Abstract
Hepatitis is a severe liver disease with worldwide distribution. This study explored the anti-inflammatory influence of Emodin on LPS-triggered liver injury in vitro and in vivo. In vitro, we discovered that LPS treatment triggered L-02 cell and primary hepatocyte inflammatory injury. Emodin weakened the LPS-triggered cell inflammatory injury and NF-κB pathway activation. Emodin alleviated LPS-stimulated decrease of miR-145 expression. Moreover, miR-145 participated in the regulation of pro-inflammatory factors expression and negatively regulated IRAK1. Besides, IRAK1 exert regulatory roles in the activation of NF-κB pathway. In vivo, we found that Emodin pre-treatment weakened the LPS-triggered increases of pro-inflammatory factors expression, up-regulations of AST and ALT level, liver cell apoptosis, reduction of miR-145 and enhancement of IRAK1. Our research verified that Emodin weakened LPS-triggered liver cell inflammatory injury in vitro and in vivo might be achieved by elevating miR-145, decreasing IRAK1 and then suppressing NF-κB pathway.
Introduction
Hepatitis is a severe liver disease with worldwide distribution [Citation1]. There are a lot of factors that take part in the occurrence of hepatitis, such as bacteria, viruses, drugs, alcohol, parasite, chemical poisons and autoimmune [Citation2–5]. The mainly pathological characteristics of hepatitis are hepatocyte injury, liver dysfunction and abnormal expressions of aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholinesterase (CHE), alkaline phosphatase (AP) and γ-glutamyl transpeptidase (GGTP) [Citation6,Citation7]. Despite the fate that medical therapy can improve the pathological features of a subset of patients with hepatitis [Citation8]. More effective therapeutic medicines and strategies are still needed.
Emodin (), an anthraquinone derivate separated from the rhizomes of Rhubarb (Rheum palmatum L.), has a wide range of therapeutic application [Citation9]. Ha et al. reported that Emodin suppressed pro-inflammatory response and inactivated histone deacetylase 1 in hypoxia rheumatoid arthritis [Citation10]. Alisi et al. indicated that Emodin prevented intrahepatic fat accumulation, inflammatory response and redox status imbalance in diet-stimulated hepatosteatosis in rats [Citation11]. Lee et al. proved that Emodin suppressed high mobility group 1 (HMGB1)-triggered inflammatory responses in vitro and in vivo [Citation12]. Moreover, Jia et al. presented that Emodin attenuated systemic and liver inflammatory response in hyperlipidemic mice stimulated by lipopolysaccharide (LPS) [Citation13]. These above findings imply that Emodin can exert anti-inflammatory influence and act as a potential therapeutic medicine in hepatitis therapy.
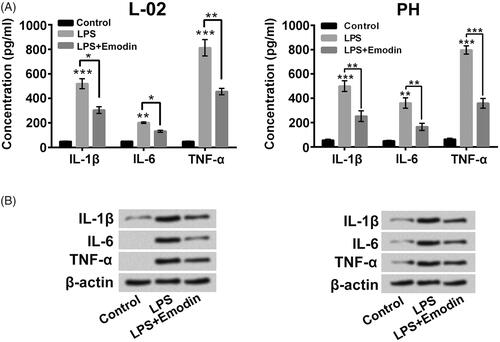
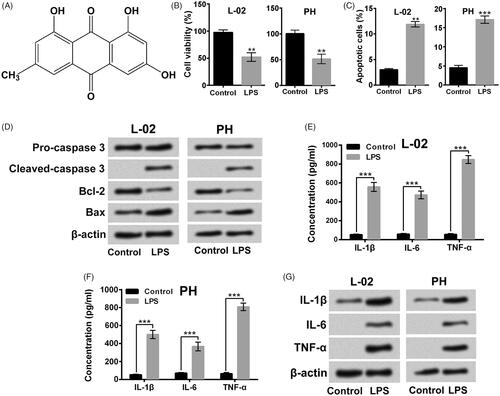
Figure 1. LPS triggered liver cell apoptosis and inflammatory injury. (A) Chemical skeleton structure of Emodin. Followed by 5 μg/ml LPS stimulation for 12 h, (B and C) viability and apoptosis of L-02 cells and primary hepatocytes (HP) were measured respectively, (D) the apoptosis-associated protein levels were tested, (E–G) the pro-inflammatory cytokines in culture supernatant and in cells were measured. **p < .01; ***p < .001.
MicroRNAs are small regulatory RNAs in eukaryotic cells, which were closely related to the modulation of multiple cellular functions and disease processes, including hepatitis [Citation14]. Bandopadhyay et al. reported that hepatitis B virus X protein (HBx) modulated microRNA-145 (miR-145) level in malignant hepatocytes [Citation15]. Gao et al. proved that silence of miR-145 triggered by HBx promoted CUL5 level and contributed to the occurrence and development of HBx-mediated hepatocellular carcinoma [Citation16].
LPS is a large molecule located in the outermost layer of Gram-negative bacteria [Citation17]. Numerous studies have demonstrated that LPS could trigger inflammatory reaction via boosting pro-inflammatory cytokines levels, such as interleukin 1-β (IL-1β), IL-6 and TNF-α [Citation17,Citation18]. Animal and cell hepatitis models triggered with LPS have been broadly used to seeking new anti-inflammatory drugs [Citation19,Citation20]. Furthermore, researches have proved that activation of NF-κB signalling pathway also contributed to the LPS-triggered inflammatory injury through entering nucleus to stimulate more inflammatory factors expressions [Citation21,Citation22].
In this research, we analyzed the anti-inflammatory influence of Emodin on LPS-triggered liver cell inflammatory injury in vitro and in vivo. The influence of Emodin on NF-κB pathway activation stimulated by LPS and the potential mechanisms involving in miR-145 were also tested.
Material and methods
Preparations of LPS and emodin solution
LPS and Emodin were both obtained from Sigma-Aldrich (catalogue numbers: L2630 and E7881, MO, USA). LPS was diluted in ultrapure water to 5 mg/ml. Emodin was diluted in Dimethyl Sulfoxide (DMSO, Sigma-Aldrich) to 20 mM. LPS and Emodin solutions were stored at −20 °C until use.
Cell culture and treatment
L-02 cells were received from China Center for Type Culture Collection, Wuhan University (Wuhan, China) and cultivated at 37 °C with 5% CO2 in RPMI-1640 medium including 10% fetal bovine serum (FBS, Gibco, CA, USA), 100 U/ml penicillin-100 mg/ml streptomycin solution (Gibco), 1 mM Glutamine (Sigma-Aldrich) and 2.5 mg/ml sodium bicarbonate (NaHCO3, Sigma-Aldrich).
Primary hepatocytes were isolated from 4 Wistar rats (2 female and 2 male, 10 weeks, 252.39–382.13 g, Experimental Animal Center of Shandong University, Jinan, China) after pentobarbital sodium anesthesia and cultivated in DMEM (Gibco) including 15% FBS and 100 U/ml penicillin-100 mg/ml streptomycin solution. All experiment processes were approved by Animal Care and Use Committee of Jining No.1 People’s Hospital in line with guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
L-02 cells and primary hepatocytes were respectively stimulated by LPS to establish cell inflammatory injury model [Citation23]. Emodin (5, 10, 15 or 20 μM) was mixed with culture medium for 24 h in the front of LPS stimulation.
Cell counting kit-8 (CCK-8) assay
CCK-8 assay offered a method to test the viability of L-02 cells and primary hepatocytes. Briefly, 1 × 104 cells were cultivated, in triplicate, in 96-well plate and stimulated by 5 μg/ml LPS and/or 5, 10, 15, 20 μM Emodin for 12 h. Then, CCK-8 solution (10 μl) was mixed into the culture medium. Followed by placing the cell plate at 37 °C for 1 h, the absorbance was tested using Micro-plate Reader at 450 nm (Molecular Device, CA, USA). Cell viability (%) was represented as the percentage of control.
Cell apoptosis assay
Annexin V-FITC/PI apoptosis detection kit (BD Bioscience, Franklin Lakes, NJ, USA) offered a method to measure L-02 cells and primary hepatocytes apoptosis. Briefly, after different exposure, the adherent and floating cells were harvested in line with the experimental design, rinsed with phosphate buffered saline (PBS), and mixed with 100 μl kit solution for 25 min at 37 °C in the dark. The percentage of apoptotic cells was measured by Beckman Coulter (CA, USA).
Enzyme-linked immunosorbent assay (ELISA)
Human IL-1β ELISA kit, human IL-6 ELISA kit, human TNF-α ELISA kit, rat IL-1β ELISA kit, rat IL-6 ELISA kit, rat TNF-α ELISA kit, mouse IL-1β ELISA kit, mouse IL-6 ELISA kit and mouse TNF-α ELISA kit (catalog numbers: RAB0273, RAB0306, RAB0340, RAB0277, RAB0311, RAB0479, RAB0274, RAB0308 and RAB0477, Sigma-Aldrich) were used to test IL-1β, IL-6 and TNF-α levels in culture supernatant of cells and serum, respectively.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNAs in cells and tissues were isolated using TRIzol™ Plus RNA Purification Kit (Invitrogen, CA, USA). High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA) provided a method to synthesis cDNA. The TaqMan™ OpenArray™ Real-Tine PCR Master Mix Assay (Applied Biosystems) was conducted to detect miR-145 expression. The Power SYBR-Green Master Mix Assay (Applied Biosystems) was performed to measure IRAK1 expression. U6 snRNA acted as the internal control for miR-145 and β-actin acted as the internal control for IRAK1. The primers information were miR-145, 5′-ACACTCCAGCTGGGCAGGTCAAAAGGGTCC-3′; U6 snRNA, 5′-TGCGGGTGCTCGCTTCGGCAGC-3′; IRAK1, 5′-GCTGTGGACACCGATACC-3′ (F) and 5′-GGTCACTCCAGCCTC TTCAG-3′ (R); β-actin, 5′-GCACCACACCTTCAATG-3′ (Forward) and 5′-TGCTTGCTGATCCACATCTG-3′ (R). Data were quantified using 2−△△Ct method [Citation24].
Cell transfection
miR-145 mimic, miR-145 inhibitor, Scramble and the negative control (NC) were all synthesized by GenePharma Corporation (Shanghai, China). The IRAK1 sequence was constructed into pcDNA3.1 plasmid and named as pc-IRAK1. shRNA targeting IRAK1 was ligated into U6/GFP/Neo plasmid and named as sh-IRAK1. Cell transfection experiment was carried out using Lipofectamine 3000 reagent (Invitrogen).
Western blotting
Total proteins were isolated from cells and liver tissues using RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific). The Pierce™ Rapid Gold BCA Protein Assay Kit (Thermo Fisher Scientific) was used to quantify the concentrations of total proteins. Western blotting system was carried out as previous described [Citation25] All antibodies were received from Abcam Biotechnology (MA, USA) and Cell Signaling Technology (MA, USA). The images of protein signals were recorded using Bio-Rad ChemiDoc™ XRS system. Data were quantified using Quantity One software (Bio-Rad Laboratories).
Acute liver injury triggered by LPS in vivo
Thirty C57BL/6 mice (5–6 weeks, 19–24 g) were obtained from Shandong Laboratory Center (Jinan, China). Followed by fed in our institution for 1 week, 10 mice acted as control and all others were triggered acute liver injury by intraperitoneal injection of LPS (40 μg/kg) [Citation26] and randomly assigned into LPS group and LPS + Emodin group. Mice in control group were treated by intraperitoneal injection of equal concentration of saline. Mice in LPS + Emodin group were pre-treated by administration orally of Emodin (50 mg/kg) for 12 h [Citation27]. All mice were anaesthetized using pentobarbital sodium and sacrificed by acute massive haemorrhage after LPS (or saline) treatment for 24 h. Then, the blood of each mouse was collected to detect the pro-inflammatory factors, ALT and AST levels in serum. Six liver tissues of each group were digested to measure the pro-inflammatory factors, apoptosis-associated proteins, and IRAK1 protein levels, as well as the miR-145 and IRAK1 RNA levels. Other four liver tissues of each group were placed in 10% formalin for TUNEL assay use.
TUNEL assay
TUNEL assay was carried out in line with the manufacturer’s instruction and previous study [Citation28]. The signals of cells were tested using microscope (Nikon, Japan). The number of TUNEL positive (+) cells was counted.
AST and ALT assay
The AST and ALT levels in serum were respectively measured using AST assay kit and ALT assay kit (catalogue number: C010-2 and C009-2, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in line with the manufacturer’s instruction.
Statistical analysis
Data were presented as the mean ± SD from three repeated experiments. Graphpad 6.0 software (Graphpad, CA, USA) provides method for statistical analysis. One-Way Analysis of Variance (ANOVA) and Student’s t-test provide methods for compute p values. p < .05 was regarded as significant difference.
Results
LPS triggered liver cell apoptosis and inflammatory injury
presented that 5 μg/ml LPS stimulation for 12 h treatment significantly lowered the viability of L-02 cells and primary hepatocytes (p < .01). The percentages of apoptotic cells after LPS stimulation were remarkably increased (, p < .01 or p < .001). The cleaved-caspase 3 and Bax levels were up-regulated after LPS treatment, while the Bcl-2 level was down-regulated after LPS treatment in L-02 cells and primary hepatocytes (). In addition, the pro-inflammatory cytokines levels in the culture supernatant of L-02 cells and primary hepatocytes were all elevated followed by LPS stimulation (, p < .001). Moreover, data in showed that LPS stimulation elevated the pro-inflammatory cytokines protein levels in L-02 cells and primary hepatocytes. The above outcomes confirmed that LPS could trigger liver cell apoptosis and inflammatory injury.
Emodin weakened the LPS-triggered liver cell apoptosis and inflammatory injury
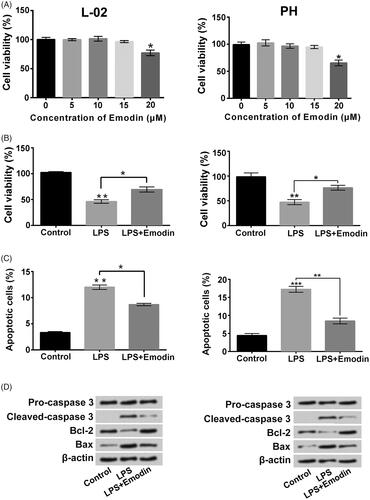
displayed that 5, 10 or 15 μM Emodin incubation had no obvious influences on L-02 cell and primary hepatocyte viability and 20 μM Emodin reduced the viability of L-02 cell and primary hepatocytes (p < .05). presented that 15 μM Emodin treatment significantly alleviated the lower of cell viability and boost of cell apoptosis caused by LPS (p < .05 or p < .01). The LPS-triggered cleaved-caspase 3 and Bax expressions increases and Bcl-2 expression decrease in L-02 cells and primary hepatocytes were also weakened by Emodin (). Furthermore, the increases of pro-inflammatory cytokineslevels in culture supernatant of L-02 cells and primary hepatocytes stimulated by LPS were all lowered by Emodin incubation ( , p < .05, p < .01, p < .001). The pro-inflammatory cytokines protein levels in L-02 cells and primary hepatocytes were also decreased after LPS + Emodin treatment (). These above findings indicated that Emodin attenuated the liver cell apoptosis and inflammatory injury stimulated by LPS.
Figure 2. Emodin weakened LPS-triggered liver cell viability loss and apoptosis. (A) Viability of L-02 cells and primary hepatocytes (PH) after 0, 5, 10, 15 or 20 μM Emodin exposure were measured. Followed by 5 μg/ml LPS and/or 15 μM Emodin exposure, (B and C) viability and apoptosis were detected respectively, (D) the apoptosis-associated protein levels were evaluated. *p < .05; **p < .01; ***p < .001.
Emodin suppressed NF-κB signalling pathway triggered by LPS in liver cells
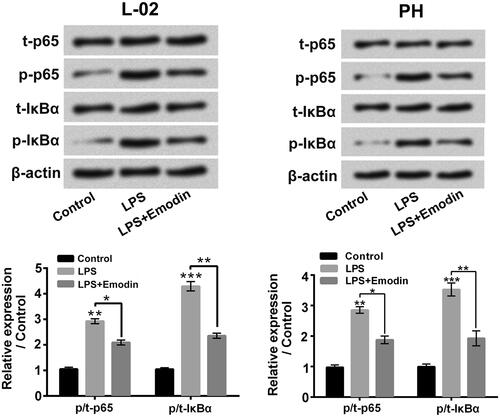
presented that LPS single stimulation boosted the p/t-p65 and p/t-IκBα rates in L-02 cells and primary hepatocytes (p < .01 or p < .001), which confirmed that LPS notably promoted NF-κB signalling pathway in liver cells. Emodin exposure obviously weakened the LPS-triggered p/t-p65 and p/t-IκBα expression rates increases in L-02 cells and primary hepatocytes (p < .05 or p < .01). These results hinted that Emodin suppressed the activation of NF-κB pathway triggered by LPS in liver cells.
Emodin slipped the LPS-triggered miR-145 expression reduction in liver cells
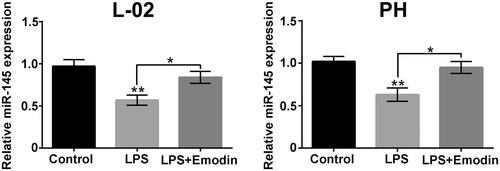
Data in displayed that LPS single stimulation noticeably reduced the miR-145 levels in L-02 cells and primary hepatocytes (p < .01). Emodin exposure slipped the LPS-triggered miR-145 expression reduction in L-02 cells and primary hepatocytes (p < .05). These results hinted that miR-145 might be associated with the regulatory activity of Emodin on LPS-triggered liver cell apoptosis and inflammatory injury.
miR-145 related to the modulation of inflammatory factor activation in liver cells
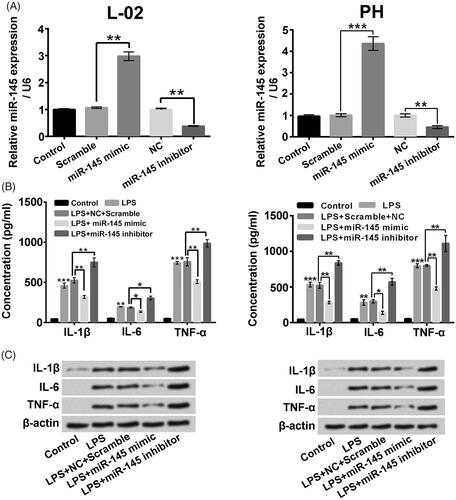
miR-145 mimic and miR-145 inhibitor were constructed into L-02 cells and primary hepatocytes, respectively, to clarify the regulatory influences of miR-145 on LPS-triggered inflammatory factors activation in liver cells. displayed that the miR-145 level was elevated after miR-145 mimic transfection and lowered after miR-145 inhibitor transfection in L-02 cells and primary hepatocytes (p < .01 or p < .001). Moreover, miR-145 mimic transfection significantly weakened the IL-1β, IL-6 and TNF-α expression increases aroused by LPS stimulation in culture supernatant of L-02 cells and primary hepatocytes (, p < .05 or p < .01). On the contrary, miR-145 inhibitor had conversed influence (p < .05 or p < .01). The elevated protein levels of pro-inflammatory cytokines in L-02 cells and primary hepatocytes triggered by LPS were similarly alleviated by miR-145 mimic transfection and potentiated by miR-145 inhibitor transfection (). These above findings confirmed that miR-145 related to the regulation of inflammatory factors expressions in liver cells.
Figure 6. miR-145 joined in the modulated of inflammatory factor expression in liver cells. (A) The miR-145 expressions in L-02 cells and primary hepatocytes (PH) after miR-145 mimic or miR-145 inhibitor transfection were measured. Followed by LPS treatment and/or miR-145 mimic (miR-145 inhibitor) transfection, (B and C) the pro-inflammatory cytokines in culture supernatant and cells were analyzed. *p < .05; **p < .01; ***p < .001.
miR-145 negatively modulated IRAK1 in liver cells
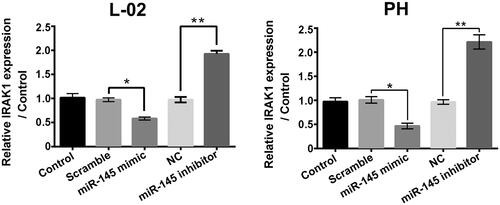
As shown in , IRAK1 mRNA levels in L-02 cells and primary hepatocytes were significantly decreased followed by miR-145 mimic transfection (p < .05) and increased followed by miR-145 inhibitor transfection (p < .01), which suggested that miR-145 negatively modulated IRAK1 in liver cells and hinted that IRAK1 might be a target gene of miR-145 in liver cells.
IRAK1 exerted modulatory activity in NF-κB signalling pathway
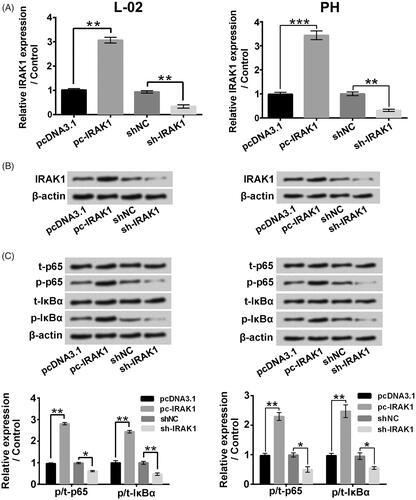
displayed that IRAK1 levels were elevated by pc-IRAK1 transfection (p < .01 or p < .001) and lowered by sh-IRAK1 transfection (p < .01). presented that pc-IRAK1 transfection significantly up-regulated the p/t-p65 and p/t-IκBα rates (p < .01) and sh-IRAK1 transfection remarkably down-regulated the p/t-p65 and p/t-IκBα rates (p < .05 or p < .01). Those above outcomes confirmed that IRAK1 exerted modulatory activity in NF-κB signalling pathway in liver cells.
Figure 8. IRAK1 exerted modulatory activity on NF-κB signalling pathway in liver cells. (A and B) The IRAK1 mRNA and protein expressions in L-02 cells and primary hepatocytes (PH) followed by pc-IRAK1 or sh-IRAK1 transfection were measured. (C) The t-p65, p-p65, t-IκBα and p-IκBα levels in L-02 cells and primary hepatocytes after pc-IRAK1 or sh-IRAK1 transfection were determined. *p < .05; **p < .01.
Emodin alleviated LPS-triggered acute liver damage in vivo
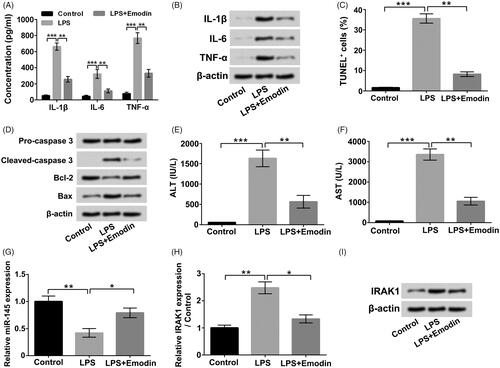
Finally, we established an animal acute liver injury model with LPS. Results in showed that LPS stimulation boosted the IL-1β, IL-6 and TNF-α levels in serum (p < .001), while the levels of IL-1β, IL-6 and TNF-α in serum were notably reduced by Emodin exposure (p < .01). found that that Emodin pre-exposure also declined the LPS-triggered increases of pro-inflammatory cytokines levels in liver tissues. presented that LPS treatment remarkably triggered liver cell apoptosis (p < .001). The enhancement rate of TUNEL positive (+) cells was distinctly reduced by Emodin pre-exposure (p < .01). illustrated that Emodin pre-exposure declined the LPS-triggered up-regulations of Cleaved caspase 3 and Bax, as well as down-regulation of Bcl-2 in liver tissues. Moreover, pointed out that LPS treatment increased the AST and ALT levels in serum (p < .001), while Emodin pre-exposure dramatically mitigated the LPS-triggered increases of ALT and AST levels in serum (p < .01). Furthermore, the miR-145 expression in liver tissues was noticeably decreased in LPS group and obviously increased in LPS + Emodin group (, p < .05 or p < .01). The IRAK1 mRNA and protein levels in liver tissues were both enhanced in LPS group and reduced in LPS + Emodin group (, p < .05 or p < .01). These outcomes verified that Emodin also could exert protective effects on LPS-triggered acute liver damage in vivo, which might also be achieved via up-regulating miR-145 and down-regulating IRAK1.
Figure 9. Emodin alleviated LPS-triggered acute liver damage in vivo. (A and B) The pro-inflammatory cytokines in serum and liver tissues were assessed. (C) The cell apoptosis in liver tissues was assessed. (D) The apoptosis-associated protein levels in liver tissues were assessed. (E and F) The AST and ALT concentrations in serum were detected. (G) The miR-145 expressions in liver tissues were measured. (H and I) The IRAK1 mRNA and protein levels in liver tissues were detected. *p < .05; **p < .01; ***p < .001.
Discussion
Plant-derived drugs have made great contribution in the prevention and therapy of a number of human diseases [Citation29,Citation30]. In this research, we found that Emodin significantly weakened the LPS-triggered liver cell inflammatory injury in vitro and in vivo. Moreover, we discovered that miR-145 and IRAK1 might play critical roles in the influences of Emodin on liver cell inflammatory injury triggered by LPS.
Inhibition of hepatocyte injury and inflammatory response plays critical roles in hepatitis treatment [Citation31]. Pro-inflammatory factors, such as TNF-α, IL-1β and IL-6, all take part in inflammatory reaction in hepatitis [Citation32]. In our experiments, LPS treatment significantly decreased human normal liver L-02 cell and primary hepatocytes viability and triggered cell apoptosis. The concentration of IL-1β, IL-6 and TNF-α in culture supernatant of liver cells, and in liver cells were all boosted with LPS treatment. Emodin co-treatment remarkably weakened the LPS-triggered liver cell viability decrease, cell apoptosis increase and pro-inflammatory factors expressions elevation. These outcomes evidenced that Emodin exerted beneficial influences on LPS-triggered liver cell inflammatory injury.
NF-κB pathway exerts critical modulatory activity in various cell functions, especially cellular inflammatory response [Citation33]. It is an essential transcription factor that joins in the modulation of pro-inflammatory factors gene expressions, including COX-2, iNOS, TNF-α, IL-1β and IL-6 [Citation22]. In our study, we discovered that LPS stimulation elevated the p/t-p65 and p/t-IκBα rates in two liver cells and Emodin co-exposure obviously declined the LPS-triggered p/t-p65 and p/t-IκBα rates elevation in liver cells. These results hinted that Emodin played influences on hepatitis cell model stimulated by LPS at least through inactivating NF-κB signalling pathway. Moreover, these outcomes were aligned with the previous experiment, which pointed out that epigallocatechin-3-gallate (EGCG) attenuated pro-inflammatory cytokines expression in LPS-stimulated L-02 cells by lowering NF-κB and MAPK pathways [Citation34].
MicroRNAs do not encode proteins but modulate intracellular protein level at post-transcriptional level [Citation35]. A variety of plant-derived medicines, including resveratrol, curcumin and ginsenosides, have been affirmed to play therapeutic effects on multiple diseases by modulating intracellular microRNAs expression [Citation36–38]. Previous studies have reported that HBx modulates the miR-145 expression in malignant hepatocytes and miR-145 was related to the pathogenesis of HBx-associated hepatocellular carcinoma [Citation15,Citation16]. In our research, miR-145 overexpression significantly declined the pro-inflammatory cytokines expression increases triggered by LPS in culture supernatant of liver cells and in liver cells. Suppression of miR-145 had adverse influences. These findings confirmed that miR-145 was associated with the modulation of inflammatory response in LPS-triggered hepatitis cell model and hinted that Emodin played anti-inflammatory activity on LPS-triggered liver cell inflammatory injury might through up-regulating miR-145.
As a member of IRAK family proteins, IRAK1 plays critical roles in regulating innate immunity and inducing inflammatory gene expression [Citation39]. Maitra et al. reported that IRAK1 contributed to the LPS-triggered reactive oxygen species (ROS) generation in macrophages [Citation40]. Joh et al. indicated that kalopanaxsaponin B inhibited LPS-triggered inflammatory response by inhibiting IRAK1 kinase [Citation41]. Li et al. proved that microRNA-146a-5p (miR-146a-5p) antagonized LPS-triggered ATP-binding cassette transporter A1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1) dysregulation in macrophages via down-regulating IRAK1 [Citation42]. In this research, we revealed that miR-145 negatively regulated IRAK1 in liver cells. Overexpression of IRAK1 significantly activated NF-κB signalling pathway in liver cells and IRAK1 suppression remarkably inactivated NF-κB signalling pathway. These outcomes verified that IRAK1 also exert modulatory roles in NF-κB signalling pathway in liver cells. Considering that NF-κB signalling pathway played critical modulatory role in the LPS-triggered inflammatory injury [Citation22], the outcomes of our study also implied that overexpression of miR-145 declined inflammatory response in LPS-triggered liver cell inflammatory injury by inhibiting IRAK1.
Finally, we also assessed the influences of Emodin on LPS-triggered acute liver damage in vivo. We demonstrated that Emodin pre-exposure could alleviate the LPS-triggered increases of pro-inflammatory factors expression, up-regulations of AST and ALT level in serum, liver cell apoptosis, as well as reduction of miR-145 and enhancement of IRAK1 in liver tissues. These results were aligned with the previous experiment, which indicated that Emodin could exert protective effects on concanavalin-A-triggered liver injury in mice [Citation27].
Taken together, our research suggested that Emodin weakened LPS-triggered human liver cell inflammatory injury in vitro and in vivo might be achieved by elevating miR-145 and declining IRAK1.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Rizzetto M. Chronic hepatitis D; at a standstill? Dig Dis. 2016;34:303–307.
- Kajiya M, Sato K, Silva MJ, et al. Hydrogen from intestinal bacteria is protective for Concanavalin A-induced hepatitis. Biochem Biophys Res Commun. 2009;386:316–321.
- Morgnanesi D, Heinrichs EJ, Mele AR, et al. A computational chemistry perspective on the current status and future direction of hepatitis B antiviral drug discovery. Antiviral Res. 2015;123:204–215.
- Czaja AJ. Diagnosis and management of autoimmune hepatitis: current status and future directions. Gut Liver. 2016;10:177–203.
- Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56:S39–S45.
- Gurung RB, Purbe B, Gyawali P, et al. The ratio of aspartate aminotransferase to alanine aminotransferase (AST/ALT): the correlation of value with underlying severity of alcoholic liver disease. Kathmandu Univ Med J. 2015;11:233–236.
- Wu SJ, Lin YX, Ye H, et al. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg (London, England). 2016;36:143–151.
- Chen Z, Liu H, Lei S, et al. LY294002 prevents lipopolysaccharideinduced hepatitis in a murine model by suppressing IkappaB phosphorylation. Mol Med Rep. 2016;13:811–816.
- Chang MH, Chang SC, Chan WH. Injurious effects of emodin on maturation of mouse oocytes, fertilization and fetal development via apoptosis. IJMS. 2012;13:13911–13925.
- Ha MK, Song YH, Jeong SJ, et al. Emodin inhibits proinflammatory responses and inactivates histone deacetylase 1 in hypoxic rheumatoid synoviocytes. Biol Pharm Bull. 2011;34:1432–1437.
- Alisi A, Pastore A, Ceccarelli S, et al. Emodin prevents intrahepatic fat accumulation, inflammation and redox status imbalance during diet-induced hepatosteatosis in rats. IJMS. 2012;13:2276–2289.
- Lee W, Ku SK, Kim TH, et al. Emodin-6-O-beta-D-glucoside inhibits HMGB1-induced inflammatory responses in vitro and in vivo. Food Chem Toxicol. 2013;52:97–104.
- Song LH, Hu MM, Liu D, et al. Thermal cracking of Enteromorpha prolifera with solvents to bio-oil. Energy Convers Manage. 2014;77:7–12.
- Liu D, Wu J, Liu M, et al. Downregulation of miRNA-30c and miR-203a is associated with hepatitis C virus core protein-induced epithelial-mesenchymal transition in normal hepatocytes and hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2015;464:1215–1221.
- Bandopadhyay M, Banerjee A, Sarkar N, et al. Tumor suppressor micro RNA miR-145 and onco micro RNAs miR-21 and miR-222 expressions are differentially modulated by hepatitis B virus X protein in malignant hepatocytes. BMC Cancer. 2014;14:721.
- Gao F, Sun X, Wang L, et al. Downregulation of microRNA-145 caused by hepatitis B virus X protein promotes expression of CUL5 and contributes to pathogenesis of hepatitis B virus-associated hepatocellular carcinoma. Cell Physiol Biochem. 2015;37:1547–1559.
- Ulmer AJ, Flad H, Rietschel T, et al. Induction of proliferation and cytokine production in human T lymphocytes by lipopolysaccharide (LPS). Toxicology. 2000;152:37–45.
- Huang X, Liu Y, Lu Y, et al. Anti-inflammatory effects of eugenol on lipopolysaccharide-induced inflammatory reaction in acute lung injury via regulating inflammation and redox status. Int Immunopharmacol. 2015;26:265–271.
- Yao J, Pan D, Zhao Y, et al. Wogonin prevents lipopolysaccharide-induced acute lung injury and inflammation in mice via peroxisome proliferator-activated receptor gamma-mediated attenuation of the nuclear factor-kappaB pathway. Immunology. 2014;143:241–257.
- Perea L, Coll M, Sanjurjo L, et al. Pentraxin-3 modulates lipopolysaccharide-induced inflammatory response and attenuates liver injury. 2017;66(3):953–968.
- Zhang J, Yang S, Chen F, et al. Ginkgetin aglycone ameliorates LPS-induced acute kidney injury by activating SIRT1 via inhibiting the NF-kappaB signaling pathway. Cell Biosci. 2017;7:44.
- Fan X, Zhang Y, Dong H, et al. Trilobatin attenuates the LPS-mediated inflammatory response by suppressing the NF-kappaB signaling pathway. Food Chem. 2015;166:609–615.
- Matsui Y, Kikuchi T, Inoue T, et al. Carapanolides J-L from the seeds of Carapa guianensis (Andiroba) and their effects on LPS-activated NO production. Molecules. 2014;19:17130–17140.
- Ish-Shalom S, Lichter A. Analysis of fungal gene expression by Real Time quantitative PCR. Methods Mol Biol (Clifton, NJ). 2010;638:103–114.
- Li R, Yin F, Guo YY, et al. Knockdown of ANRIL aggravates H2O2-induced injury in PC-12 cells by targeting microRNA-125a. Biomed Pharmacother. 2017;92:952–961.
- Tsai T-H, Tam K, Chen S-F, et al. Deletion of caveolin-1 attenuates LPS/GalN-induced acute liver injury in mice. J Cell Mol Med. 2018;22:5573–5582.
- Xue J, Chen F, Wang J, et al. Emodin protects against concanavalin A-induced hepatitis in mice through inhibiting activation of the p38 MAPK-NF-kappaB signaling pathway. Cell Physiol Biochem. 2015;35:1557–1570.
- Kyrylkova K, Kyryachenko S, Leid M, et al. Detection of apoptosis by TUNEL assay. Methods Mol Biol. 2012;887:41–47.
- Sengupta T, Vinayagam J, Singh R, et al. Plant-derived natural products for Parkinson's disease therapy. Adv Neurobiol. 2016;12:415–496.
- Fridlender M, Kapulnik Y, Koltai H. Plant derived substances with anti-cancer activity: from folklore to practice. Front Plant Sci. 2015;6:799.
- Dai G, Sun B, Wu L, et al. Comparative pharmacokinetics of three alkaloids in normal and acute hepatitis rats after oral administration of Yanhuanglian total alkaloids extract. Biomed Chromatogr. 2018;32:e4329.
- Kasprzak A, Seidel J, Spachacz R, et al. Intracellular expression of pro-inflammatory cytokines (IL-1alpha, TNF-alpha, and IL-6) in chronic hepatitis C. Roczniki Akademii Medycznej w Bialymstoku (1995). 2004;49:207–209.
- Leeman JR, Gilmore TD. Alternative splicing in the NF-kappaB signaling pathway. Gene. 2008;423:97–107.
- Liu Q, Qian Y, Chen F, et al. EGCG attenuates pro-inflammatory cytokines and chemokines production in LPS-stimulated L02 hepatocyte. Acta Biochim Biophys Sinica. 2014;46:31–39.
- Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379.
- Sheth S, Jajoo S, Kaur T, et al. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PloS One. 2012;7:e51655.
- Zheng J, Wu C, Lin Z, et al. Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA-mediated control of DNA methylation–a novel mechanism suppressing liver fibrosis. FEBS J. 2014;281:88–103.
- Chan LS, Yue PY, Wong YY, et al. MicroRNA-15b contributes to ginsenoside-Rg1-induced angiogenesis through increased expression of VEGFR-2. Biochem Pharmacol. 2013;86:392–400.
- Su J, Richter K, Zhang C, et al. Differential regulation of interleukin-1 receptor associated kinase 1 (IRAK1) splice variants. Mol Immunol. 2007;44:900–905.
- Maitra U, Singh N, Gan L, et al. IRAK-1 contributes to lipopolysaccharide-induced reactive oxygen species generation in macrophages by inducing NOX-1 transcription and Rac1 activation and suppressing the expression of antioxidative enzymes. J Biol Chem. 2009;284:35403–35411.
- Joh EH, Jeong JJ, Kim DH. Kalopanaxsaponin B inhibits LPS-induced inflammation by inhibiting IRAK1 Kinase. Cell Immunol. 2012;279:103–108.
- Li X, Ji Z, Li S, et al. miR-146a-5p antagonized AGEs- and P.g-LPS-induced ABCA1 and ABCG1 dysregulation in macrophages via IRAK-1 downregulation. Inflammation 2015;38:1761–1768.