Abstract
Nanomedicine is a rapidly emerging field and is reported to be a promising tool for treating various diseases. Green synthesized nanoparticles are documented to possess a potent anticancer effect. Rabdosia rubescens is a Chinese plant which is also one of the components of PC-SPES and used to treat prostate cancer. In the present study, we synthesized the gold nanoparticles from R. rubescens (RR-AuNP) and analyzed its anticancer activity against the lung carcinoma A549 cell lines. Since lung cancer is reported to be with increased morbidity and decreased survival rate. The biosynthesized RR-AuNP were confirmed using UV-Visible spectrophotometer, size and shape of RR-AuNP were assessed by DLS, TEM and EDX. The biomolecules present in RR-AuNP and its topographical structure were detected using FTIR, SAED and AFM analysis. MTT assay was performed to detect the IC50 dose of RR-AuNP and its apoptotic effect was assessed by detecting the caspases activation, ROS generation. The anticancer effect of RR-AuNP was confirmed by DAPI staining, TUNEL assay and its molecular mechanism were confirmed by assessing the apoptotic signalling molecules protein expression. Our results illustrate that RR-AuNP showed a strong absorption peak at 550 nm and the RRAuNP were polydispersed nanospheres with size of 130 nm. RR-AuNP IC50 dose against A549 lung carcinoma cell line was detected to be at 25 µg/ml. The results of DAPI staining, TUNEL and immunoblotting analysis confirms both the 25 µg/ml and 50 µg/ml of RR-AuNP possess potent anticancer and apoptotic effect, suggesting that RR-AuNP that it may be a persuasive molecule to treat lung cancer.
Introduction
Nanotechnology, a blooming field with various applications had widened its wings as nanomedicine. Recently, researchers are focused on the usage of nanoparticles for treating various diseases. Since nanoparticles possess unique properties like nanoscale size, targeted delivery and high efficacy [Citation1], fascinated researchers explore it as nanomedicine. Noble metals like silver, gold, copper and platinum were mostly preferred for the synthesis of nanoparticles. These nanoparticles were synthesized by physical, chemical and biological methods [Citation2,Citation3]. Synthesis of nanoparticles using chemical reduction method leads to the generation of toxic compounds that get adsorbed to the surface of the particles which make it has not applicable for treating patients [Citation4].
Therefore, researches were focused on the synthesis of eco-friendly, biocompatible nanoparticles using biological method [Citation5] since the biological methods are inexpensive, can be synthesized easily and also the stability of biosynthesized nanoparticles are greater compared to other methods. The naoparticles were synthesized by reducing the noble metals using plant extract, microorganisms hence it is nontoxic as well potent nanoparticles which can be used in the fields of biological labelling [Citation6], photothermal therapy [Citation7] and nanomedicine [Citation8]. Among all noble metals, gold is mostly preferred for the synthesis of nanoparticles due to its inimitable intense plasma resonance property [Citation9]. Biosynthesis of gold nanoparticle using plant extract can be potent drug to treat the world wide deadly disease cancer.
PC-SPES is a herbal supplement composed eight different herbal plant extract of Chinese origin. PC-SPES possess potent anticancer activity. It is used to treat prostate cancer and induced apoptosis in hormone-dependent breast cancer cells [Citation10,Citation11]. Rabdosia rubescens is one of the plant extracts used in the preparation of PC-SPES. Rabdosia rubescens prescribed in traditional Chinese treatment for various ailments like throat infection, pharyngitis and stomach ache (HDNCM, 199), and it is also consumed as herbal tea by Chinese [Citation12,Citation13]. Rabdosia rubescens are rich in diterpenoids which is the main bioactive component apart from that it possesses flavonoids, phenolic acids, triterpenoids and volatile oil [Citation14,Citation15]. The antitumour property of R. rubescens was due to the constituents’ oridonin and poncidin [Citation16]. Therefore, the current investigation was expected to biosynthesis gold nanoparticle using the leaves extract of R. rubescens.
It is estimated by the year 2027, the death rate is to be increased by 70%, most of the cancer deaths is due to lung, breast, prostate and colorectum. Lung cancer contributes one-fourth of the cancer death rates, and it also possesses poor survival rate of 17.8% only [Citation17]. National Cancer Center (NCC) reported that the lung cancer incidence rate in China is about 781,000 people every year [Citation18]. Chemotherapy and radiotherapy are treatments given to patients with lung cancer [Citation19], which increases the survival rate of cancer patients but it leads to various side effects since the drugs are not targeted only to the cancer cells [Citation20]. The metabolism and drug clearance rate of allopathic drugs are the serious draw backs for its usage [Citation21]. Nanoparticles can trounce this and can be a potent alternative for existing anticancer drugs. Hence, the current study is designed to evaluate the potency of biosynthesized R. rubescens gold nanoparticles against lung cancer in vitro.
Methodology
Chemicals
Tetrachloroauric(III) acid (520918), cell culture medium RPMI 1640 medium (R8758), Foetal Bovine Serum, Trypsin, Pencillin-Streptomycin solution and Thiazolyl blue formazan (MTT) were procured from Sigma Aldrich, USA.
Preparation of aqueous R. rubescens (RR) extract
Fresh R. rubescens leaves were procured from local farm and authenticated before preparation of gold nanoparticles. The leaves were thoroughly washed with distilled water and dried under shade for 15 days till it gets completely dried off. Using electrical mixer grinder, the dried leaves were ground to fine powder. 100 g R. rubescens leaves powder was boiled for 30 min at 60 °C in 1 l of distilled of water. The extract was then filtered and used for further analysis.
RR-AuNP synthesis
Rabdosia rubescens gold nanoparticles (RR-AuNP) were synthesized by adding 10 ml of R. rubescens aqueous extract to 190 ml of 1 mM Gold(III) chloride trihydrate solution and incubated at room temperature for 15 min. Synthesis of RR-AuNP was confirmed by colour change of solution from yellow to ruby red [Citation22].
Characterization of RR-AuNP
Ultraviolet-visible spectroscopic analysis (UV)
The bioreducing property of RR-AuNP was evaluated using Ultraviolet-Visible Spectrophotometer. The sample was assessed for the period of 30 days and the OD values were measured.
RR-AuNP DLS evaluation
Dynamic Light Scattering and Zeta Potential using Zeta-PAL (Brookhaven, USA) were performed to evaluate the size of RR-AuNP. 50 µg/ml of RR-AuNP were mixed with deionized water and subjected to run in Zeta-PAL for 30 s at a temperature of 25 °C, refractive index of 1.33, electric field 13.9 V/cm and voltage of 5 volts. The experiment was repeated for three times. The values were recorded, and using Smoluchowski coagulation equation, the zeta potential means were calculated.
RR-AuNP SAED evaluation
Selected Area Diffraction Pattern of RR-AuNP was assessed using Zetasizer Pro (Malvern Panalytical Ltd, UK) to detect the structure of biosynthesized gold nanoparticles using Malvern Zetasizer instrument. The patterns obtained SAED analysis and the distance between planes was using the formula λL Rd = (1) where λL is a constant of the microscope, R is the ring radius, and d is the interplanar distance
RR-AuNP FTIR analysis
The functional groups present in the RR-AuNP were identified using FTIR instrument Invenvio (Bruker). 2 mg RR-AuNP were mixed with 200 mg of potassium bromide and the mixture was placed in sample holder for FTIR analysis. The mixture was analyzed at the spectra range from 4000 to 500 cm−1 (Spectrum 2000, Perkin Elemer Waltham, USA). At resolution of 4 cm/scan, about 50 scans were done and the obtained data were analyzed using WINFIRST software, Mattson, USA.
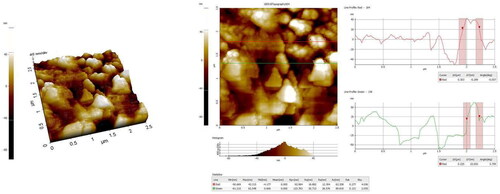
RR-AuNP AFM estimation
The size of biosynthesized RR-AuNP was estimated using an atomic force microscope. The sample was placed on the probe made up of silica and the using nitrogen gas the sample was dried and assessed for the size.
RR-AuNP TEM-EDX analysis
High resolution transmission electron microscopic analysis was performed to detect the size, structure and the chemical compounds of biosynthesized RR-AuNP. TEM-Electron Diffraction X ray analysis was performed to detect the presence of gold ions in the RR-AuNP. The sample was ultrasonicated by mixing it with methanol and then assessed for EDAX by placing it on the carbon film at the volt of 200 kV (JEOL instrument).
RR-AuNP cytotoxicity analysis
Culturing of lung carcinoma A549 cells
Human Adenocarcinomic alveolar basal epithelial (A549) cells and Human Medical Research Council cell strain 5 (MRC-5) lung cancer cells were procured from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China) and ATCC, USA, respectively. The cells were cultured in Dulbecco’s modified Eagle medium (DMEM)/F12 medium throughout the experimental period. The medium was supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic (penicillin-streptomycin) solution was added to prevent cells from contamination. The cells were incubated in CO2 incubator maintained at 37 °C with 5% CO2 supply. The culture medium was replaced for every 48 h or if the medium colour changes to yellow. Upon reaching 80% confluency, the cells were subcultured using 0.25% trypsin along with 0.02% ethyldiaminetetaacetic acid (Trypsin EDTA).
Cell cytotoxicity assay (MTT assay)
The dose-dependent cytotoxic effect of RR-AuNP was analyzed by treating the lung carcinoma A549 cells and Human lung fibroblast cells MRC-5 with different doses of RR-AuNP ranging from 1 to 50 µg/ml and then subjected to MTT assay [Citation23]. The mitochondrial membrane disruption induced by RR-AuNp was measured by assessing the blue crystals formazan formed due to the diminution of MTT via mitochondrial succinic dehydrogenease secreted by liver cells. The cultured cells were trypsinized, and the cells were counted using cell counter, about 105 numbers of cells were loaded on the 96-well cell culture plates. The lung cancer cells were treated with diverse concentrations of RR-AuNP’s varying from 0 to 50 µg/ml and incubated at a temperature of 37 °C with 5% CO2 for a period of 24 h. The viability of cells was estimated using MTT assay, and the CC50 dose of RR-AuNP’s was calculated for further analysis.
Analysis of RR-AuNP’s anticancer activity
DAPI staining
The anticancer activity of the biosynthesized RR-AuNP was assessed by analyzing the morphology of cells incubated with RR-AuNP using DAPI (4′,6-diamidino-2-phenylindole dihydrochloride) stain and TUNEL (Terminal deoxynucleotidylTransferase (TdT) dUTP Nick-End Labeling) assay. 2 × 105 cells were inoculated in 6 well plates and the plates were treated with biosynthesized gold nanoparticles (15 and 20 µg/ml RR-AuNP), the cells were incubated at a temperature 37 °C for a period of 24 h with 5% CO2. The cells were then fixed for 15 min with 2.5% glutaraldehyde and 0.1% Triton X-100. Fixed cells were then subjected to DAPI staining and incubated for 5 min at the temperature of 37 °C. The stained lung cancer cells were then washed with ice-cold Phosphate Buffered Saline and observed in NIKON Eclipse 80i fluorescent microscope, Japan.
TUNEL assay
After incubation period, the control and treated cells were fixed with paraformaldehyde for 15 min, and then rinsed with PBS. After rinsing, the cells were added with 70% ethanol and incubated in ice for 30 min; the cells were then washed with 50 µl of DNA labelling solution (Abcam) and incubated at 37 °C for 1 h. On completion of incubation, the cells were washed with PBS and treated with PI solution for 30 min, kept at dark place. The stained cells were then observed using NIKON Eclipse 80i fluorescent microscope, Japan.
RR-AuNP effect on caspases activity
Caspases which are key initiators of apoptosis, the RR-AuNP effect on caspases were assessed using the kits procured from Abcam, USA. The substrate p-NA emits light when it cleaved the caspases 3 identifies and cleaves the sequence DEVD, whereas the caspase 9 identifies and cleaves the sequence LEHD present on the labelled p-NA substrate thereby emitting light which was measured at 400 nm.
RR-AuNP activity on reactive oxygen species generation
2',7'-Dichlorodihydrofluorescein diacetate (H2DCFDA) is a cell permeate which is a reduced form of fluorescein is an active indicator of reactive oxygen species. In the presence of reactive oxygen species, the non-fluorescent H2DCFDA is reduced to highly fluorescent substance 2',7'-dichlorofluorescein (DCF) which can be quantified at 530 nm [Citation24]. The anticancer agents generate reactive oxygen species to induce apoptosis in cancerous cells. The levels of reactive oxygen species synthesized by RR-AunNP can be detected with H2DCFDA kit (Invitrogen, USA). A549 cells were cultured in 6 well plates and same cells were treated with two different concentration of 15 and 20 µg/ml RR-AuNP’s for 24 h. After the treatment period, the cells were incubated for 30 min at 37 °C with indicator dye 80 mM H2DCFDA. The plates were then subjected to ELISA reader and the fluorescence intensity exhibited by each was measured at 530 nm. The values obtained for each group were normalized with the concentration of protein respectively.
RR-AuNP anti-apoptotic activity
Western blotting analysis
A549 lung carcinoma cells were cultured in the 60 mm petridishes and treated with RR-AuNP (control, 15 µg/ml, 20 µg/ml) to assess the apoptotic protein expression using immunoblotting analysis. After 24 h of treatment period, the cells were harvested and lysed with ice cold RIPA buffer. The cell lysate was centrifuged at 15,000 rpm for 15 min and the clear supernatant was separated and used for further protein analysis. The cell lysate of each group was subjected to protein estimation and 40 µg of protein from each group were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred to PVDF membrane using the current of 100 mV. The transferred membranes were then blocked with 5% non-fat dry milk for 2 h to avoid non-specific binding of antibodies. The blocked membranes were then incubated with anti-human mouse monoclonal BECN-1 (sc-48341), Bax (sc-7480), Bcl-2 (sc-7382), caspase-3 (sc-7272), Bid (sc-56025) and housekeeping β-actin (sc-47778) antibodies at a dilution of 1:1000 for 12 h at 4 °C. The protein bands separated on the membrane were detected using secondary mouse antibody conjugated with horseradish peroxidase and visualized with chemiluminescence ECL kit, quantified using ChemiDoc XRS molecular imager.
Statistical analysis
The experiments were performed in triplicate, and the results were analyzed with one-way ANOVA followed by the post hoc Student’s Newman Keul’s test using Graph Pad Prism 4 software to detect the statistical significance between the groups. The results were expressed as mean ± standard deviation (SD). Statistically significant difference was considered to be p values <.05
Results
RR-AuNP characterization
UV-Visible spectroscopic and DLS analysis
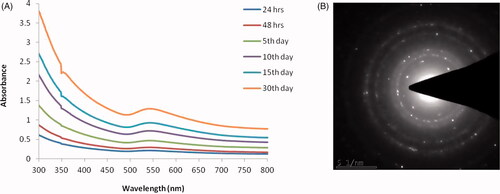
The ultraviolet-visible spectroscopic analysis confirms the synthesis of RR-AuNP. The absorbance peak was observed between 350 and 700 nm, and the highest absorbance peak was detected at 550 nm on 30th day () signifying the synthesis of gold nanoparticles increases, respectively, to the incubation period. The structure of RR-AuNP was analyzed by SAED pattern, it exhibits ring-like structure which signifies the RR-AuNP synthesized is of crystalline nature nanoparticles ().
TEM and EDX analysis
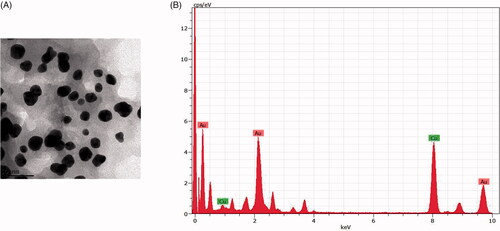
The shape and size of the RR-AuNP synthesized were assessed by high-resolution TEM analysis. of two different magnifications exhibits the polydispered spherical shaped particles. The EDX results confirm the presence of gold nanoparticles in the sample, and it also shows the weak peaks of copper which may be due to the presence other materials bound to the surface of RR-AuNP ().
FTIR& SAED analysis:
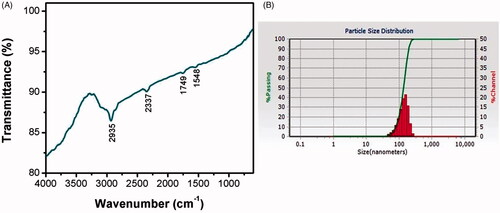
The biomolecules present in the RR-AuNP sample and their capping efficacy were measured by FTIR analysis (). The maximum peak range of RR-AuNP was observed between 1500 and 3000 cm−1, with distinctive peaks at 1548, 1749, 2337, 2935 cm−1 which is due to the reduction of gold ions by R. rubescens. The peak at 2935 cm−1 indicates the existence of hydroxyl group of polyphenols compound from R rubescens, the stretch at 2337 cm−1 specifies the presence of esters, aldehydes and ketones. The thickness of the shell capping the nanoparticles and their actual size was measured using DLS analysis. The average size of the RR-AuNP was found to be approximately 130 nm ().
RR-AuNP anticancer activity
RR-AuNP IC50 dose analysis
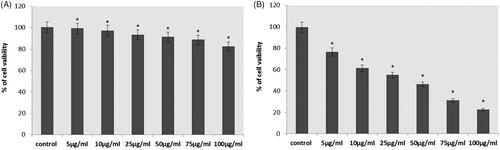
illustrates the cytotoxic effect of different concentration of RR-AuNP against the lung carcinoma A549 cell line and Human lung fibroblast cells MRC-5 assessed using MTT assay. The IC50 dose of synthesized RR-AuNP was obtained at the dose of 50 µg/ml; hence, for the further experiment, the dose of 50 µg/ml and still lower concentration of 25 µg/ml were selected. There is no toxicity was observed in MRC-5 cells.
Figure 5. Cytotoxicity effect of gold nanoparticles synthesised from R. rubescens (RR-AuNP) against lung carcinoma cell line A549 and Human lung fibroblast cells MRC-5 cells treated with different doses of RRAuNP. (A) Human lung fibroblast cells MRC-5 cells; (B) lung carcinoma cell line A549. Each bar represents mean ± SEM of three independent observations. Significant at *p < .05.
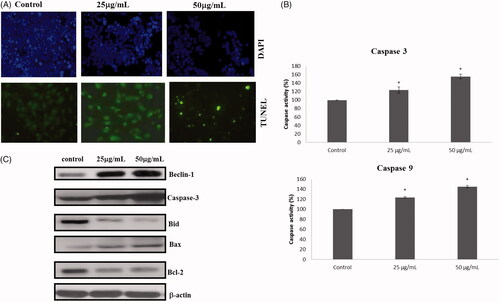
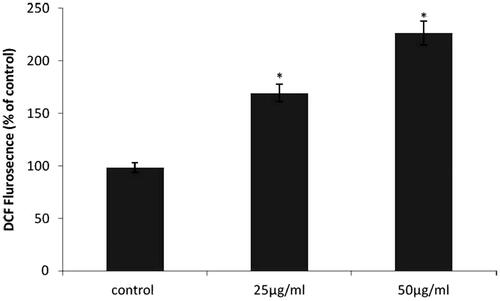
ROS generation of RR-AuNP in A549 cells
shows the intracellular level of reactive oxygen species of control and RR-AuNP treated lung carcinoma A549 cells. About, 50% and 100% rise in ROS content was observed in 25 and 50 µg/ml RR-AuNP treated cells, respectively, which indicate the instigation of apoptosis by the biosynthesized RR-AuNP which was reason for increased number of apoptotic cells observed in DAPI staining.
RR-AuNP apoptotic effect
RR-AuNP apoptotic induction against lung carcinoma A549 cell line was analyzed by DAPI staining & TUNEL assay. shows the DAPI stained images of control and RR-AuNP treated cells. Intact nucleus was observed in the control cells, whereas in 25 µg/ml and 50 µg/ml RR-AuNP decreased number of viable cells. The results were further confirmed with TUNEL which signifies apoptotic cells with increased green fluorescence.
RR-AuNP effect on induction of caspases
Caspases plays a vital role in apoptosis, mainly Caspases 3 is the initiator caspases and caspase 9 are executor caspases. depicts the levels of caspases present in control and lung carcinoma cells treated with different concentration of RR-AuNP. Both the levels of initiator and the executor caspases were significantly elevated in lung carcinoma A549 cells treated with 25 and 50 µg/ml of RR-AuNP, about 1.5 fold increases in caspases levels were detected in the cells treated with 25 µg/ml of RR-AuNP.
Apoptotic effect of RR-AuNP on A549 lung carcinoma cells
represents the expressions of apoptotic protein expression in control and RR-AuNP treated lung carcinoma A549 cells assessed by immunoblotting analysis. These results when compared to control there is noticeable increase in proapoptotic proteins Beclin-1, Bid, Bax, Caspase 3, whereas the Bcl2 protein expression was significantly decreased in both 25 and 50 µg/ml of RR-AuNP treated A549 cells indicating the initiation of apoptosis in lung carcinoma cells.
Discussion
Cancer management is still a challenging task which is to be achieved even though mile stones were reached in treatment of cancer still the survival rate of lung cancer remains to be low. The lung cancer patients were treated with cisplatin or with along with other chemodrugs which ensures the survival rate of only about 20–26% [Citation24]. Hence, it is need of today to discover a persuasive drug to treat lung cancer with nil or less side effects. Nanomedicine is one such boon for treating cancer patient [Citation25], but the synthesis of nanoparticles using chemical leads to the generation of toxic molecules which still worsens the situation of cancer patients. Therefore, we synthesized gold nanoparticles via biological method using R. rubescens, a Chinese herb, in the current study.
The gold nanoparticles generation was confirmed by the ultraviolet-visible spectroscopy analysis which illustrates a maximum peak of 550 nm till the 30th day, which is the characteristic absorption peak of gold nanoparticle [Citation26]. The size and shape of the nanoparticles were exhibited depending upon the SPR property of the metal [Citation27]. FTIR analysis of RR-AuNP showed peak at 1548, 1749, 2337, 2935 cm−1. The peaks in the spectrum imply the presence of esters, ethers and aromatic compounds. The peak at 2935 cm−1 may be due to the OH groups present in alcohols and phenolics [Citation28]. The peak observed was due to the decrease of gold content which may be due to the presence of polyphenols that binds the gold with carbonyl group and cap the gold nanoparticle hence preventing from combination of nanoparticles. The functional components of R. rubescens could have bound to AuCl4 and decreased it to the formation of RR-AuNP. TEM observation was done to analyze the shape of R. rubescens biosynthesised gold nanoparticles, which showed spherical shaped nanoparticles. The molecular composition of RR-AuNP was assessed by EDX using TEM images and it confirms the presence of gold ions.
Chinese tradition medicine prescribes R. rubscens as anticancer drug and it is one of the ingredients in PC-SPES used to treat prostate cancer. Anticancer activity of RR-AuNP against lung carcinoma A549 cell line was assessed using MTT assay. CC50 value was obtained at dose of 50 µg/ml which inhibited the growth of 50% A549; hence, this dosage was selected for the analysis of anticancer effect of RRAuNP. To investigate the property of lower dose of RR-AuNP, 25 µg/ml were also chosen. The cytotoxic effect of RR-AuNP was due to the active components ponicidin and oridonin, two diterpenoids that possess significant anti-angiogenic activity [Citation29], and also due to the interface of gold atoms which deter the intracellular proteins and phosphate, nitrogen groups in DNA [Citation30].
The anticancer property of gold nanoparticles is due to the ability of gold metal to reduce the expressions of signalling proteins which includes Ras, Akt, tumour markers, etc. [Citation31]. The apoptotic signalling proteins which are the key pathway deregulated in most of the cancers, therefore, we assessed the effect of RR-AuNP on apoptotic signalling proteins.
Apoptotic cells were identified by their unique morphology with condensed nucleus, chromatin and fragmented. The late apoptotic cells are seen with blebbed plasma membrane, and it also loses the membrane integrity [Citation32]. Apoptotic induction by RR-AuNP was assessed using DAPI staining; the increased decreased blue fluorescence confirms the increased number of apoptotic cells. It was further confirmed with TUNEL assay which showed increased green fluorescence indicator of DNA breakage, breakdown of DNA is the characteristic event of apoptosis [Citation33].
The main biochemical changes observed in apoptosis are the increase in ROS level, activation of caspases, breakdown of DNA and protein and changes in the membrane [Citation34]. The increase in intracellular ROS levels and the caspases 3, 9 activities were noted in the lung cancer cells treated with RR-AuNP which indicates the apoptotic induction in lung carcinoma A549 cell line, which is also confirmed by DAPI staining and TUNEL assay.
Apoptotic pathway is classified into two pathways as extrinsic pathway which depends on the death receptor activation for the apoptotic initiation and the intrinsic pathway which otherwise called as mitochondrial pathway [Citation35]. The activators of intrinsic pathway are increased ROS levels and cytosolic calcium levels [Citation36]. RR-AuNP increased the levels of intracellular ROS in the A549 cell; hence, we assessed the protein expression of intrinsic apoptotic signalling molecules in control and RR-AuNP treated cell line. Bcl2 family of protein consists of both proapoptotic and antiapoptotic protein. In the present study, there is increase in proapoptotic protein expression of Bid, Bax and decrease in antiapoptotic protein expression of Bcl2 [Citation37]. The increase in levels of caspase 3, in RR-AuNP treated A549 cells confirms the anticancer activity of RR-AuNP. It was also further confirmed by increase in the protein expression of Beclin 1 a mammalian orthologue of yeast Atg6. Bcl2 inhibits the expression of Beclin 1 by binding to the BH3 domain of Beclin 1 thereby controlling the induction of apoptosis [Citation38]. RRAuNp decreased Bcl2 protein expression which would have increased the expression of Beclin-1 leading to the apoptosis induction of lung carcinoma A549 cell line.
Conclusion
To conclude, we have synthesized eco-friendly, biocompatible gold nanoparticle from Chinese traditional medicinal herb R. rubescens leaves extract. Synthesized RR-AuNP was primarily identified with the ruby red colour change, and it is confirmed using UV-Visible spectroscopic analysis which shows the absorption peak at 550 nm. The crystalline structure and size of RR-AuNP were confirmed with SAED and DLS analysis. The size and shape were further confirmed with FTIR, and the presence of gold nanoparticles is confirmed with EDX analysis. The apoptotic property of RR-AuNP was confirmed by estimation of intracellular ROS levels, caspases levels and it further confirmed with DAPI staining, TUNEL assay. The anticancer activity of RR-AuNP was authentically proved by estimating the protein expression of apoptotic signalling proteins. Thereby, it is proven that biosynthesized RR-AuNP is potent anticancer agent against lung carcinoma A549 cell line.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Suganthy N, Sri Ramkumar V, Pugazhendhi A, et al. Biogenic synthesis of gold nanoparticles from Terminalia arjuna bark extract: assessment of safety aspects and neuroprotective potential via antioxidant, anticholinesterase, and antiamyloidogenic effects. Environ Sci Pollut Res Int. 2018;25:10418–10433.
- Schluesener JK, Schluesener HJ. Nanosilver: application and novel aspects of toxicology. Arch Toxicol. 2013;87:569–576.
- Poulose S, Panda T, Nair PP, et al. Biosynthesis of silver nanoparticles. J Nanosci Nanotechnol. 2014;14:2038–2049.
- Chandran SP, Chaudhary M, Pasricha R, et al. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22:577–583.
- Sadhasivam S, Shanmugam P, Yun K. Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf B Biointerf. 2010;1;81:358–362.
- Leng C, Wu J, Xu Q, et al. A highly sensitive disposable immunosensor through direct electro-reduction of oxygen catalyzed by palladium nanoparticle decorated carbon nanotube label. Biosens Bioelectron. 2011;27:71–76.
- Jain SK, Gupta Y, Jain A, et al. Mannosylated gelatin nanoparticles bearing an anti-HIV drug didanosine for site-specific delivery. Nanomedicine. 2008;4:41–48.
- Aswathy J, Jahnavi S, Krishna R, et al. Targeted labeling of cancer cells using biotin tagged avidin functionalized biocompatible fluorescent nanocrystals. J Nanosci Nanotech. 2011;11:7611–7620.
- Philip D. Mangifera indica leaf-assisted biosynthesis of well-dispersed silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 2011;78:327–331.
- Di Paola RS, Zhang H, Lambert GH, et al. Clinical and biologic activity of an estrogenic herbal combination (PC-SPES) in prostate cancer. N Engl J Med. 1998;339:785–791.
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31.
- National Pharmacopoeia Committee. Pharmacopoeia of People's Republic of China 2015. Beijing, China: National Pharmacopoeia Committee; 2015.
- Zhao J, Deng JW, Chen YW, et al. Advanced phytochemical analysis of herbal tea in China. J Chromatogr A. 2013;1313:2
- Takeda Y, Otsuka H. Recent advances in the chemistry of diterpenoids from Rabdosia species. Stud Nat Prod Chem. 1995;15:111e85.
- Liu C, Zhao Z. Advances in research of Rabdosia rubescens (Hemsl.) Hara. Chin Pharm J. 1998;33:577e81.
- Sun HD, Lin ZW, Qin CQ, et al. Studies on the chemical constituents of antitumor plant Rabdosia rubescens (Hemsl.) Hara. Acta Bot Yunn. 1981;3:95e100.
- National Cancer Institute. SEER Cancer Statistics Review, 1975–2011. Available online: http://seer.cancer.gov/csr/1975_2011/
- National Cancer Center; 2017. National Cancer Registry Programme. Three Year Report of Population Based Cancer Registries: 2014–2017.
- Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460.
- Li X, Kabolizadeh P, Yan D, et al. Improve dosimetric outcome in stage III non-small-cell lung cancer treatment using spot-scanning proton arc (SPArc) therapy. Radiat Oncol. 2018;13:35.
- Sinha R, Kim GJ, Nie S, et al. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5:1909–1917.
- Sindhu K, Rajaram A, Sreeram KJ, et al. Curcumin conjugated gold nanoparticle synthesis and its biocompatibility. Rsc Advances. 2014;4:1–18.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
- Klastersky J, Sculier JP, Bureau G, et al. Cisplatin versus cisplatin plus etoposide in treatment of advanced non-small cell lung cancer. Lung cancer working party, Belgium. JCO 1989;7:1087–1092.
- Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760.
- Govindaraju K, Basha SK, Kumar VG, et al. Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J Mater Sci. 2008;43:5115–5122.
- Fayaz MA, Tiwary CS, Kalaichelvan PT, et al. Blue orange light emission from biogenic synthesized silver nanoparticles using Trichoderma viride. Colloid Surf B Biointerf. 2010;75:175–178.
- Ali DM, Sasikala M, Gunasekaran M, et al. Biosynthesis and characterization of silver nanoparticles using marine Cyanobacterium, Oscillatoria willei NTDM01. Digest J Nanomater Biostruct. 2011;6:385–390.
- Sato K, Mochizuki M, Saiki I, et al. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol Pharm Bull. 1994;17:635–639.
- Blagoi YP, Galkin VL, Gladchenko GO, et al. Metal complexes of nucleic acids in solutions. Naukova Dymka, Kiev (in Russian). 1991. p. 272.
- Martins D, Frungillo L, Anazzetti MC, et al. Antitumoral activity of L-ascorbic acid-poly-D,L-(lactide-co-glycolide) nanoparticles containing violacein. Int J Nanomed. 2010;5:77–85.
- Kroemer G, El-Deiry WS, Golstein P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12:1463–1467.
- Vaux D, Silke J. Mammalian mitochondrial IAP-binding proteins. Biochem Biophy Res Commun. 2003;203:449–504.
- Kumar V, Abbas AK, Fausto N, et al. Robins and Cotran: pathologic basis of disease. 8th ed. Philadelphia: Saunders Elsevier; 2010, p. 25–32
- O’Brien MA, Kirby R. Apoptosis: a review of pro-apoptotic and antiapoptotic pathways and dysregulation in disease. J Vet Emerg Crit Care. 2008; 18:572–585.
- Karp G. Cell and molecular biology: concepts and experiments. 5th ed. New Jersey: John Wiley and Sons; 2008, p. 653–657.
- Reed JC. Bcl-2 family proteins: regulators of apoptosis and chemoresistance in haematologic malignancies. Semin Haematol. 1997;34:9–19.
- Kang R, Zeh HJ, Lotze MT, et al. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571