensive attention as a means to treat nerve injuries. This study focused on the potential application of graphene oxide (GO) composite conductive materials combined with electrical stimulation (ES) in nerve repair. A conductive poly(L-lactic-co-glycolic acid) (PLGA)/GO composite membrane was prepared, and its properties were tested using a scanning electron microscope (SEM), a contact angle meter, and a mechanical tester. Next, neural stem cells (NSCs) were planted on the PLGA/GO conductive composite membrane and ES was applied. NSC proliferation and differentiation and neurite elongation were observed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, immunofluorescence, and PCR, respectively. The results showed that the PLGA/GO membrane had good hydrophilicity, mechanical strength, and protein adsorption. ES combined with the PLGA/GO membrane significantly promoted NSC proliferation and neuronal differentiation on the material surface and promoted significant neurite elongation. Our results suggest that ES combined with GO-related conductive composite materials can be used as a new therapeutic combination to treat nerve injuries.
Introduction
Treatment for central nervous system injuries and subsequent rehabilitation remain challenging worldwide. Achieving satisfactory results from traditional surgery or hormone shock is difficult [Citation1]. Therefore, more effective therapeutic methods are urgently needed. Electrical stimulation (ES) promotes the repair and regeneration of various tissues, including bone, nerve and epidermis [Citation2–4]. A recent paper in Nature revealed that ES could even restore motor function in patients with lower limb paralysis after spinal cord injury, further confirming the great potential of ES for application in repairing nerves [Citation5]. ES can regulate the body’s potential as well as physiological functions, including molecular transport, signal transduction and embryonic development, through various ion channels. ES also promotes physiological activities, such as the proliferation, migration and differentiation of various cell lines [Citation6–8]. ES can also increase neuronal axonal elongation and growth [Citation9,Citation10]. With the rapid development of tissue engineering in the field of nerve repair and regeneration, adjusting the physical and chemical properties of biomaterials to enable new functional properties has become a research priority. Among biomaterials, conductive polymer materials are of interest because they can simulate electronic or ionic conductivity and mediate the stimulation of electrical currents to regulate cell and tissue growth. Applying ES combined with composite biomaterials that have excellent biocompatibility and conductivity will further enhance ES’s biological effects and a new combination of ES with physical intervention has great potential in tissue engineering, particularly for nerve repair.
Many conductive materials, such as polypyrrole, polyaniline and graphene oxide (GO), are widely used in nerve regeneration and have achieved satisfactory results [Citation11,Citation12]. Among them, GO is a special single-layer two-dimensional lattice composed of sp2 and sp3 hybrid carbon atoms produced by graphene oxidation. GO has extraordinary conductivity, very high chemical stability, and excellent biocompatibility. Graphene and its derivatives can effectively promote the adhesion, proliferation, and differentiation of various cells, including embryonic, pluripotent, and mesenchymal stem cells [Citation13,Citation14]. In addition, GO can induce the differentiation of NSCs and embryonic stem cells into neurons and promote neuronal axonal differentiation and growth [Citation15,Citation16]. Therefore, GO has many potential uses in nerve repair, and as an excellent conductive carrier, GO combined with ES may further enhance the growth and neuronal differentiation of NSCs growing on its surface.
Thus, this study synthesized the electrically active poly(L-lactic-co-glycolic acid) (PLGA)/GO nanomaterial. The PLGA/GO composite material’s ultrastructure, hydrophilicity, and mechanical strength were detected using a scanning electron microscope (SEM), a contact angle meter and a mechanical tester. Next, the protein adsorption of the material was measured using the bovine serum albumin (BSA) model protein. Finally, the PLGA/GO membrane’s effect on NSC proliferation and growth and neurite elongation under ES conditions was systematically studied to further explore the potential application and feasibility of ES combined with conductive polymer composite materials in nerve repair.
Materials and methods
Manufacture of the PLGA/GO membrane
GO and PLGA were mixed to prepare the electrically active PLGA/GO nanocomposite membrane using the solution evaporation method. First, GO and PLGA were dissolved in chloroform. The weight percentages (wt%) of GO were 0.1 wt%, 1 wt% and 5 wt%. These solutions were magnetically stirred and sonicated until fully mixed. Some of the solutions were plated onto siliconized glass slides for subsequent cell experiments, while the remainder was plated onto Petri dishes to be air-dried for subsequent characterization. The obtained PLGA/GO membrane was vacuum-dried for 48 h for later use.
Characterization
Scanning electron microscopy (SEM, XL30 ESEM-FEG, FEI) was used to observe the surface morphology of the membrane. X-ray diffraction (XRD, D8 ADVANCE, Bruker, Germany) was used to determine the elemental composition. To analyze the surface hydrophobicity, the static water contact angle was measured to evaluate the surface wettability of the membrane using the sessile drop method on a contact angle system (VCA 2000, AST, Bellerica, USA). The mechanical properties of the matrices were tested by a universal mechanical testing machine (Instron 1121, High Wycombe, UK).
Adsorption efficiency of protein
BSA was chosen as a model protein to evaluate the adsorption efficiency of protein on the composite membrane. Briefly, the membrane was first exposed to 10 ml of BSA solution (2 mg/mL) by means of a rotator at 150 rpm for 2 h. The amounts of BSA loaded on the membrane were determined through the concentration and reduction of BSA within the samples using a BCA protein assay.
Neural stem cell cultures
Neural stem cells (NSCs) were obtained from the cerebral cortex of embryonic mice (E13-14). The dissociated cells were cultured in growth medium at a density of 50,000 cells/cm2 and incubated at 37 °C in a 5% CO2 humidified incubator. The growth medium contained neurobasal medium (GIBCO/Invitrogen, USA), B27 neural supplement (GIBCO/Invitrogen, USA), 100 ng/mL penicillin-streptomycin (Gibco-Invitrogen, USA), 20 ng/mL epidermal growth factor (EGF, Sigma, USA) and 20 ng/mL basic fibroblast growth factor (bFGF, Sigma). NSCs were passaged once and the average diameter of the neurospheres was greater than 200 μm.
Cell proliferation assay
To determine the optimal ES parameter and PLGA/GO weight ratio for the subsequent experiments, an MTT assay was performed to evaluate NSC proliferation. First, a pair of L-shaped platinum electrodes separated by 10 mm were submerged in 70% ethanol for 10 min, washed with PBS 3 times, and exposed to UV light for 3 h. Then, the electrodes were placed in 24-well culture plates to produce an electric field. A function signal generator (Suing, Guangdong, China) was connected to the electrodes to create a signal source. The digital oscilloscope of the signal generator was used to monitor output signals. NSCs were exposed to 100 mV of ES for 1 h/day. The ES frequency was programmed at 20, 100, and 500 Hz with a stable 50% duty cycle and rectangular pulses. NSCs with a density of 20,000 per well were cultured on the surface of poly-D-lysine-pretreated slides for 1 and 3 days and then assessed by the (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich, St. Louis, USA). In brief, the MTT stock solution was added to each well and the cells were incubated at 37 °C for 4 h. Then, acidified isopropanol was added to each well. The optical density (OD) was measured at 540 nm on a full-wavelength microplate reader (Infinite M200, TECAN, AUSTRIA). In addition, to investigate the suitable wt% of GO in the composite membrane, NSCs were also cultured on PLGA/GO membrane (0, 0.1%, 1%, 5% wt%) without ES for 1 and 3 days followed by the MTT assay. After the optimal ES parameter and wt% of GO were both confirmed, the membranes were divided into four groups: the PLGA group, PLAG/GO group, PLGA + ES group and PLGA/GO + ES group. The proliferation of NSCs in the different groups under ES was finally measured at 1, 3, and 7 d by the MTT assay.
Immunofluorescence staining
NSCs cultured for 7 days under differentiation conditions were fixed with 4% paraformaldehyde in PBS for 30 min. Differentiation of NSCs was induced by foetal bovine serum (FBS, Life Technologies, Waltham, USA) in the defined medium. Then, the cells were treated with 0.1% Triton X-100 (Sigma) for 10 min and blocked with 10% goat serum (Sigma) for 2 h. The primary antibodies were then added and the samples incubated overnight at 4 °C. Afterward, the samples were washed with PBS three times and incubated with the secondary antibodies for 2 h at room temperature, followed by 4′,6-diamidino-2-phenylindole (DAPI) staining. The primary antibodies included Tuj-1 as a marker for neurons (1:400 dilution, Abcam, Cambridge, UK) and GFAP as a marker for astrocytes (1:500 dilution, Abcam). Photos were taken under a confocal laser scanning microscope (LSM 780, ZEISS, Cambridge, UK). Then, we chose 30 differentiated neurons (Tuj-1 positive cells) randomly to measure the neurite length as previously described [Citation17]. If the longest neurite of the chosen cell extended beyond the image border, another new image was taken to measure the entire length of the process. Finally, Image-Pro Plus software (MediaCybernatics LP, Maryland, USA) was used to analyze the neurite length.
Quantitative real-time PCR analysis
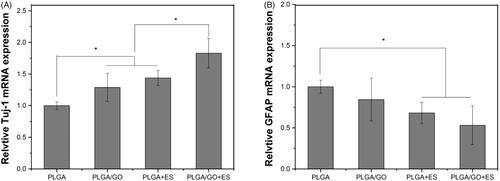
To further quantitatively analyze the expression of the target genes of Tuj-1 and GFAP, we performed real-time polymerase chain reaction (RT-PCR). The total RNA concentration and purity were determined by a Nanodrop Assay (Tecan M200). RNA samples were reverse-transcribed into cDNA for qPCR using the PrimeScriptTM Reagent Kit with gDNA Eraser (Takara Bio, Kusatsu, Japan). The primer sequences for the target genes are listed in . The PCR amplification was performed as follows: initial heating at 95 °C for 10 min, followed by 40 cycles at 95 °C for 30 s, 58 °C for 60 s and 72 °C for 60 s. Each gene expression value was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene. The results are reported as the relative gene expression.
Table 1. List of genes and primer nucleotide sequences.
Statistical analysis
All quantitative data were analyzed with GraphPad Prism 5.0 (SanDiego, CA, USA) and presented as the mean ± standard deviation. Multiple group comparisons were performed using one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test. Two-group comparisons were tested by Student’s t-test. A value of p < .05 was considered to be significant.
Results and discussion
Characterization of PLGA/GO membrane
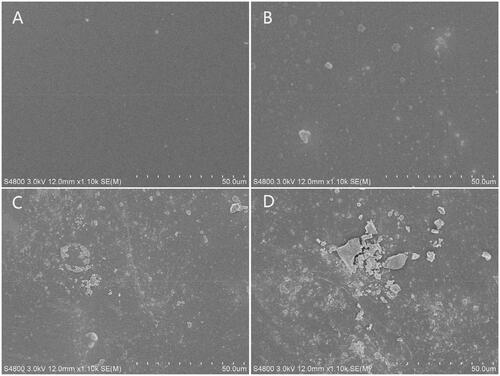
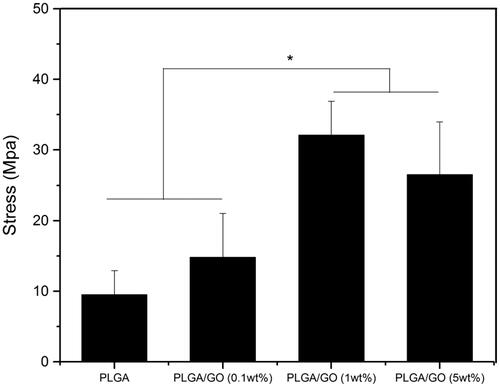
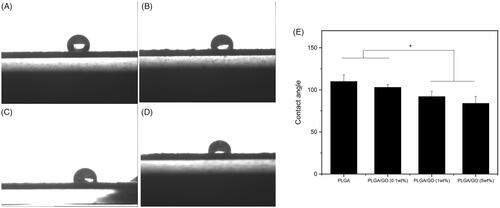
To confirm the optimal wt% of GO in the composite material, PLGA/GO membranes containing 0.1, 1, and 5 wt% GO were prepared. In addition, the materials’ ultrastructure, hydrophilicity, and mechanical strength were characterized. shows the SEM results, which indicated that the PLGA membrane surface was smooth with no obvious folds (). After the addition of GO, the PLGA/GO membrane surface became rough and uneven. In addition, as the wt% of GO increased, fold-like bumps on the membrane surface also increased and some areas had bulk bulges due to massive GO enrichment in these areas (). A rough surface is generally more favourable for protein attachment and cell adhesion [Citation18,Citation19]. Therefore, the roughness of the PLGA/GO membrane surface is beneficial for cell growth on it. Good mechanical properties can maintain the stability and extension of nerve tissue scaffolds. Our results indicated that the tensile strengths of the PLGA membrane and PLGA/GO (0.1 wt%) membrane did not significantly differ, while high tensile strength was achieved by the PLGA/GO (1 wt%) membrane and PLGA/GO (5 wt%) membrane, which was significantly higher than that of the other 2 cases (p < .05, . This increase might be attributed to the interfacial interaction between the oxygen-containing functional part of the GO and the hydroxyl or amino groups of the substrate, thus improving the surface interaction between the GO and the polymer materials [Citation20]. Finally, we evaluated the material’s hydrophilicity in terms of the contact angle (). The results showed that the hydrophilicity of PLGA/GO was significantly lower than that of PLGA and tended to decrease as the GO wt% increased, also suggesting a positive correlation between the material’s surface hydrophilicity and its GO content. The material’s increased hydrophilicity benefits cell adhesion and proliferation on its surface [Citation21,Citation22].
Protein adsorption
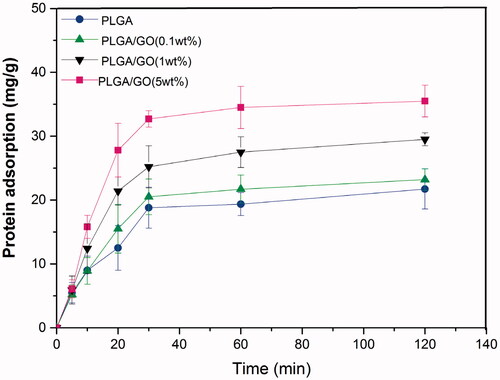
BSA was used as a model protein to measure the material’s protein adsorption ability. As shown in the figure, the BSA adsorption levels of the PLGA/GO (1 wt%) and PLGA/GO (5 wt%) groups after 2 h of incubation were much higher than those of the other 2 groups (p < .05, ), whereas the PLGA and PLGA/GO (0.1 wt%) groups did not significantly differ. Furthermore, the protein adsorption level of the PLGA/GO (5 wt%) group was the highest among all groups. Our results indicated that the addition of GO was conducive to protein adsorption on the material. GO physically binds to proteins through π-electron clouds, H-bonding, and electrostatic attraction [Citation23,Citation24]. Combining these results with our previous results, we speculate that GO increased the material’s surface roughness and enhanced its hydrophilicity, both of which were conducive to BSA adhesion on the surface. We believe that this physical bonding provides maximum protection to the protein activity and is conducive to bonding with active proteins, such as growth factors, to further increase the biological activity of the conductive polymer materials.
Cell proliferation
To confirm the appropriate ES frequency and GO wt% in the polymer for subsequent studies, the proliferation of NSCs growing on the material surface was analyzed using MTT, as shown in . The results indicated that after 1 day of providing ES to the NSCs, the ES (100 Hz) group did not significantly differ from the ES (500 Hz) group, but the results for both groups were significantly higher than those of the control group. After 3 days, the result for the ES (100 Hz) group was significantly higher than the results for the other 3 groups (p < .05, ), suggesting that the proliferation effect of the NSCs was the most significant at an ES frequency of 100 Hz. Therefore, 100 Hz was selected as the frequency parameter for subsequent ES. Currently, the mechanism underlying the promotion of NSC proliferation by ES remains unclear. ES can promote the migration and attachment of cell-matrix proteins on the material surface, thus facilitating cell adhesion [Citation2]. The results showed that the adhesion and proliferation rates of stem cells could be improved by increasing the roughness of the membrane surface. In addition, ES can up-regulate DNA polymerase-related proteins, such as proliferating cell nuclear antigen (PCNA), and promote proliferation signal transduction-related extracellular signal-regulated kinases, which may also be associated with the proliferation promotion of ES for NSCs [Citation25].
Figure 5. NSC proliferation assay. (A) Proliferation of NSCs cultured under ES at different frequencies for 1 and 3 days. (B) Proliferation of NSCs cultured on the PLGA and PLGA/GO membranes (0.1 wt%, 1 wt%, 5 wt% GO) for 1 and 3 days. (C) Proliferation of NSCs cultured on the PLGA and PLGA/GO membrane with or without ES treatment for 1, 3, or 7 days. (∗, p < .05, n = 3).
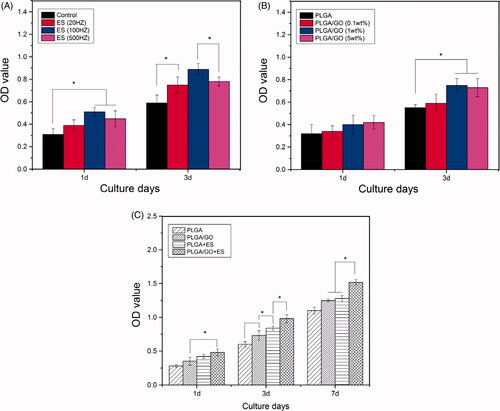
Next, we planted NSCs on the surfaces of composite materials with different GO wt% and the results are shown in . The results indicated that although the OD value in the PLGA/GO (5 wt%) group after 1 d was slightly higher than that in the other 3 groups, the 4 groups did not significantly differ (p>.05, ). The OD values in the PLGA/GO (1 wt%) and PLGA/GO (5 wt%) groups did not significantly differ after 3 d, but these values were significantly higher than those in the PLGA group. Therefore, the PLGA/GO membrane containing 5 wt% GO was selected for subsequent studies. Our previous results showed that the hydrophilicity of PLGA/GO was enhanced as the GO wt% increased and materials with better hydrophilicity favoured cell adhesion and growth. In addition, GO adsorbed many proteinaceous substances and even cells through its chemical groups and electrostatic attraction, which was also conducive to cell adhesion and growth [Citation26,Citation27].
However, we found that the proliferation effect on the NSCs on the PLGA/GO membrane did not continuously increase as the GO content increased. NSC growth did not differ significantly between the PLGA/GO membranes with 5 and 1% wt% GO. Our previous results indicated that the roughness and hydrophilicity of the PLGA/GO (5 wt%) groups were both higher than those of the PLGA/GO (1 wt% group), suggesting that cells were more likely to adhere in the PLGA/GO (5 wt%) group. Cell adhesion is the primary condition for cell proliferation and is directly associated with cell proliferation. However, the proliferation effect in the PLGA/GO (5 wt%) group was not significant compared with that in the PLGA/GO (1 wt%) group, which, as our analysis showed, might be associated with the cytotoxicity of GO. Although GO has reliable biocompatibility and has been extensively applied in biological experiments, high GO concentrations could also lead to cytotoxicity. GO can bind to cell membrane receptors to interfere with normal cellular metabolism and nutritional uptake to further induce cell death [Citation28]. In addition, the exposure of cells to excessive GO can induce reactive oxygen species (ROS) production to cause oxidative stress injury. Oxidative stress can induce the injury of cellular DNA and mitochondria to cause cell apoptosis/death [Citation29,Citation30]. Furthermore, GO can also interact with genes that encode key enzymes and proteins associated with cell growth to influence cell activity [Citation31]. Therefore, the higher hydrophilicity and rough surface area of the PLGA/GO (5 wt%) might be accompanied by stronger cytotoxicity. Consequently, in contrast to the increased promotion of NSC proliferation as the GO wt% increased from 0.1 to 1%, the promotion of NSC proliferation by the PLGA/GO membrane was not significantly increased as the GO wt% increased from 1 to 5%.
To investigate the effects of the single and combined use of the material and ES on NSC proliferation, the PLGA, PLGA/GO, PLGA + ES, and PLGA/GO + ES groups were established. NSC proliferation was determined after 1, 3, and 7 d. The results revealed no significant differences between the PLGA/GO and PLGA + ES groups after 1 d and 7 d. After 3 d and 7 d, the results for these two groups were significantly higher than those for the PLGA group. In addition, the OD values for the PLGA/GO + ES group at all culture time points were the highest among the four groups at 7 d (p < .05, ), indicating that ES can be combined with the PLGA/GO conductive composite material to further enhance NSC proliferation on the material surface.
Neurite growth
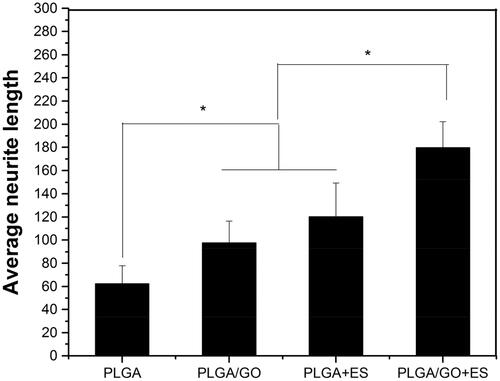
To investigate the effect of ES combined with PLGA/GO on neurite growth, the neurite length was quantitatively analyzed after NSC differentiation using immunofluorescence. As shown in and , Tuj-1-positive neuronal cells were selected and the average neurite length of selected neurons were measured. The average neurite length result was (62.4 ± 15.4 μm) in the PLGA group was significantly shorter than that in the other 3 groups (p < .05, ). The neurite lengths in the PLGA/GO group (97.5 ± 18.9 μm), PLGA + ES group (137 ± 13.2 μm), and PLGA/GO + ES group (179.8 ± 24.5 μm) showed an increasing trend and the pairwise comparison results showed significant differences. The immunofluorescence of NSC differentiation into GFAP-positive astrocyteswere shown in . ES can change the local electric fields of extracellular matrix (ECM) proteins to adsorb proteins (such as adhesion proteins) closely associated with neurite growth on the material surface to promote neurite elongation after NSC differentiation [Citation32]. Furthermore, ES can significantly change the cell membrane potential and induce cell membrane depolarization [Citation33]. Cell membrane depolarization assembles actin filaments in growth cones to promote neurite growth. GO’s promotion of neurite elongation after differentiation might be due to GO acting on focal adhesion kinase (FAK) and p38 mitogen-activated protein kinase (MAPK) to cascade and mediate the Rack1- and Cdk5-related cellular pathways and further up-regulate the expression of proteins associated with neurite growth, such as NFL, thus eventually promoting elongation [Citation34]. Recent studies also examined the neurite lengths of PC12 cells on GO composite materials and speculated that GO conductance and surface chemistry were associated with its promotion of neurite growth [Citation35].
Figure 6. Immunofluorescence staining of neurons derived from NSC differentiation. The Tuj-1-positive cells are shown in red, while the cell nuclei, counterstained with DAPI, are shown in blue. All scale bar lengths are 100 μm.
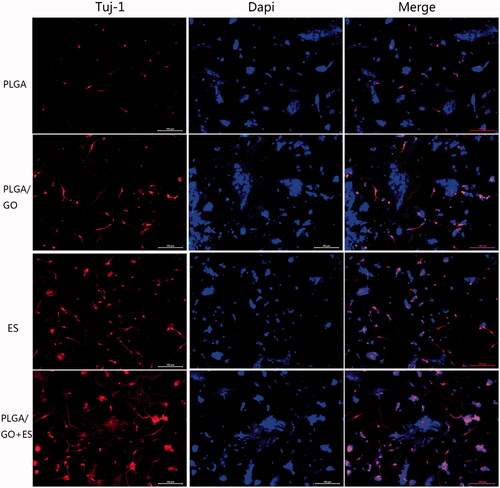
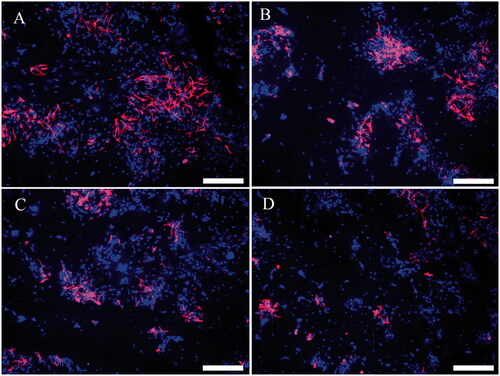
Figure 8. Immunofluorescence of NSC differentiation into astrocytes. Differentiated astrocytes in the PLGA group (A), PLGA/GO group (B), PLGA + ES group (C), and PLGA/GO + ES group (D). Immunostaining markers are GFAP (red) for neurons and DAPI (blue) for nuclei. The scale bar was 200 μ.
Our results also indicated that although ES and PLGA/GO both promote neurite elongation, the effect of the ES was significantly stronger than that of the PLGA/GO. When ES acted on the PLGA/GO conductive composite material, this elongation promotion was further enhanced. Thus, the interaction between the ES and the conductive materials rich in electric charges may have further increased the material surface’s electrical activity and further activated the conductive materials’ mechanism for promoting neurite elongation.
Cell differentiation
To further investigate the effect of ES combined with PLGA/GO on NSC differentiation, we performed quantitative and qualitative analyses using PCR and immunofluorescence. As shown in , the PCR results indicated that both the PLGA/GO and PLGA + ES groups significantly promoted NSC differentiation into neurons and, to some extent, inhibited NSC differentiation into astrocytes, although the two groups were not significantly different. The Tuj-1 protein expression level was the highest in the PLGA/GO + ES group, while the GFAP expression level did not differ significantly from that in the PLGA + ES group and was significantly lower than that in the PLGA and PLGA/GO groups. These results suggested that the combined application of PLGA/GO and ES maximally induced NSC differentiation into neurons and inhibited NSC differentiation into astrocytes. The immunofluorescence results indicated that NSCs on the surfaces of different materials successfully differentiated into neurons and astrocytes. In addition, changes in the numbers of positive cells from our immunofluorescence results were generally consistent with the PCR results. Our results first confirm that ES and GO independently promote NSC differentiation into neurons, which is consistent with results in the literature [Citation36,Citation37]. The GO-chitosan nanocomposite material can enhance osteogenic and neuronal differentiation of human mesenchymal stem cells (hMSCs), possibly because the nanocomposite material’s unique asymmetric nanomorphology increases cellular contact and communication as well as interactions between the cells and the substrate [Citation38]. In addition, poly(ε-caprolactone) (PCL)/GO hybrid nanofibre scaffolds partially promote neuronal differentiation and significantly inhibits astrocyte differentiation [Citation39]. This study confirmed that the PLGA/GO composite material significantly promoted NSC differentiation into neurons; however, the inhibitory effect on astrocytes was not significant. When ES was combined with PLGA/GO, the NSCs exhibited clearer neuronal differentiation and the astrocytes were significantly reduced. Many biomaterial characteristics, such as flexibility, size, surface morphology, and physical and chemical properties, influence stem cell differentiation. This study confirmed that PLGA/GO can be used as a conductive material to effectively promote NSC differentiation and showed better NSC differentiation effects after being combined with ES. Controlling the fate of NSCs is a great challenge in repairing nerves. Previous studies have typically used chemical inducers and growth factors, such as soluble proteins and ECM molecules [Citation40], to promote neuronal stem cell differentiation. This study obtained satisfactory results by combining ES and the PLGA/GO conductive material. This novel combination will provide new ideas for future nerve tissue repair.
Figure 9. Quantitative real-time PCR analysis of the gene expression of Tuj-1 (A) and GFAP (B) after NSCs were cultured for 7 days. (∗, p < .05, n = 3).
The application of graphene and its derivative-based composite biomaterials in the central and peripheral nervous system has received increasing attention. In addition to exhibiting well-recognized excellent physicochemical properties, conductivity and biocompatibility, graphene-based biomaterials can not only promote the proliferation of NSCs and differentiate into neurons [Citation41] but also effectively facilitate Schwann cell adhesion and proliferation in vitro [Citation13]. Several studies in vivo not only showed that graphene-based scaffolds can effectively reduce local inflammation, enhance angiogenesis and axonal regeneration after implantation in the spinal cord injury site but also proved that graphene-based scaffolds can promote axonal regeneration and remyelination to treat peripheral nerve injury [Citation13,Citation42]. Moreover, the manufacture of graphene-based composite scaffolds is flexible. In addition, pure GO scaffolds [Citation43], PLGA/GO composite nanofibres [Citation41], and graphene coating films [Citation39] can all perform the specific biological functions of graphene. Therefore, we believe that graphene-based material has great potential for nerve repair. The present study further confirmed that the combination of ES- and GO-based biomaterials can effectively promote NSC proliferation and neuronal differentiation. Since ES has already been widely used in the clinic, the potential clinical application of this novel combination also deserves more attention.
Conclusion
Although studies in related fields have mainly focused on nerve repair using biomaterials carrying growth factors or stem cells, this study confirmed that adding the conductive polymer material GO to a composite material gave it greater hydrophilicity and mechanical strength. ES and PLGA/GO composite membranes can be used alone to promote proliferation, differentiation, and neurite elongation in NSCs. Combining these two factors to treat nerve damage may further promote nerve regeneration. Nevertheless, this study had some limitations. First, this study used in vitro cell experiments for validation; thus, further in vivo animal experiments are required. Next, we did not perform further in-depth investigations into the mechanism underlying the promotion of NSC differentiation into neurons by ES and conductive polymer materials. However, our results still suggest that this simple and safe physical intervention using ES with conductive composite materials containing GO will become a new combination of nerve repair treatments. ES has been extensively applied in clinical practice for rehabilitation therapy after nerve injury, thus further increasing the clinical application potential of ES + GO conductive materials.
supplymentary_data.doc
Download ()Disclosure statement
The authors declare no competing financial interests and non-financial interests.
Additional information
Funding
References
- Ahuja CS, Nori S, Tetreault L, et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80: S9–S22.
- Qi Z, Xia P, Pan S, et al. Combined treatment with electrical stimulation and insulin-like growth factor-1 promotes bone regeneration in vitro. PLoS One. 2018;13:e0197006.
- James ND, McMahon SB, Field-Fote EC, et al. Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol. 2018;17:905–917.
- Feldman DS. The feasibility of using pulsatile electromagnetic fields (PEMFs) to enhance the regenerative ability of dermal biomaterial scaffolds. J Funct Biomater. 2018;9:66.
- Wagner FB, Mignardot JB, Le Goff-Mignardot CG, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71.
- Huang Y, Li Y, Chen J, et al. Electrical stimulation elicits neural stem cells activation: new perspectives in CNS repair. Front Hum Neurosci. 2015;9:586.
- Rajabi AH, Jaffe M, Arinzeh TL. Piezoelectric materials for tissue regeneration: a review. Acta Biomater. 2015;24:12–23.
- Zhang Z, Klausen LH, Chen M, et al. Electroactive scaffolds for neurogenesis and myogenesis: graphene-based nanomaterials. Small. 2018;14:1801983.
- Senger JLB, Verge VMK, Macandili HSJ, et al. Electrical stimulation as a conditioning strategy for promoting and accelerating peripheral nerve regeneration. Exp Neurol. 2018;302:75–84.
- Goganau I, Sandner B, Weidner N, et al. Depolarization and electrical stimulation enhance in vitro and in vivo sensory axon growth after spinal cord injury. Exp Neurol. 2018;300:247–258.
- Palza H, Zapata PA, Angulo-Pineda C. Electroactive smart polymers for biomedical applications. Materials (Basel). 2019;12:277.
- Hitscherich P, Aphale A, Gordan R, et al. Electroactive graphene composite scaffolds for cardiac tissue engineering. J Biomed Mater Res. 2018;106:2923–2933.
- Wang J, Cheng Y, Chen L, et al. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019;84:98–113.
- Zhang Q, Esrafilzadeh D, Crook JM, et al. Electrical stimulation using conductive polymer polypyrrole counters reduced neurite outgrowth of primary prefrontal cortical neurons from NRG1-KO and DISC1-LI mice. Sci Rep. 2017;7:42525.
- Guo W, Qiu J, Liu J, et al. Graphene microfiber as a scaffold for regulation of neural stem cells differentiation. Sci Rep. 2017;7:5678.
- Kim TH, Lee KB, Choi JW. 3D graphene oxide-encapsulated gold nanoparticles to detect neural stem cell differentiation. Biomaterials. 2013;34:8660–8670.
- Jiang D, Du J, Zhang X, et al. miR-124 promotes the neuronal differentiation of mouse inner ear neural stem cells. Int J Mol Med. 2016;38:1367–1376.
- Yamashita D, Machigashira M, Miyamoto M, et al. Effect of surface roughness on initial responses of osteoblast-like cells on two types of zirconia. Dent Mater J. 2009;28:461–470.
- Zareidoost A, Yousefpour M, Ghaseme B, et al. The relationship of surface roughness and cell response of chemical surface modification of titanium. J Mater Sci Mater Med. 2012;23:1479–1488.
- Yuan M, Xiong C, Jiang L, et al. The preparation, characterization, mechanical and antibacterial properties of GO-ZnO nanocomposites with a poly(l-lactide)-modified surface. Materials (Basel). 2018;11:323.
- Wang P, Yu T, Lv Q, et al. Fabrication of hydroxyapatite/hydrophilic graphene composites and their modulation to cell behavior toward bone reconstruction engineering. Colloids Surf B Biointerfaces. 2019;173:512–520.
- Vlcek J, Lapcik L, Havrdova M, et al. Flow induced HeLa cell detachment kinetics show that oxygen-containing functional groups in graphene oxide are potent cell adhesion enhancers. Nanoscale. 2019;11:3222–3228.
- Lee WC, Lim CH, Shi H, et al. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5:7334–7341.
- Li H, Fierens K, Zhang Z, et al. Spontaneous protein adsorption on graphene oxide nanosheets allowing efficient intracellular vaccine protein delivery. ACS Appl Mater Interfaces. 2016;8:1147–1155.
- Hernandez-Bule ML, Paino CL, Trillo MA, et al. Electric stimulation at 448 kHz promotes proliferation of human mesenchymal stem cells. Cell Physiol Biochem. 2014;34:1741–1755.
- Patila M, Pavlidis IV, Kouloumpis A, et al. Graphene oxide derivatives with variable alkyl chain length and terminal functional groups as supports for stabilization of cytochrome c. Int J Biol Macromol. 2016;84:227–235.
- Shen H, Liu M, He H, et al. PEGylated graphene oxide-mediated protein delivery for cell function regulation. ACS Appl Mater Interfaces.2012;4:6317–6323.
- Guo X, Mei N. Assessment of the toxic potential of graphene family nanomaterials. J Food Drug Anal. 2014;22:105–115.
- Chong Y, Ma Y, Shen H, et al. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials 2014;35:5041–5048.
- Waiwijit U, Kandhavivorn W, Oonkhanond B, et al. Cytotoxicity assessment of MDA-MB-231 breast cancer cells on screen-printed graphene-carbon paste substrate. Colloids Surf B Biointerfaces. 2014;113:190–197.
- Chatterjee N, Eom HJ, Choi J. A systems toxicology approach to the surface functionality control of graphene-cell interactions. Biomaterials. 2014;35:1109–1127.
- Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials. 2001;22:1055–1064.
- Zou Y, Qin J, Huang Z, et al. Fabrication of aligned conducting PPy-PLLA fiber films and their electrically controlled guidance and orientation for neurites. ACS Appl Mater Interfaces. 2016;8:12576–12582.
- Lee JS, Lipatov A, Ha L, et al. Graphene substrate for inducing neurite outgrowth. Biochem Biophys Res Commun. 2015;460:267–273.
- Convertino D, Luin S, Marchetti L, et al. Peripheral neuron survival and outgrowth on graphene. Front Neurosci. 2018;12:1.
- Heo C, Yoo J, Lee S, et al. The control of neural cell-to-cell interactions through non-contact electrical field stimulation using graphene electrodes. Biomaterials. 2011;32:19–27.
- Park SY, Park J, Sim SH, et al. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv Mater Weinheim. 2011;23:H263–H267.
- Kenry, WC Lee, KP Loh, et al. When stem cells meet graphene: opportunities and challenges in regenerative medicine. Biomaterials. 2018;155:236–250.
- Shah S, Yin PT, Uehara TM, et al. Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv Mater. 2014;26:3673–3680.
- Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441.
- Pan S, Qi Z, Li Q, et al. Graphene oxide-PLGA hybrid nanofibres for the local delivery of IGF-1 and BDNF in spinal cord repair. Artif Cells Nanomed Biotechnol. 2019;47:651–664.
- Qian Y, Zhao X, Han Q, et al. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat Commun. 2018;9:323.
- Palejwala AH, Fridley JS, Mata JA, et al. Biocompatibility of reduced graphene oxide nanoscaffolds following acute spinal cord injury in rats. Surg Neurol Int. 2016;7:75.