?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To investigate the effect of Sulfiredoxin-1 (Srxn1) on astrocyte injury induced by hydrogen peroxide (H2O2).
Methods
Observing the changes of H2O2 on contents of lactate dehydrogenase (LDH), malondialdehyde (MDA), superoxide dismutase (SOD) and apoptosis after transfected Srxn1 siRNA into astrocytes. The protein expression of Notch 1, NICD and Hes1, the content of LDH and MDA, the activity of SOD and apoptosis rate of astrocytes after inhibiting or activation of Notch signalling pathway were detected by Western blot, ELISA and flow cytometry, respectively.
Results
Knockdown of Srxn1 could promote the secretion of LDH and MDA, decrease the activity of SOD and aggravate apoptosis of astrocytes induced by H2O2. The results of Western blot, ELISA assay and flow cytometry indicated that activation of the Notch signalling pathway attenuated the effect of Srxn1 on H2O2-induced oxidative damage and apoptosis of astrocytes.
Conclusion
Srxn1 may protect astrocytes from oxidative stress injury induced by H2O2 by activation of Notch signalling pathway.
Introduction
Astrocyte is the most abundant cell type in the brain, with various physiological functions, such as, stable intercellular communication, maintaining extracellular environment stability, forming the blood–brain barrier, anti-oxidative stress, synthetic neurotransmitter, structural support, neuronal metabolism, etc [Citation1–3]. In recent years, more and more studies have shown that astrocytes can produce a large number of growth factors, promote neuronal repair, and are associated with various diseases such as Parkinson’s disease, Alzheimer’s disease, neurodegenerative diseases, and epilepsy [Citation4,Citation5]. Epilepsy is a common neurological disease and the second largest disease after stroke; According to statistics, there are about 350,000 new epilepsy cases every year in China, and about one-third of them show drug resistance [Citation6]. Patients with refractory epilepsy are those who have been treated with at least two different antiepileptic drugs for more than 2 years, while the number of seizures has not decreased or even increased, and the treatment effect is poor [Citation7]. Sulfiredoxin-1 (Srxn1) is an endogenous small molecule of protein eukaryotes obtained through evolutionary selection, which is associated with the function of antioxidation and anti-apoptosis and can protect nerve cells from oxidative damage induced by hydrogen peroxide [Citation8]. In this study, the H2O2 model was established in astrocytes of neonatal rats within 24 h of Sprague Dawley (SD) rats. The expression of Srxn1 was silenced by RNAi technique, and the effects of Srxn1 on oxidative damage and apoptosis of astrocytes were observed.
Materials and methods
The study was conducted in accordance with the Basic & Clinical Pharmacology &Toxicology policy for experimental and clinical studies.
Isolation and culture of astrocytes
100 SD newborn rats purchased from Shanghai Experimental Animal Center of Chinese Academy of Sciences (Shanghai, China) within 24 h were taken from male and female, and the cerebral cortex of the suckling mice was isolated. The tissues were minced, incubated in 0.25% trypsin for 20 min, and digested into single cells. The cells were cultured in a 75 cm2 culture bottle using DMEM/F12 medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum; the neurons and microglia were removed by rapid fluid exchange, differential adherence and shaker shaking. Astrocytes were identified by immunocytochemistry and morphology, cultured for 6–8 days and subcultured for subsequent experiments.
Cell transfection
The astrocytes in the logarithmic growth phase were trypsinized and suspended, adjusted to a concentration of 2 × 105 cells/mL and inoculated into a 24-well plate, and the cells were grown to a confluency degree of about 50% for transfection. The control siRNA and the Srxn1 siRNA were transfected into astrocytes by LipofectamineTM 2000 transfection reagent (Invitrogen) and then continued to culture for 48 h. Control siRNA sequence: 5′-TTCTCCGAACGTGTCACGT-3′; Srxn1 siRNA sequence: 5′-CATCCACACCAGACTTGCAGT-3′.
Experimental grouping
Astrocytes were divided into blank control (NC) group, hydrogen peroxide-inducing (H2O2) group, siRNA control (H2O2+si-con) group, knockdown Srxn1 (H2O2+si-Srxn1) group, Notch pathway inhibitor control (H2O2+PBS) group, Notch pathway inhibitor (H2O2+DAPT) group, Notch pathway activator control (H2O2+si-Srxn1 + PBS) group, transfected Srxn1 siRNA combined with Jagged1 (R&D systems Company) (H2O2+si-Srxn1 + Jagged 1) group. Treatment method: inhibition of Notch pathway: DAPT was added to the cell culture medium and the final concentration was adjusted to 50 μmol/L, and the culture was continued for 24 h; Transfected Srxn1 siRNA with Jagged 1: Srxn1 siRNA was transfected into astrocytes and Jagged 1 (5 mg/L) was added to the cell culture medium for 24 h. After pretreatment, the cells in above groups (except NC group) were replaced with fresh medium, added with H2O2 and adjusted the final concentration to 100 μmol/L, and then cultured for 6 h.
Western blot
The cells of NC group, H2O2 group, H2O2+si-con group and H2O2+si-Srxn1 group were collected, and RIPA lysate (Beyotime, Shanghai, China) containing protease inhibitor was added to lyse the cells on ice, centrifuged at 12,000 g for 20 min at 4 °C, and the supernatant was taken. The protein sample concentration was measured by BCA kit (Beyotime). After SDS-PAGE electrophoresis, the appropriate amount of protein was transferred onto PVDF membrane, and the blocking experiment was conducted in 5% skimmed milk at room temperature for 1 h. Adding the corresponding primary antibody (Abcam, Cambridge, 1:1000), incubating at 4 °C overnight, and then adding HRP labeled secondary antibody (Abcam,1:5000), incubating at room temperature for another 1 h after washing the membrane with TBST 3 times, and the ECL kit (Solarbio) showed a band with β-actin as an internal reference and the experiment was repeated 3 times.
Detection of LDH, MDA content and SOD activity in cells by LISA assay
The cells were collected in each group and centrifuged to obtain a supernatant. The contents of LDH, MDA and SOD activity in cell supernatant were detected by using lactate dehydrogenase (LDH) ELISA kit, malondialdehyde (MDA) ELISA kit and superoxide dismutase (SOD) ELISA kit (Wuhan, China), respectively.
Flow cytometry for apoptosis
Cells were collected in each group and centrifugally collected after trypsin digestion at 4 °C and 300 g. 5 μL of Annexin V-FITC and 10 μL of PI were added according to the instructions of Annexin V-FITC/PI Apoptosis Detection Kit (Solarbio, Beijing, China), gently mixed, and then incubated at room temperature for 15 min in the dark, finally apoptosis was detected by flow cytometry (BD Biosciences, San Jose, CA, USA).
Statistical analysis
The comparisons between two groups were compared via student’s t test and among multiple groups were conducted by one-way analysis of variance (ANOVA) followed by SPSS 22.0 and GraphPad Prism 7. All data were expressed as ± s. P < .05 was with statistical significance.
Results
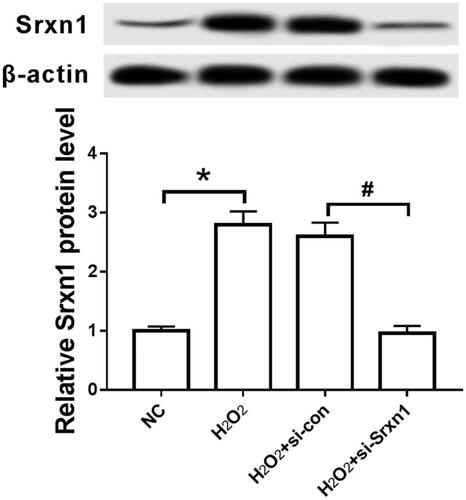
Effect of H2O2 on the expression of Srxn1 protein in astrocytes
The expression of Srxn1 protein was detected by Western blot. The results are shown in : Compared with NC group, the expression of Srxn1 protein in H2O2-treated astrocytes was significantly up-regulated (P < .05). After transfecting with Srxn1 siRNA by RNAi method for pretreatment, the expression level of Srxn1 protein in group H2O2+si-Srxn1 was significantly lower than that in group H2O2+si-con (P < .05).
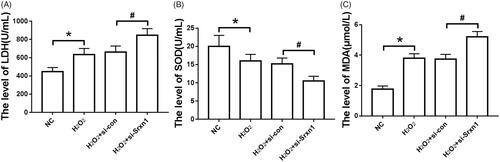
Effects of knockdown of Srxn1 on LDH, SOD and MDA in astrocytes induced by H2O2
It can be seen from that in H2O2-induced astrocytes, LDH release and MDA content were significantly increased (P < .05), and SOD activity was significantly decreased (P < .05). After transfected with Srxn1 siRNA, the release of LDH, MDA content and SOD activity in astrocytes induced by H2O2 increased or decreased significantly (P < .05).
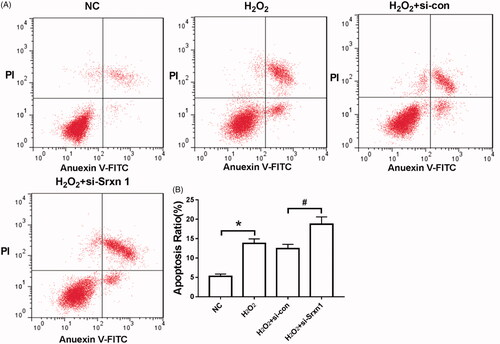
Effect of knockdown of Srxn1 on H2O2 induced apoptosis in astrocytes
Apoptosis was detected by flow cytometry. The results () showed that the apoptotic rate of astrocytes induced by H2O2 was significantly higher than that of NC group (P < .05). Knockdown of Srxn1 promoted H2O2-induced apoptosis in astrocytes (P < .05).
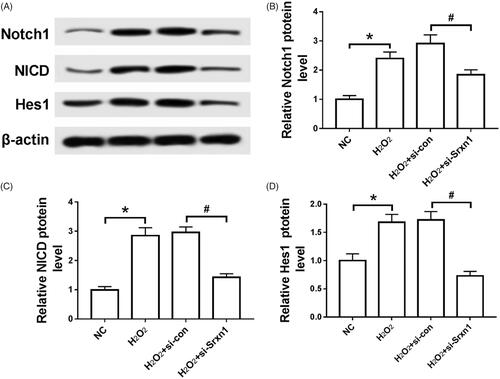
Effect of knockdown Srxn1 on H2O2 induced notch pathway in astrocytes
Compared with NC group, the expression of Notch 1, NICD and Hes1 protein in astrocytes induced by H2O2 was significantly up-regulated (P < .05). After knockdown of Srxn1, the expression levels of Notch 1, NICD and Hes1 in astrocytes induced by H2O2 were significantly decrease compared with H2O2+si-con group (P < .05) ().
Figure 4. Knockdown of Srxn1 inhibits H2O2 induced Notch pathway in astrocytes. Note: (A) the electrophoresis of Notch 1, NICD and Hes1 protein in each group; (B) the expression level of Notch 1 protein in each group; (C) the expression level of NICD protein in each group; (D) the expression level of Hes1 protein in each group. Compared with NC group, *P <.05; compared with H2O2+si-con group, #P < .05.
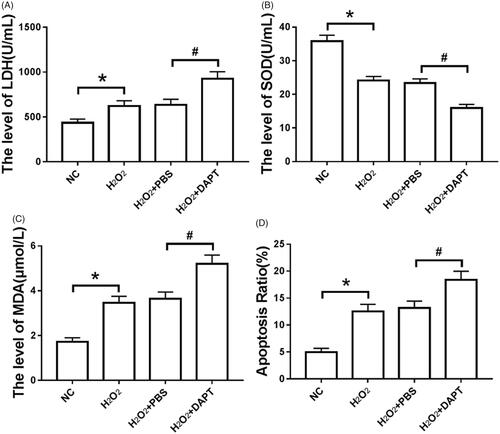
Inhibition of notch singling pathway on oxidative damage and apoptosis of astrocytes induced by H2O2
After treatment with H2O2, The release of LDH and MDA content in astrocytes increased (P < .05), the activity of SOD decreased (P < .05), and the apoptosis rate increased (P < .05). After pretreatment with DAPT, oxidative damage and apoptosis of astrocytes induced by H2O2 were aggravated (P < .05) ().
Figure 5. Inhibition of Notch singling pathway on oxidative damage and apoptosis of astrocytes induced by H2O2. Note: (A) the release amount of LDH in each group; (B) the detection of SOD activity in each group; (C) the change of MDA content in each group; (D) the apoptosis rate of each group. *P < .05 compared with NC group; #P < .05 compared with H2O2+PBS group.
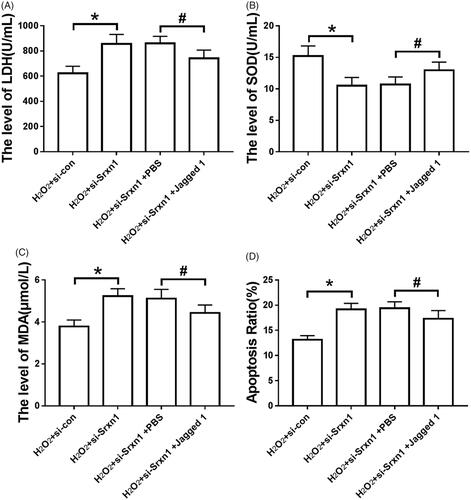
Effects of knocking down Srxn1 and activating notch pathway on oxidative damage and apoptosis of astrocytes induced by H2O2
After knocking down Srxn1 in astrocytes, LDH release and MDA content in astrocytes induced by H2O2 reinforced significantly (P < .05), SOD activity weakened significantly (P < .05), and apoptosis rate increased significantly (P < .05). Jagged 1 pretreatment could attenuate the effect of Srxn1 knockdown on H2O2-induced oxidative damage and apoptosis of astrocytes (P < .05) ().
Figure 6. Jagged 1 can attenuate the effect of knockdown Srxn1 on H2O2 induced oxidative damage and apoptosis in astrocytes. Note: (A) LDH release in astrocytes induced by hydrogen peroxide after Jagged 1 pretreatment and knockdown of Srxn1; (B) SOD activity in astrocytes induced by hydrogen peroxide after Jagged 1 pretreatment and knockdown of Srxn1; (C) MDA content in astrocytes induced by hydrogen peroxide after Jagged 1 pretreatment and knockdown of Srxn1; (D) hydrogen peroxide after Jagged 1 pretreatment and knockdown of Srxn1. 2 apoptosis rate induced by astrocytes. Compared with H2O2+si-con group, *P < .05; compared with H2O2+si-Srxn1 + PBS group, *P < .05.
Discussion
Epilepsy is a repetitive transient brain dysfunction syndrome caused by abnormal discharge of neurons, while the pathogenesis of epilepsy is still unintelligible. It is generally believed that head trauma, central nervous system infection, and family inheritance are the main risk factors for epilepsy [Citation9]. During epileptic seizures, neurons may undergo changes such as DNA cleavage, chromatin shrinkage, and increased cell membrane permeability. Long-term seizures of epilepsy can lead to permanent neuronal damage [Citation10]. Studies [Citation11,Citation12] have shown that in epileptic patients, the morphology and number of astrocytes have changed significantly, the dysfunction of glutamine synthase and adenosine kinase affects the release of glutamate from astrocytes. However, the frequency of seizures can be reduced by regulating glutamate transporter 1 and extracellular glutamate content in astrocytes.
Srxn1 belongs to the conservative Sulfiredoxin antioxidant family and has neuroprotective effect. According to reports [Citation13,Citation14], silencing of Srxn1 expression in astrocytes may up-regulate inflammatory cytokine levels, promote inflammatory responses, and aggravate H2O2-induced cells apoptosis; It can also reduce SOD activity, inhibit glutathione (an important free radical scavenger and antioxidant) production and promote LDH release, and aggravate oxidative stress damage in cells. Zhang et al. [Citation15] found that overexpression of Srxn1 can inhibit the expression of apoptosis-related proteins and cytochrome C release, regulate the P13K/AKT signalling pathway and alleviate myocardial cell injury induced by ischemia-reperfusion; Li et al. [Citation16] showed that Srxn1 could enhance the proliferation and differentiation of cardiac stem cells and reduce the apoptosis of cardiomyocytes induced by oxidative stress by reducing the production of reactive oxygen species and maintaining the balance of mitochondrial membrane potential. In rat astrocytes, there is evidence that knockdown of Srxn1 promotes H2O2-induced LDH release in astrocytes, inhibits the expression of antioxidant enzyme peroxiredoxins, and aggravates cellular oxidative stress damage. In this study, the H2O2 model was established by using SD neonatal rat astrocytes. It was found that the expression of Srxn1 protein and the content of LDH and MDA were significantly up-regulated, in addition, the apoptosis rate was also notably elevated, whereas the activity of SOD was obviously reduced. After knocking down Srxn1 through RNAi, the release of LDH and the MDA contents, SOD activity and cell apoptosis rate increased or decreased significantly.
The Notch signalling pathway consists of 4 Notch receptors, 5 Notch ligands and DNA binding protein CSL. When the Notch ligand binds to the receptor in different cells, its C-terminal cleavage product releases localized signal cytoplasmic region (NICD) from gamma-secreting complex enzymatic digestion. NICD transfers to the nucleus and binds to DNA binding protein CSL to form a transcriptional complex, which further activates target genes such as Hes and Hrt to exert biological effects [Citation17]. A number of studies [Citation18,Citation19] have shown that Notch signalling pathway is associated with proliferation, apoptosis and tumor development of cervical cancer, prostate cancer, and other cancer cells. Moreover, inhibition of the Notch signalling pathway protects endothelial cells from inflammatory damage [Citation20]. In addition, Notch signalling pathway plays a regulatory role in cellular oxidative stress injury. For example, Yang et al. [Citation21] took human umbilical vein endothelial cells as the research subject, after using DAPT to inhibit Notch signalling pathway, H2O2-induced apoptosis rate decreased, cell viability and migration ability increased. Mo and others [Citation22] found that knocking down Notch 1 could increase H2O2 induced apoptosis.
In conclusion, knockdown of Srxn1 in H2O2-induced astrocytes can promote oxidative stress damage in cells, which may be mediated by regulating Notch signalling pathway. Activation of Notch signalling pathway may attenuate H2O2-induced oxidative stress injury. This study may provide new ideas for the protection of oxidative stress damage in astrocytes.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19:182–189.
- Bélanger M, Allaman I, Magistretti P. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab.2011;14:724–738.
- Argaw AT, Asp L, Zhang J, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122:2454–2468.
- Chandrasekaran A, Avci HX, Leist M, et al. Astrocyte differentiation of human pluripotent stem cells: new tools for neurological disorder research. Front Cellular Neurosci. 2016;10:1–27.
- Tang J, Zhang J, Ma J, et al. Astrocyte calcium wave induces seizure-like behavior in neuron network. Sci China Technol Sci. 2017;60:1011–1018.
- Singh A, Trevick S. The epidemiology of global epilepsy. Neurol Clin. 2016;34:837–847.
- Brodie MJ. Pharmacological treatment of drug-resistant epilepsy in adults: a practical guide. Curr Neurol Neurosci Rep. 2016;16:82–91.
- Li Q, Yu S, Wu J, et al. Sulfiredoxin-1 protects PC12 cells against oxidative stress induced by hydrogen peroxide. J Neurosci Res. 2013;91:861–870.
- Ogunrin OA, Obiabo OY, Obehigie E. Risk factors for epilepsy in Nigerians - a cross-sectional case-control study. Acta Neurol Scand. 2014;129:109–113.
- Devinsky O, Vezzani A, Najjar S, et al. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36:174–184.
- Steinhäuser C, Grunnet M, Carmignoto G. Crucial role of astrocytes in temporal lobe epilepsy. Neuroscience 2016;323:157–169.
- Wang X, Sha L, Sun N, et al. Deletion of mTOR in reactive astrocytes suppresses chronic seizures in a mouse model of temporal lobe epilepsy. Mol Neurobiol. 2016;54:1–13.
- Yu S, Wang X, Lei S, et al. Sulfiredoxin-1 protects primary cultured astrocytes from ischemia-induced damage. Neurochem Int. 2015;82:19–27.
- Zhou Y, Duan S, Zhou Y, et al. Sulfiredoxin-1 attenuates oxidative stress via Nrf2/ARE pathway and 2-Cys Prdxs after oxygen-glucose deprivation in astrocytes. J Mol Neurosci. 2015;55:941–950.
- Zhang JK, He ZY, Guo JH, et al. Sulfiredoxin-1 protects against simulated ischaemia/reperfusion injury in cardiomyocyte by inhibiting PI3K/AKT-regulated mitochondrial apoptotic pathways. Bioscience Rep. 2016;36:1–11.
- Li X, He P, Wang X, et al. sulfiredoxin-1 enhances cardiac progenitor cell survival against oxidative stress via the upregulation of the ERK/NRF2 signal pathway. Free Rad Biol Med. 2018;123:8–19.
- Hori K, Sen A, Artavanis-Tsakonas S. Notch signaling at a glance. J Cell Sci. 2013;126:2135–2140.
- Sun L, Liu M, Sun GC, et al. Notch signaling activation in cervical cancer cells induces cell growth arrest with the involvement of the nuclear receptor NR4A2. J Cancer. 2016;7:1388–1395.
- Pedrosa AR, Graça JL, Carvalho S, et al. Notch signaling dynamics in the adult healthy prostate and in prostatic tumor development. Prostate. 2016;76:80–96.
- Zhao J, Liang Y, Song F, et al. TSG attenuates LPC-induced endothelial cells inflammatory damage through notch signaling inhibition. Iubmb Life. 2016;68:37–50.
- Yang Y, Duan W, Liang Z, et al. Curcumin attenuates endothelial cell oxidative stress injury through Notch signaling inhibition. Cell Signal. 2013;25:615–629.
- Mo JS, Yoon JH, Ann EJ, et al. Notch1 modulates oxidative stress induced cell death through suppression of apoptosis signal-regulating kinase 1. Proc Natl Acad Sci USA. 2013;110:6865–6870.