Abstract
Objective
To explore the effects of miR-193b on cell proliferation, migration, invasion and tumourigenicity of renal cell carcinoma, and the underlying molecular mechanisms.
Methods
The expression of miR-193b and IGF1R was detected by quantitative real-time polymerase chain reaction (qRT-PCR). MTT assay was used to detect cell viability. The migration and invasion abilities were measured by transwell assay. Western blot was used to detect the protein expression of IGF1R. Murine xenograft model was established using Caki-1cells transfected with miR193b.
Results
The expression of miR-193b was significantly down-regulated in renal cell carcinoma tissues and cells while the expression of IGF1R was obvious increased in tissues. Overexpression miR-193b or knockdown of IGF1R significantly inhibited the abilities of cells proliferation, migration and invasion in renal cell carcinoma. MiR-193b directly targeted IGF1R and inhibited its expression in vitro and vivo. Up-regulation miR-193b inhibits cells proliferation, migration and invasion of renal cell carcinoma by targeting IGF1R. In addition, overexpression miR-193b significantly inhibited tumour growth in nude mice.
Conclusion
miR-193b can inhibit the growth and metastasis of renal cell carcinoma by targeting decreasing IGF1R expression, which provides a new target for the prevention and treatment of renal cell carcinoma.
Introduction
Renal cell carcinoma (RCC) is one of the most common malignant tumours in the urinary system [Citation1]. According to the research, the incidence of kidney cancer has increased in recent years, only after bladder cancer. At present, radical nephrectomy is the main treatment for renal cell carcinoma while there is still no effective treatment for patients with advanced renal cell carcinoma, metastasis and recurrence. Nowadays, molecular targeted therapies have been applied in the treatment of renal cell carcinoma, which has improved the prognosis of patients [Citation2,Citation3]. However, some patients are resistant to molecular targeted drugs, and the overall survival remains poor. MiR-193b is a kind of microRNA (miRNA). A large number of studies have confirmed that miR-193b is abnormally elevated in various solid malignancies, indicating its cancer-promoting effect in the progression of tumours. However, the role of miR-193b in renal cell carcinoma has not been fully proved.
Insulin-like growth factors (IGFs) are important members of growth factors. IGF1R is widely expressed in various types of cells in living organisms. Its signal transduction pathway is closely related to the occurrence and development of malignant tumours [Citation4,Citation5]. IGF1R mediates the malignant proliferation, invasion and metastasis of tumour cells through a variety of signal transduction pathways, and also mediates the angiogenesis and anti-apoptotic effects of tumour cells [Citation6]. Studies have reported that IGF1R inhibitors have anti-renal cancer effects [Citation7], but the specific mechanism of action is not fully understood.
In this study, we detected the expression of miR-193b and IGF1R in RCC tissues and cells and observed the effects on proliferation, migration and invasion of RCC. In addition, murine xenograft model was established to measure the effects of miR-193b on the growth of RCC. In the end, we found miR-193b can inhibit the growth and metastasis of renal cell carcinoma by targeting decreasing IGF1R expression, which provides a new target for the prevention and treatment of renal cell carcinoma.
Materials and methods
Materials
Experimental animal
Twelve SPF BALB/C nude mice (18–20 g) were purchased from Hunan Slake Laboratory Biology Co., Ltd. Study was carried out in accordance with the regulations of the Laboratory Animal Management and Ethics Committee.
Laboratory reagents and equipment
Human renal tubular epithelial cells HK2, renal cancer cells Caki-1, 786-O, ACHN were purchased from ScienCell, USA; tissue specimens were collected from 45 cases of renal cancer specimens and corresponding adjacent normal tissues from September 2017 to September 2018 in our hospital. The study was approved by the medical ethics committee of the hospital, and all patients and their families signed informed consent. RPMI 1640 culture medium, fetal bovine serum, CCK-8 reagent and trypsin were purchased from GIBCO, USA; LipofectamineTM2000, CBA protein quantification kit and reverse transcription kit were purchased from Dalian Takara Company; the dual luciferase reporter assay kit was purchased from Promega, USA; Matrix glue and Transwell chamber were purchased from Coming company, USA; the gel imaging analyzer was purchased from Kodak Company; The semi-dry film transfer machine was purchased from BIO-RAD, USA; the ABI 7500 real-time PCR system was purchased from ABI, USA; The cell culture incubator was purchased from Forma Scientific, USA; the PCR instrument was purchased from BIO-RAD, USA.
Methods
Cell culture
Human renal tubular epithelial cells HK2, renal cell carcinoma cells Caki-1, 786-O and ACHN were cultured in RPMI 1640 medium containing 10% fetal bovine serum. The cells were cultured in an incubator at 37 °C and 5% CO2.
Cell transfection
MiR-193b mimics (miR-193b), miRNA negative control (miR-con), inhibitor targeting miR-193b (anti-miR-193b) and their corresponding negative control (anti-con), small interfering RNA (siRNA) targeting IGF1R (si- IGF1R), siRNA negative control (si-con), PcDNA 3.1 (Ctrl) and PcDNA 3.1-IGF1R (IGF1R) were transfected into Caki-1 cells using LipofectamineTM2000 for 24 h. After successful transfection, the cells in each group were used for subsequent experiments.
QRT-PCR assay
An appropriate amount of cells in the logarithmic growth phase 1.2.2 was taken. The cells were lysed with Trizol solution and extracted RNA according to the instructions of the RNA extraction kit and then quantified. Next, cDNA was synthesized using reverse transcription kit according to the instructions. The detection of IGF1R and miR-193b was carried out according to the instructions of the qRT-PCR kit operation. The relative expression levels of IGF1R and miR-193b were calculated using 2-ΔΔCt.
Western blot assay
Proper amount of 1.2.2 cells in logarithmic growth phase were taken and lysed in RIPA lysis buffer. The protein was quantified using BCA method. Total proteins were subjected to SDS-PAGE gel and transferred to PVDF transfection and blocked for 2 h. Subsequently, the membranes were incubated with primary antibody at 4 °C overnight. The next day, the membrane was washed and incubated with secondary antibody at 37 °C for 2 h. Finally, the developing mixture was added and exposed. The expression of the target protein IGF1R is represented by the ratio of the grey value of the target band to the grey value of β-actin.
CCK-8 assay
Cells were seeded into 96-well plates at a density of 5000 cells per well and then incubated with 20 μl of CCK-8 solution after cultured at 24, 48, 72, 96, 120 h. The absorbance was measured at 490 nm with a microplate reader. Cell proliferation is directly proportional to the absorbance of cells.
Transwell assay
100 μl cell suspension (1 × 105 cells/mL) was seeded in the upper chambers. 600 μl of serum-containing medium was added to the lower chamber and cultured overnight. The chamber was removed, and the noninvasive cells in the upper chamber were wiped with a cotton swab, washed three times with PBS, fixed for 30 min and stained with 0.1% crystal violet for about 20 min. The migrating cells attached to the lower surface of the chamber were observed under a microscope, and five fields of view were randomly taken and counted. Cell invasion detection needs to apply appropriate thickness of matrix glue on the upper surface of the chamber, and the rest should be operated according to the experimental steps of migration detection.
Luciferase reporter gene detection assay
An appropriate amount of cells in logarithmic growth phase 1.2.2 in each group was taken and operated according to the dual luciferase reporter gene detection kit technical manual requirements. The psiCHECK2 vector uses firefly luciferase activity as an internal reference. The expression of psiCHECK2-IGF1R-3′UTR WT and psiCHECK2-IGF1R-3′UTR MUT was used as a control to observe the binding ability of miR-193b to IGF1R.
Nude mouse tumour formation experiment
The SPF nude mice were divided into NC group and miR-193b group, six rats in each group. 0.2 ml of cancer cells with a concentration of 1 × 10 6/mL were absorbed with a 1 ml syringe, tumours were inoculated on the right forelimb of nude mice, and the cells were kept in the SPF environment. The longest diameter A (mm) and the shortest diameter B (mm) of the tumours were measured with vernier callipers 5 days a week from the second day. The volume of tumours was calculated by V = a*b2*0.52 and the mean value was taken. On the 30th day, large tumours were formed subcutaneously in nude mice. The nude mice were sacrificed, and tumour samples were weighted and applied for further experiment.
Statistical analysis
All data in the experiment were analyzed using SPSS 21.0 software. Measurement data were expressed as mean ± standard deviation (x ± s). Data between groups were compared using one-way analysis of variance. Pairwise comparisons were performed using SNK-q test. The difference was statistically significant with p < .05.
Results
Expression of miR-193b and IGF1R in renal cell carcinoma
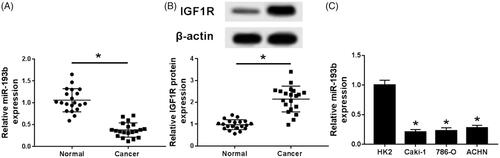
qRT-PCR was used to detect the expression of miR-193b. Compared with normal adjacent tissues, the expression of miRNA-193b in renal cell carcinoma tissues was significantly decreased (). In addition, miR-193b in renal cell carcinoma cells, including Caki-1, 786-O and ACHN also decreased compared with renal epithelial cells (). Western blot was used to detect the expression of IGF1R protein in renal cell carcinoma and adjacent normal tissues and a significantly increased of IGF1R protein was found in renal cell carcinoma ().
Figure 1. Expression of miR-193b and IGF1R in renal cell carcinoma. (A) Expression of miR-193b in renal cancer tissues and paracancerous tissues; (B) expression of IGF1R in renal cancer tissues and paracancerous tissues; (C) expression of miR-193b in renal cancer cells and renal tubular epithelial cells; *p < .05.
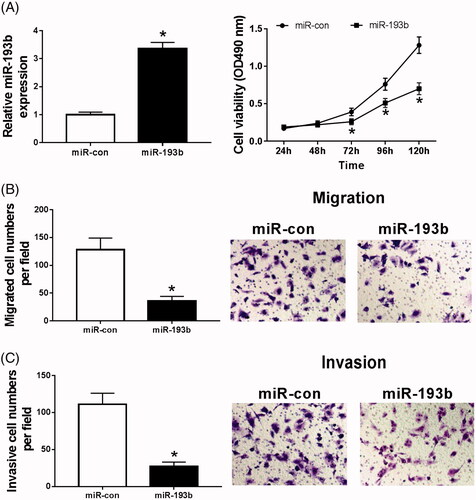
Overexpression of miR-193b inhibits proliferation, migration and invasion of renal cancer cells
MiR-193b mimics were transfected into Caki-1 cells and the transfection efficiency was measured (). After transfected, we found the abilities of cells proliferation (), migration () and invasion () were significantly suppressed. Therefore, overexpression of miR-193b inhibits proliferation, migration and invasion of renal cell carcinoma.
Figure 2. Overexpression of miR-193b inhibits proliferation, migration and invasion of renal cancer cells. (A) The effect of over-expression of miR-193b on the expression of miR-193b in CaKi-1 cells; (B) the effect of over-expression of miR-193b on the activity of CaKi-1 cells; (C,D) the effect of over-expression of miR-193b on the migration and invasion of Ki-1 cells; *p < .05.
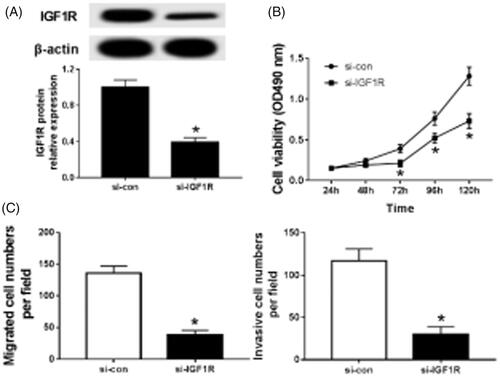
Knockdown of IGF1R inhibits proliferation, migration and invasion of caki-1 cells
Caki-1 cells were transfected with si-IGF1R or si-con and the transfection efficiency was measured (). After transfected, the abilities of cells proliferation (), migration () and invasion () were significantly inhibited. It can be seen that knockdown of IGF1R inhibits proliferation, migration and invasion of renal cancer cells.
Figure 3. Knockdown IGF1R inhibits proliferation, migration and invasion of Caki-1 cells. (A)The effect of knocking down IGF1R on the expression of IGF1R in CaKi-1 cells; (B) the effect of knocking down IGF1R on the activity of CaKi-1 cells; (C) the effect of knocking down IGF1R on the migration and invasion of CaKi-1 cells; *p < .05.
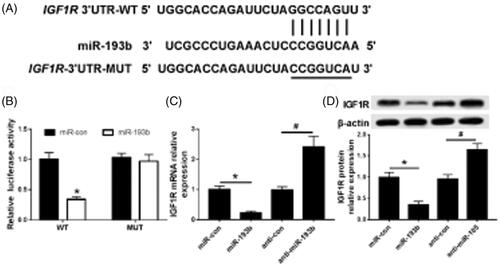
IGF1R is a target of miR-193b
The potential seed sites of miR-193b and IGF1R were shown in . Luciferase reporter analysis indicated that the luciferase activity was depressed in Caki-1 cells co-transfected with IGF1R-wt and miR-193b while there was no change in IGF1R-mut group (). In addition, the mRNA () and protein () expression of IGF1R were detected in Caki-1 cells transfected with miR-193b or miR-con or anti- miR-193b or anti-con, and the data showed miR-193b inhibited the expression of IGF1R.
Figure 4. miR-193 targets IGF1R. (A) MiR-193 targeting combined with sequence information of IGF1R gene 3'UTR; (B) MiR-193b targeting on luciferase activity in CaKi-1 cells; (C) MiR-193b targeting on the expression of IGF1R in CaKi-1 cells; (D) MiR-193b targeting on the expression of IGF1R protein in CaKi-1 cells; compared with the group of MiR-con, *p < .05, compared with the group of anti-MiR-con, #p < .05.
Overexpression of miR-193b inhibits proliferation, migration and invasion of renal cancer cells via IGF1R
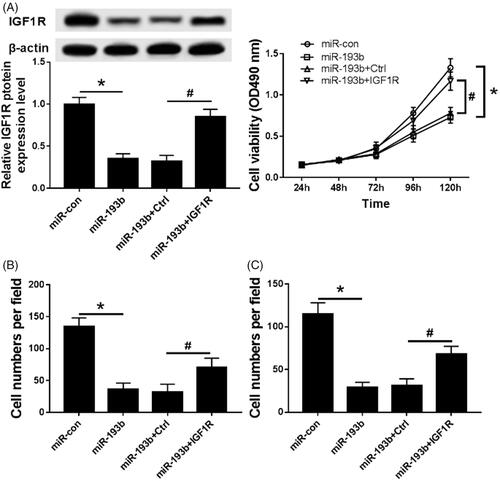
MiR-193b or miR-con or miR-193b and Ctrl or miR-193b and IGF1R were transfected into the Caki-1 cells. Compared with the miR-con group, the expression of IGF1R was significantly decreased in the miR-193b group () and the abilities of cells proliferation (), migration () and invasion () were significantly inhibited. Whereas overexpression of IGF1R partially reverses the inhibitory effect of miR-193b on proliferation, migration and invasion of Caki-1 cells. It can be seen that overexpression of miR-193b inhibits proliferation, migration and invasion of renal cancer cells via IGF1R
Figure 5. Overexpression of IGF1R partially reverses the inhibitory effect of miR-193b on proliferation, migration and invasion of Caki-1 cells. (A) The effect of overexpression of IGF1R on the expression of IGF1R in CaKi-1 cells; (B) The effect of overexpression of IGF1R on the activity of CaKi-1 cells; (C) The effect of overexpression of IGF1R on the migration and invasion of Ki-1 cells; Compared with the MiR-con group, *p < .05; Compared with the MiR-193b + Ctrl group, #p < .05.
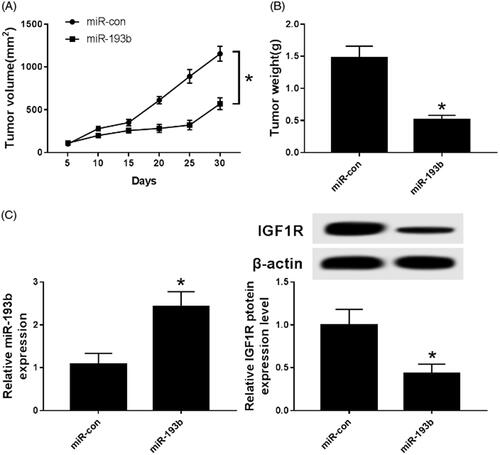
Effect of over expression of miR-193b on tumourigenic ability in nude mice
The Caki-1 cells transfected with miR-193b mimics were subcutaneously transplanted into nude mice and labelled as miR-193b group nude mice. Nude mice transplanted with Caki-1 cells transfected with miR-con were labelled as the miR-con group. Compared with the MiR-con group, the size () and weight () of tumours in the MiR-193b group was significantly decreased. Subsequently, the expression of miR-193b and IGF1R were detected and the data showed an up-regulation of miR-193b () while down-regulation of IGF1R (). It is obvious that over expression of miR-193b inhibits tumour growth in nude mice.
Figure 6. Effect of overexpression of miR-193b on tumourigenic ability of nude mice. (A) The effect of over-expression of miR-193b on the tumourigenic volume of nude mice; (B) the effect of over-expression of miR-193b on the tumourigenic weight of nude mice; (C) the expression of miR-193b in tumourigenic tissue of nude mice; (D) the expression of IGF1R protein in tumourigenic tissue of nude mice; *p < .05.
Discussion
miRNAs are endogenous, short chain, non-coding small molecule RNA with a size of 20–25 nt. It regulates target gene expression at post-transcriptional level through partial or complete binding of base complementary pairing with target gene 3 Eucalyptus UTR [Citation8]. miRNAs are involved in the occurrence and development of various cancer diseases, including kidney disease and renal cell carcinoma [Citation9]. MiR-193 acts as a tumour suppressor gene in oesophagal cancer, ovarian cancer, breast cancer and liver cancer [Citation10–13]. Trevisani et al. [Citation14] showed that the expression of miR-193b-3p was increased in renal cell carcinoma patients who received nephrectomy and was highly correlated with the risk of developing chronic kidney disease. In this study, qRT-PCR was used to detect the expression of miR-193b in renal cell carcinoma and renal cancer cells, and low expression of miR-193b was observed. CKK-8 and Transwell experiments showed that over-expression of miR-193b could inhibit the proliferation, migration and invasion of renal cell carcinoma cells.
IGF-1 receptor (IGF1R) is a transmembrane tyrosine kinase receptor, which plays a vital role in cell proliferation, growth, differentiation as well as apoptosis. IGF-1 and insulin share the downstream signalling pathway in normal cells and cancer cells [Citation15]. IGF1R can promote malignant transformation and induce cell proliferation, differentiation but inhibit cell apoptosis [Citation22]. It acts as an oncogene in several cancers, including invasive bladder cancer, breast cancer, pancreatic cancer and ovarian cancer [Citation16–18]. As early as 1995, Kellerer et al [Citation19] reported that the expression of IGF1R in renal cell carcinoma increased more than twice, and there was no significant correlation between the increase of insulin receptor tyrosine kinase activity and the change of insulin receptor homotype expression. Recent investigations by Eich et al [Citation20] in Japan showed that IGF1R was positively expressed in upper urothelial carcinoma (UTUC) and associated with tumour recurrence, progression, total mortality and specific mortality. This study provided evidence for IGF1R as a potential therapeutic target for UTUC. Sichani et al. [Citation21] showed a significant negative correlation between IGF1R and the survival time of RCC patients undergoing radical nephrectomy. The death risk of clear cell renal cell carcinoma (ccRCC) patients with high expression of IGF1R increases by 70% and has higher chemical resistance. Silencing IGF1R increases the chemical sensitivity of ccRCC cells. This study examined the expression of IGF1R in renal cell carcinoma and renal cell carcinoma and found that IGF1R was highly expressed in renal cell carcinoma. Knock-down of IGF1R has similar effect to over-expression of miR-193b on renal cell carcinoma, and over-expression of IGF1R can reverse the inhibitory effect of miR-193b on proliferation, migration and invasion of renal cell carcinoma.
In recent years, the abnormal expression of miRNA in tumours plays a more and more important role. It can participate in the occurrence and development of tumours through a variety of target genes and signalling pathways. The relationship between miRNA and target genes is complex. One miRNA can target multiple target genes simultaneously and participate in different physiological regulation. One target gene can be the common target of multiple miRNA. Li et al. [Citation23] showed that the IGF1R is a key target gene of miR-193b in the study of osteoarthritis. Tumour necrosis factor-α can inhibit the expression of hsa-circ-0045714 in chondrocytes, thereby up-regulating the expression of miRNA-193b. Both IGF1R and hsa-circ-0045714 can promote the expression of collagen II and aggregating proteoglycan, and up-regulate the proliferation of chondrocytes. MiR-193b can inhibit the expression of collagen II and aggregation proteoglycan, down-regulate the proliferation of chondrocyte but enhance its apoptosis. Over-expression of IGF1R can reverse the effect of miR-193b while over-expression of miR-193b or silence of IGF1R can inhibit the function of hsa-circ-0045714. In this study, double luciferase reporter gene assay was used to verify that IGF1R is the target of miR-193b, and miR-193b can negatively regulate IGF1R. In-depth study of in vitro xenotransplantation of CaKi-1 cells overexpressing miR-193b in nude mice showed that the growth of tumours was significantly inhibited, and the expression of IGF1R in tumours was significantly down-regulated, suggesting that miR-193b could inhibit the growth and metastasis of renal carcinoma by targeting negative regulation of IGF1R.
In summary, miR-193b has a series of biological behaviours that inhibit the proliferation, migration and invasion of renal cancer cells and inhibit the growth and metastasis of tumours in vivo. This mechanism may be related to the targeted negative regulation of IGF1R and provide support for miR-193b targeted therapy for renal cell carcinoma.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Capitanio U, Montorsi F. Renal cancer. Lancet. 2016;387:894–906.
- Morris MR, Latif F. The epigenetic landscape of renal cancer. Nat Rev Nephrol. 2017;13:47–60.
- Schmidt LS, Linehan WM. Genetic predisposition to kidney cancer. Semin Oncol. 2016;43:566–574.
- López IP, Rodriguez-de la Rosa L, Pais RS, et al. Differential organ phenotypes after postnatal Igf1r gene conditional deletion induced by tamoxifen in UBC-CreERT2; Igf1r fl/fl double transgenic mice. Transgenic Res. 2015;24:279–294.
- Werner H, Sarfstein R. Transcriptional and epigenetic control of IGF1R gene expression: implications in metabolism and cancer. Growth Horm IGF Res. 2014;24:112–118.
- Liu JP, Baker J, Perkins AS, et al. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993;75:59–72.
- Rota LM, Albanito L, Shin ME, et al. IGF1R inhibition in mammary epithelia promotes canonical Wnt signaling and Wnt1-driven tumors. Cancer Res. 2014;74:5668–5679.
- Yamaguchi R, Harada H, Hirota K. VHL-deficient renal cancer cells gain resistance to mitochondria-activating apoptosis inducers by activating AKT through the IGF1R-PI3K pathway. Tumor Biol. 2016;37:13295–13306.
- Chandrasekaran K, Karolina DS, Sepramaniam S, et al. Role of microRNAs in kidney homeostasis and disease. Kidney Int. 2012;81:617–627.
- Chan CM, Lai KKY, Ng EKO, et al. Serum microRNA-193b as a promising biomarker for prediction of chemoradiation sensitivity in esophageal squamous cell carcinoma patients. Oncol Lett. 2018;15:3273–3280.
- Zhang J, Qin J, Su Y. miR-193b-3p possesses anti-tumor activity in ovarian carcinoma cells by targeting p21-activated kinase 3. Biomed Pharmacother. 2017;96:1275–1282.
- Yang Z, Zhuang Q, Hu G, et al. MORC4 is a novel breast cancer oncogene regulated by miR-193b-3p. J Cell Biochem. 2019;120:4634–4643.
- Yin W, Nie Y, Chen L, et al. Deregulation of microRNA-193b affects the proliferation of liver cancer via myeloid cell leukemia-1. Oncol Lett. 2018;15:2781–2788.
- Trevisani F, Ghidini M, Larcher A, et al. MicroRNA 193b-3p as a predictive biomarker of chronic kidney disease in patients undergoing radical nephrectomy for renal cell carcinoma. Br J Cancer. 2016;115:1343–1350.
- Solarek W, Czarnecka AM, Escudier B, et al. Insulin and IGFs in renal cancer risk and progression. Endocr Relat Cancer. 2015;22:R253–R264.
- Iida M, Tsuboi K, Niwa T, et al. Compensatory role of insulin-like growth factor 1 receptor in estrogen receptor signaling pathway and possible therapeutic target for hormone therapy-resistant breast cancer. Breast Cancer. 2019;26:272–281.
- Chen B, Li Q, Zhou Y, et al. The long coding RNA AFAP1-AS1 promotes tumor cell growth and invasion in pancreatic cancer through upregulating the IGF1R oncogene via sequestration of miR-133a. Cell Cycle. 2018;17:1949–1966.
- Zorea J, Prasad M, Cohen L, et al. IGF1R upregulation confers resistance to isoform-specific inhibitors of PI3K in PIK3CA-driven ovarian cancer. Cell Death Dis. 2018;9:944.
- Kellerer M, von Eye Corleta H, Mühlhöfer A, et al. Insulin- and insulin-like growth-factor-I receptor tyrosine-kinase activities in human renal carcinoma. Int J Cancer. 1995;62:501–507.
- Eich ML, Tregnago AC, Faraj SF, et al. Insulin-like growth factor-1 receptor expression in upper tract urothelial carcinoma. Virchows Arch. 2019;474:21–27.
- Sichani MM, Yazdi FS, Moghaddam NA, et al. Prognostic value of insulin-like growth factor-I receptor expression in renal cell carcinoma. Saudi J Kidney Dis Transpl. 2010;21:69–74.
- Tracz AF, Szczylik C, Porta C, et al. Insulin-like growth factor-1 signaling in renal cell carcinoma. BMC Cancer. 2016;16:453
- Li BF, Zhang Y, Xiao J, et al. Hsa-circ-0045714 regulates chondrocyte proliferation, apoptosis and extracellular matrix synthesis by promoting the expression of miR-193b target gene IGF1R. Hum Cell. 2017;30:311–318.
